Review Article - Interventional Cardiology (2021)
Cerebral autoregulation in hypertensive patients: A review
- Corresponding Author:
- Michel Ferreira Machado
Department of Neurology, Hospital das Clínicas, University of São Paulo Medical School, Av. Dr. Enéas de Carvalho Aguiar, 255, 05403-000 São Paulo, Brazil,
E-mail: michelfmachado83@gmail.com
Received date: April 14, 2021; Accepted date: April 28, 2021 Published date: May 05, 2021
Abstract
Cerebral Autoregulation (AR) consists of a complex mechanism characterized by the ability of the brain microcirculation to contract and dilate in response to variations in Blood Pressure (BP), aiming at maintaining a constant Cerebral Blood Flow (CBF). Vascular changes induced by Systemic Arterial Hypertension (SAH), such as thickening of the smooth muscle layer and intimal proliferation, result in reduced luminal diameter and increased cerebrovascular resistance (CVR). Here, the mechanisms of AR and the repercussions of these histological changes on the microcirculatory capacity to maintain constant CBF in these patients will be reviewed. The material for this review was taken mostly from electronic journals. To collect publications, PubMed e Cochrane database of systematic reviews were used.
Keywords
Blood cerebral flow • Cerebrovascular autoregulation • Hypertension • Ultrasonography • Doppler
Introduction
The human brain accounts for about 2% of body weight, receives 15%-20% of cardiac output and is responsible for approximately 20% of total body oxygen consumption. Much of this energy requirement is provided from glucose metabolism, which during its oxidative or aerobic process, promotes the generation of 38 molecules of adenosine triphosphate (ATP), in order to ensure the proper functioning of the Na+/K+ pump and promote ionic homeostasis. Neuronal cells, however, have different thresholds of electrical and metabolic dysfunction, which helps to understand, for example, ischemic penumbra as an area of brain tissue, in which these cells are able to maintain basic metabolic processes, remaining electrically “quiescent”, that is, at a still potentially reversible stage. From that stage, the progressive reduction in Cerebral Blood Flow (CBF), if not restored, can initiate a chain of events that will determine neuronal death [1].
As a result, to prevent or delay the onset of this harmful chain of events, the brain tissue has an active mechanism in order to always keep the supply of oxygen and nutrients adequate to satisfy its high energy consumption, called pressure autoregulation or autoregulation (AR) [2].
Here, the mechanisms of AR, cerebral microvascular changes secondary to chronic arterial hypertension and their impact on the microcirculatory capacity to maintain constant CBF in these patients will be reviewed.
Literature Review
Pressure autoregulation or cerebral autoregulation
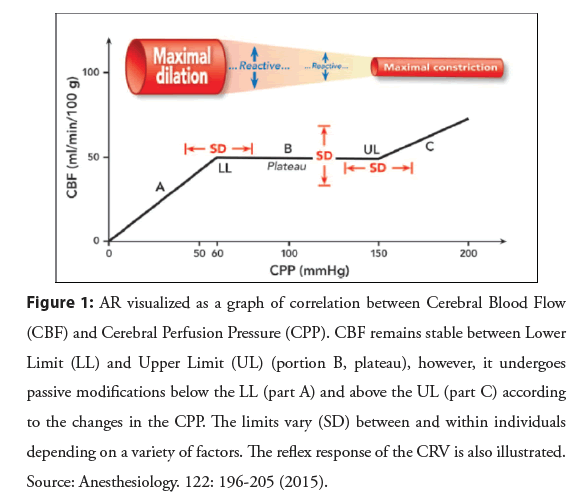
The process by which CBF remains relatively constant, despite changes in Cerebral Perfusion Pressure (CPP) and which depends on the reflex response of cerebrovascular resistance (CRV) to these changes, is known as pressure autoregulation or autoregulation (AR)2 (Figure 1).
Figure 1: AR visualized as a graph of correlation between Cerebral Blood Flow (CBF) and Cerebral Perfusion Pressure (CPP). CBF remains stable between Lower Limit (LL) and Upper Limit (UL) (portion B, plateau), however, it undergoes passive modifications below the LL (part A) and above the UL (part C) according to the changes in the CPP. The limits vary (SD) between and within individuals depending on a variety of factors. The reflex response of the CRV is also illustrated. Source: Anesthesiology. 122: 196-205 (2015).
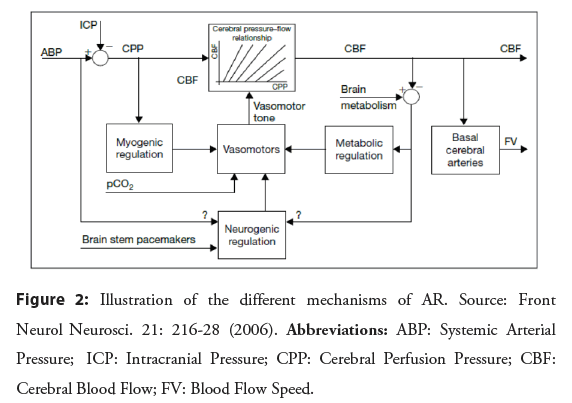
Pre-capillary arterioles and pial branches are classically known as the site responsible for AR. These arterial segments are extremely sensitive to changes in gas concentrations and changes in Blood Pressure (BP) values, serving as the first line of defense in maintaining cerebral perfusion. Three different mechanisms are proposed to be responsible for this cerebrovascular response to changes in CPP [2,3]:
• Myogenic mechanism: related to changes in transmural pressure (instead of intravascular pressure alone) which affect the forces under the Smooth Muscle Cells (SMC) of the pre-capillary arterioles and pial branches, which, depending on their degree of stretching, contract or relax promoting rapid control of blood flow [2,3].
• Neurogenic mechanism: depends on sympathetic (vasoconstrictor) and parasympathetic (vasodilator) autonomic activity, regulated by means of sensory fibers that arise outside the brain and are found in the large conductance vessels, pial arteries and penetrating arterioles [4].
• Metabolic mechanism: products of neuronal and endothelial metabolism exert a vasodilatory or vasoconstrictive influence on the SMC of the arterial wall, controlling the pressure of autoregulation from the primary determinant of regional flow, which is the local metabolic activity [5].
The neurogenic and metabolic mechanisms have a longer latency period to complete their actions [6]. Despite this, it is through the continuous vasoconstrictor and vasodilator activity regulated simultaneously by these three different mechanisms, that AR is able to maintain blood flow adequate to the needs of the brain [7], as illustrated in Figure 2.
Dynamic model for assessing autoregulation
Sudden changes in BP induce concomitant changes in CBF, but it starts to recover and reestablishes its baseline value in a shorter time interval than necessary for the reestablishment of BP [8]. The existence of this time interval (latency) until recovery of the CBF present before the sudden changes in BP indicates that the autoregulatory capacity of the brain vasculature is not perfect, being dependent on the severity and speed of the BP modification and therefore, among other reasons, it should not be analyzed only as a stationary phenomenon [9].
Thus, by recognizing the important role of time in the BP × CBF relationship, the dynamic study of AR enabled a better understanding of its physiology and became the model of choice, in most situations, for its clinical evaluation [6].
One of the first techniques used to induce a rapid change in BP in order to analyze AR using the dynamic model (dynamic AR) was the femoral cuff technique (thigh cuff test) [9]. The technique consisted of using wide cuffs (20 cm) that were placed around both thighs and inflated to values of 20-40 mmHg above systolic pressure for at least 2 minutes. The rapid reduction in peripheral resistance, resulting from the deflation of the cuffs, led to an equal reduction in Cerebral Blood Flow Speed (CBFS) and BP, which remained low for about 5-10s until the baroreceptor mechanism restored it to its previous value. With regard to CBFS, in physiological situations, the time required for its restoration to baseline values would be less than that necessary for the normalization of BP, but if AR was compromised, CBFS would take the same time interval as BP for return to their initial values [9].
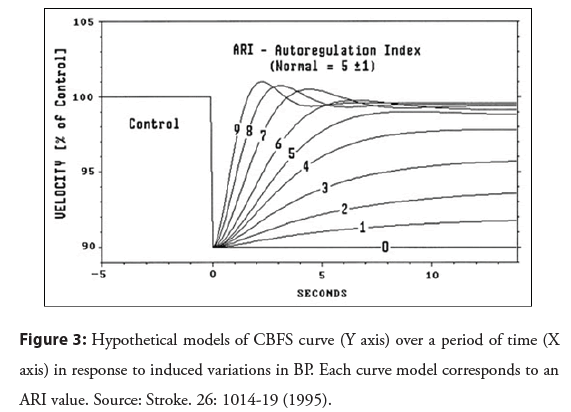
The thigh cuff test was used by Tiecks et al. [10] when analyzing dynamic AR. The authors used the mean values of CBFS and BP recorded in the 30 seconds following the deflation of the cuffs to calculate autoregulation index (ARI) that would reflect the changes, every second, in the CRV in relation to the changes in BP. Based on the actual BP curve over that period of time, a computer program created a hypothetical concomitant CBFS curve, assuming that the CBFS varied directly in proportion to the BP variations. Another 10 possible CBFS curve models were also created and each corresponded to an ARI value, being 0 (absence of AR) and 9 (best existing AR response). Each of these curve models was then compared to the actual CBFS records to identify the one that best represented the actual CBFS curve and, therefore, would correspond to the individual’s ARI value (Figure 3) [10].
Figure 3: Hypothetical models of CBFS curve (Y axis) over a period of time (X axis) in response to induced variations in BP. Each curve model corresponds to an ARI value. Source: Stroke. 26: 1014-19 (1995).
In addition, based on the instant analysis of this linear relationship between BP and CBF, processed by different mathematical models, it is possible to obtain other different hemodynamic parameters: Critical Closing Pressure (CrCP) and area-resistance product (RAP). The first refers to the theoretical value of BP with which the CBF is non-existent, functions as a marker of the tone of the pre-capillary arteriole and can represent the metabolic mechanism of AR [11]. The second corresponds to the CRV and, therefore, can represent the myogenic mechanism of AR [12]. The evaluation of these parameters in the context of AR seems to be more appropriate to interpret the brain’s circulatory response.
Autoregulation in hypertensive patients
Some clinical practice situations may be associated with some degree of impairment of any of the cerebral hemodynamic variables and, therefore, AR and depending on the severity, correlate with the more or less favorable neurological outcome. One of these situations is Systemic Arterial Hypertension (SAH).
Almost a century after the discovery of William Harvey and shortly after Stephen Hales performed the first known BP measurement in history on his mare, a young professor of medicine in Berlin, Samuel Schaarschmidt, who died prematurely in 1747, identified and suggested how to treat a clinical condition defined as “spastic constriction of the arteries”, what today is called “essential” or “primary” hypertension. However, the histological bases of Schaarschmidt’s discovery were already known even before BP was measured in humans, as Richard Bright (1836) and George Johnson (1968) demonstrated that chronic “Bright’s disease” was characterized by hypertrophy of the heart wall, arteries and arterioles [13].
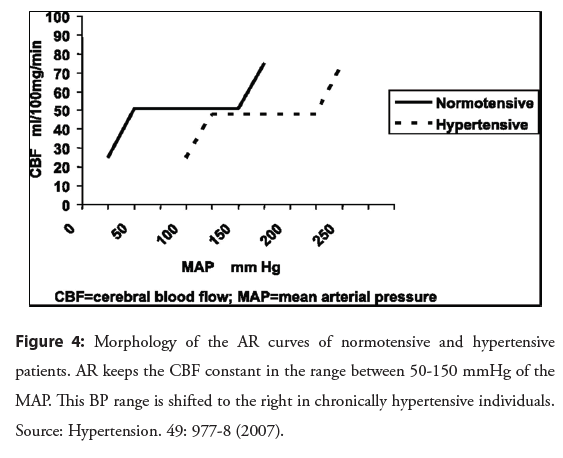
This arterial hypertrophy and remodeling are adaptive responses present in the early stages of SAH, which reduce stress on the arterial wall and protect arterioles, capillaries and venules from this increase in BP. In the chronic phase of the disease, additional changes occur that include the accumulation of fibrous proteins, elastin and collagen, degeneration of SMC and reduced endothelial nitric oxide synthesis This contributes to increase the CRV, which can have an impact on autoregulatory control, resulting in the deviation towards higher values of the upper and lower limits of the AR (Figure 4) [14,15].
Although transmural hemodynamic stress is the main determinant of these arterial changes found in hypertensive patients, some intracellular signaling cascades activated by the Renin-Angiotensin- Aldosterone System (RAAS) can also stimulate the remodeling of peripheral resistance arteries. In this regard, the process mediated by the production and performance of reactive oxygen species, such as superoxide, linked to angiotensin II (Angio II) and aldosterone [16] and mineralocorticoid receptors that stimulate the enzymatic activity of metalloloproteinases, inducing the degradation of the extracellular matrix, one of the necessary mechanisms for the remodeling to occur [17].
RAAS also play, independently of arterial remodeling, an important role in AR. AngioII helps to regulate BP through vasoconstriction secondary to the stimulation of angiotensin 1 (AT1) receptors of vascular SMC. Once present in the microcirculation and cerebral arteries, Angio II receptors and the Angiotensin-Converting Enzyme (ACE) indicate that CBF can also be modulated by this hormone [18].
Another structural alteration related to SAH, but specific to the brain tissue, is the rarefaction of its arterioles and capillary network. Sokolova et al. [19] using different experimental models of secondary hypertension (such as renal artery ligation or subcutaneous administration of deoxycorticosterone acetate saline (DOCA) in rats with unilateral nephrectomy) and spontaneously hypertensive rats identified a 25%-50% reduction in the number of pial arteries and intracerebral capillaries (diameter 1.4-5.6 μm).
Even though it has not been studied directly in hypertensive patients, this seems to be another mechanism, regardless of the change in AR pressure limits, which may contribute to cerebral hypoperfusion in chronic SAH, since the loss of capillaries in the white matter is already observed even before a significant reduction in CBF and the appearance of subcortical lesions [20].
The evaluation of the relationship between the magnitude of the impact of spontaneous BP changes on CBF has traditionally been carried out by the transfer function analysis, which establishes that CRV responds quickly to changes in BP to mitigate changes on CBF, each heartbeat [9,21]. Even so, with an increase in the thickness of the cerebral arterioles normally found in hypertensive patients, there is a greater transmural passive transmission of BP, which could cause some AR involvement and damage to the surrounding brain tissue [21].
Thus, to better understand the behavior of AR in these patients, Serrador et al. [22] through dynamic analysis, sought to correlate the transfer function with the CRV. The hypothesis generated was that if the AR was intact, changes in BP would cause minimal changes in the CBF and the gain in the transfer function would be low. On the other hand, if it were compromised, there would be a greater impact on the CBF and the gain in the transfer function would be high.
The authors found that both hypertensive and normotensive individuals presented reductions in the gain of the transfer function, but interestingly the hypertensive patients suffered less with variations in BP, as their AR curve shifted to the right (requiring greater reductions to trigger a symptom), has less vasoreactivity to CO2 (which means that, for example, they do not present a change in CBF, despite the hypocapnia of orthostasis) and are less influenced by the action of vascular tone (which is known to interfere both in transmission and in attenuation of BP). Thus, any hypertensive patient could undergo a safe reduction in BP, without having to worry about cerebral hypoperfusion and early neurological symptoms [22].
As a result, interest in evaluating the behavior of AR began to grow no longer at each heartbeat, as was done by analyzing the transfer function, but within the same heartbeat, valuing the variables instantaneous, CrCP and RAP, generated from the BP × CBF relationship, in order to improve the understanding of the complex brain vascular regulatory system [23].
To this end, Robertson et al. [23] investigated whether the CrCP and the RAP were more sensitive than the analysis of the transfer function in detecting minimal changes in AR, based on a model in which these variables were evaluated as regulators of AR, aiming at better identify differences between young and normal or hypertensive adults.
Using this model, the authors found that RAP, representing CRV, is high in hypertensive patients compared to young people, but CrCP, an indicator of arteriolar wall tension, is similar among them. Furthermore, the reduction in CrCP has been shown to be the primary regulator of CRV in response to the hypotensive stimulus [23].
Therefore, despite the well-known arteriolar stiffening, hypertensive individuals really maintain their ability to attenuate changes in CBF secondary to changes induced in BP, suggesting that chronic adaptation due to SAH is associated with changes in vascular distensibility and not with an increase in blood pressure smooth muscle activity (that is, it would result from a structural and non-functional mechanism) and this increase in distensibility results in greater attenuation and lower pulse pressure transmission, minimizing damage related to hyperdynamia [23,24]. But while much is known about the crucial role of the arterial system in regulating CBF, little attention is paid to the role of the Cerebral Venous System (CSV) in maintaining perfusion to meet the brain’s metabolic demands. Cerebral Veins (CV) are collapsible because of their specific cytoarchitectural structure and as a result, they undergo marked changes in their transverse configuration when transmural pressure is slightly positive or negative. Transmural pressure can fall either by decreasing intraluminal pressure (ILP) or by increasing External Pressure (EP) or both. In turn, changes in the variables that make up the Bernoulli-Poiseuille equation (upstream or downstream resistance to flow, flow rate, acceleration-deceleration, negative gravitational pressure) directly influence the ILP. Thus, the slight negativity of transmural pressure can affect blood flow both by reducing the cross-sectional area of the CV, and by variations, for example, in viscous resistance and acceleration-deceleration, secondary to this venous collapse [25].
Based on the physical principles of Monro-Kellie’s doctrine, according to which within the intracranial compartment, the sum of brain volume, cerebrospinal fluid (CSF) and cerebral blood volume always remains constant, an increase in one of these components should cause a reduction in one or the two remaining, the blood circulating in the skull therefore has an unchanging volume at all times [26]. Thus, arterial vasodilation can only be produced by a reduction in the volume of CV, which can be achieved by a reduction in venous pressure [25]. The CVS is mainly affected because it maintains a pressure that is not normally very different from that of the CSF [27].
Despite this, according to Yu et al. [28], volume of cerebral venous blood should not be considered simply as a volume buffer, limited to a passive physiological response of non-contractile veins. Thus, despite this passive behavior of CVs, their action on autoregulatory vascular mechanisms cannot be underestimated. Studies on AR and cerebral hemodynamics and their main findings are summarized in Table 1.
| Reference | Participants | Main findings/Conclusions |
|---|---|---|
| Strandgaard. [8] | Untreated hypertensive (n=13); treated hypertensive (n=9); normotensive volunteers (n=10). | Chronic SAH induces thickening of the wall of cerebral arterioles, in such a way that the greater the degree of hypertension, the greater the lower limit of AR. |
| Brown, et al. [29] | 12 rhesus monkeys: (n=6) nitroprusside group and (n=6) trimethaphan group. | The static model of AR assesses its efficiency, that is, changes in CVR in response to induced changes in BP. |
| Aaslid, et al. [9] | Normotensive volunteers (n=10). | The AR capacity of the brain vasculature is not perfect, it depends on the severity and speed of BP modification and should not be analyzed only as a stationary phenomenon. |
| Panerai. [6] | Not applicable | The dynamic study should be the model of choice, in most situations, for the clinical assessment of AR. |
| Serrador, et al. [22] | Normotensive (n=22); controlled hypertensive (n=20); uncontrolled hypertensive with or without blood pressure-lowering medications (n=18). | Hypertensive patients suffer less from BP variations, as they have their autoregulation curve shifted to the right, less vasoreactivity to CO2 and are less influenced by the action of vascular tone. |
| Robertson, et al. [23] | Young adults (n=23); normotensive adults (n=22); controlled hypertensive adults (n=23); uncontrolled hypertensive adults (n=12). | Chronic adaptation resulting from SAH is associated with changes in vascular distensibility and not with an increase in smooth muscle activity (that is, it would result from a structural and non-functional condition). |
| Yu, et al. [28] | 16 Yorkshire landrace pigs: Doppler group (n=7); Xe-CBF group (n=9). | The action of the venous system on vascular autoregulatory mechanisms cannot be underestimated. |
Table 1: Studies on autoregulation (AR) and cerebral hemodynamics and their main findings.
That said, in addition to the physiological mechanisms that make up dynamic AR, it is possible to state that blood flow in the cerebrovascular bed is also regulated according to the basic principles of fluid mechanics, so that the dynamic pressure-flow relationship can, therefore, be influenced by changes in CRV and/ or changes in the compliance of the vascular system as a whole [21,29].
Conclusion
Hypertensive patients, despite numerous structural and functional vascular changes, have their ability to autoregulate intact, keeping the CBF adequate to neuronal metabolic demand and protecting brain tissue from damage related to pressure variability.
References
- Doberstein C, Martin NA. Cerebral blood flow in clinical neurosurgery. In: Youmans JR. Neurological surgery. 4th ed. Philadelfhia: WB Saunders. 519-69 (1996).
- Willie CK, Tzeng YC, Fisher JA, et al. Integrative regulation of human brain blood flow. J Physiol. 592(5): 841-59 (2014).
- Paulson OB, Stdrangard S, Edvinson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 2(2): 161-92 (1990).
- Van Lieshout JJ, Secher NH. Sympathetic activity does/does not influence cerebral blood flow. J Appl Physiol. 105(4): 1364-8 (2008).
- Johnson PC. Autorregulation of blood flow. Circulation Research. 59(5): 483-95 (1956).
- Panerai RB. Assessment of cerebral pressure autoregulation in humans: A review of measurement methods. Physiol Meas. 19(3): 305-38 (1998).
- Kleinfeld D, Blinder P, Drew PJ, et al. A guide to delineate the logic of neurovascular signaling in the brain. Front Neuroenergetics. 3: 1-9 (2011).
- Strandgaard S. Autoregulation of cerebral Blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation. 53(4): 720-7 (1976).
- Aaslid R, Lindegaard KF, Sorteberg W, et al. Cerebral autoregulation dynamics in humans. Stroke. 20: 45-52 (1989).
- Tiecks F, Lam A, Aaslid R, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 26(6): 1014-19 (1995).
- Aaslid R, Lash SR, Bardy GH, et al. Dynamic pressure-flow velocity relationships in the human cerebral circulation. Stroke. 34: 1645-9 (2003).
- Panerai RB, Moody M, Eames PJ, et al. Cerebral blood flow velocity during mental activation: Interpretation with different models of the passive pressure-velocity relationship. J Appl Physiol 99(6): 2352-62 (2005).
- Folkow B, Hallbäck-Nordlander M. Interaction between functional and structural elements in primary hypertension. In: Amery A, Fagard R, Lijnen P, Staessen J, editors. Hypertensive Cardiovascular Disease: Pathophysiology and Treatment. Dev Cardiovasc Med. 16: p.231-248 (1982).
- Baumbach GL, Heistad DD. Cerebral circulation in chronic arterial hypertension. Hypertension. 12(2): 89-95 (1988).
- Wolinsky H. Long-terms effects of hypertension on the rat aortic wall and their relation to concurrent aging changes. Circ Res. 30(3): 301-9 (1972).
- Park JB, Touyz RM, Chen X, et al. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am J Hypertens. 15(1): 78-84 (2002).
- Rigsby CS, Ergul A, Dobos VP, et al. Effects of spironolactone on cerebral vessel structure in rats with sustained hypertension. Am J Hypertens. 24(6): 708-15 (2011).
- Brecher P, Tercyak A, Chobanian AV. Properties of angiotensin converting enzyme in intact cerebral micro vessels. Hypertension. 3(2): 198-204 (1981).
- Sokolova IA, Manukhina EB, Blinkov SM, et al. Rarefication of the arterioles and capillary network in the brain of rats with different forms of hypertension. Microvasc Res. 30(1): 1-9 (1985).
- Joutel A, Monet-Lepretre M, Gosele C, et al. Cerebrovascular dysfunction and microcirculation rarefaction precedes white matter in lesions a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 120(2): 433-45 (2010).
- Zhang R, Zuckerman JH, Giller CA, et al. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 274: H233-41 (1998).
- Serrador JM, Sorond FA, Vyas M, et al. Cerebral pressure-flow relations in hypertensive elderly humans: transfer gain in different frequency domains. J Appl Physiol. 98(1): 151-9 (2005).
- Robertson AD, Edgell H, Hughson RL. Assessing cerebrovascular autoregulation from critical closing pressure and resistance area product during upright posture in aging and hypertension. Am J Physiol Heart Circ Physiol. 307(2): H124-33 (2014).
- Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension. 21(6): 816-26 (1993).
- Ursino M, Lodi CA. A simple mathematical model of the interaction between intracranial pressure and cerebral hemodynamic. J Appl Physiol. 82(4):1256-69 (1997).
- Carmelo A, Ficola A, Fravolini ML, et al. ICP and CBF regulation: A new hypothesis to explain the “windkessel” phenomenon. Acta Neurochir. 81(81): 112-6 (2002).
- Schlesinger B. The venous system of the brain with special reference of the Galenic system. Brain. 62: 274-91 (1939).
- Yu Y, Chen J, Si Z, et al. The hemodynamic response of the cerebral bridging veins to changes in ICP. Neurocrit Care. 12(1): 117-23 (2010).
- Brown F, Crockard H, Johns L, et al. The effects of sodium nitroprusside and trimethaphan camsylate on cerebral blood flow in rhesus monkeys. Neurosurgery. 2(1): 31-4 (1978).