Review Article - Interventional Cardiology (2013) Volume 5, Issue 3
Challenges of biological valve development
- Corresponding Author:
- Robert D Simari
Division of Cardiovascular Diseases & Department of Internal Medicine
Mayo Clinic, Rochester, MN, USA
Tel: +1 507 284 3727
Fax: +1 507 538 6418
E-mail: simari.robert@mayo.edu
Abstract
Keywords
aortic valve, bioreactor, decellularization, heart valve, pulmonic valve, recellularization, scaffold, stem cell, tissue engineering
Cardiac valves are dynamic structures that open and close approximately 3 billion-times over an average lifetime [1]. The ability of cardiac valves to allow unobstructed forward flow of blood and prevent regurgitation depends on the overall structural integrity, mobility and pliability of the valve cusps/leaflets. Valves must continually adapt and undergo structural, as well as functional, changes throughout the life of the individual in order to prevent deterioration and malfunction [2,3]. Pathologic valve failure most commonly results from maladaptive structural and functional changes in response to changing physiologic conditions. The definitive treatment for valve failure is replacement; currently, the options for replacement include mechanical and bioprosthetic valves.
The development of mechanical and bioprosthetic valvular prostheses have enhanced the survival and quality of life for patients with valvular dysfunction. Despite the benefits afforded by prosthetic valves, there are still significant limitations to those currently available. The goal of biological valve tissue engineering is to overcome the limitations of the currently available prosthetic valves. The ideal tissue-engineered heart valve (TEHV) is one that can grow with the patient, remodel in response to physiologic challenges and does not require anticoagulation. This ideal valve would become a living/growing organ within the patient, one that is able to maintain homeostasis in the harsh, ever changing environment of the human cardiac cycle and eliminate the need for subsequent valve replacements owing to growth of the patient or degeneration of the prosthesis.
Current valve replacement options & unmet needs
The two types of prosthetic valves that are currently available are mechanical and bioprosthetic. Mechanical valves have similar properties and have come in four major designs over the years. The ball-in-cage type of mechanical heart valve (e.g., Starr–Edwards) was one of the first developed and is no longer commercially available. The hinged bileaflet type (e.g., St Jude Medical, MN, USA) is currently frequently used. Other types of mechanical heart valves include tilting disk (e.g., Bjork–Shiley, Shiley Corp., CA, USA; and Medtronic-Hall, Medtronic Inc., MN, USA) and trileaf let (e.g., Roscardioinvest). The leaflets of mechanical valves are usually constructed of pyrolytic carbon owing to its excellent biocompatibility, low thrombogenicity and high durability, making it superior to the metals and plastics that have previously been used. The major benefits to using mechanical valves for valve replacement are their excellent durability (20–30 years) and flow dynamics, which are similar to the native valve. The major drawback of mechanical valves is the need for chronic anticoagulation using vitamin K antagonists [4].
Bioprosthetic heart valves are frequently secured in a support frame (stented), although less commonly, they can be without the support frame (stentless). They are available in three main types, determined by the tissue from which they are made. These materials are most commonly cusps from porcine aortic valves, bovine pericardium and cadaveric homografts. Typically bovine and porcine bioprosthetic valves are glutaraldehyde fixed, whereas cadaveric homografts are not. The major benefits of bioprosthetic valves are that they have a very low incidence of thromboembolism and lifelong anticoagulation is usually not necessary. In addition, they have flow dynamics similar to native valves and they can be delivered percutaneously in some patients [5]. The major drawbacks of bioprosthetic valves are their limited durability, with a significant failure rate at 10 years in all patients, and an even higher failure rate in pediatric and young adult populations [4,6]. A major reason for the decreased durability of bioprosthetic valves is that they are fixed in glutaraldehyde to eliminate antigenicity. The glutaraldehyde fixation process affects the valve durability owing to devitalization of the tissue and subsequent accelerated calcification.
While there is significant overlap between the valve-replacement needs of adult and pediatric patients, there are also important differences. The most significant difference is the need for growth of the replacement valve in the pediatric population. Pediatric aortic valve disease has many etiologies (e.g., congenital, rheumatic and infectious) with the final common pathway being valuvlar stenosis or regurgitation. In addition, congenital heart disease often requires the replacement of the pulmonic valve in the right ventricular outflow tract, which is uncommon in adult patients. Aortic valve replacement in children has classically been a procedure of last resort, being performed only when multiple other treatments have failed. A major reason for the delay or avoidance of aortic valve replacement in children is the inadequate performance of the valve substitutes currently available. The use of mechanical and fixed-tissue valves in the pediatric population is limited by their inability to grow, repair and remodel, necessitating repeat surgeries to enlarge the valve as the patient grows. Bioprosthetic valves (porcine or pericardial) used in children are limited by their rapid calcification and requirement for early reoperation. Mechanical prostheses, while durable, are susceptible to other complications, including hemolysis and thromboembolism. The use of anticoagulation with vitamin K antagonists, which is required with all mechanical valves, presents significant additional risks and challenges in the pediatric population and in the case of females who are, or desire to become, pregnant [7]. Owing to the limited options in the pediatric population, the Ross procedure is commonly used in children requiring a replacement aortic valve, whereby the autologous pulmonic valve is used to replace the aortic valve and an allogenic pulmonic valve is used to replace the pulmonic valve [8,9]. This procedure has improved outcomes in pediatric patients by placing a homograft in the higherpressure aortic-valve position. The benefits of using a pulmonary autograft in the aortic position is that the living graft can grow with the child, decreasing the need for reoperation and eliminating the need for anticoagulation that would be required with the use of a mechanical valve. Despite its benefits, this procedure has its complications and is clearly not optimal, as it requires sacrificing a healthy valve to treat the diseased valve and, in the end, requires two valves being replaced to treat a single diseased valve [10,11].
In adults, aortic stenosis (AS) is the most common form of degenerative valve disease. The treatment of choice for severe AS is replacement of the aortic valve. The prevalence of AS increases with age and it is seen in 2.8% of all patients over 75 years of age [12]. The frequency of AS continues to increase with the general aging of the population in North America and Europe. Owing to the frequency of AS aortic valve replacement, procedures are commonplace with over 200,000 performed worldwide each year in adults alone [13]. The choice of which prosthetic valve to use in adults usually revolves around the age of the individual and the individual factors associated with chronic anticoagulation use. Owing to the limited durability of bioprosthetic valves, they are frequently reserved for older individuals and those that have contraindications to chronic anticoagulation.
Strategies of biological valve development
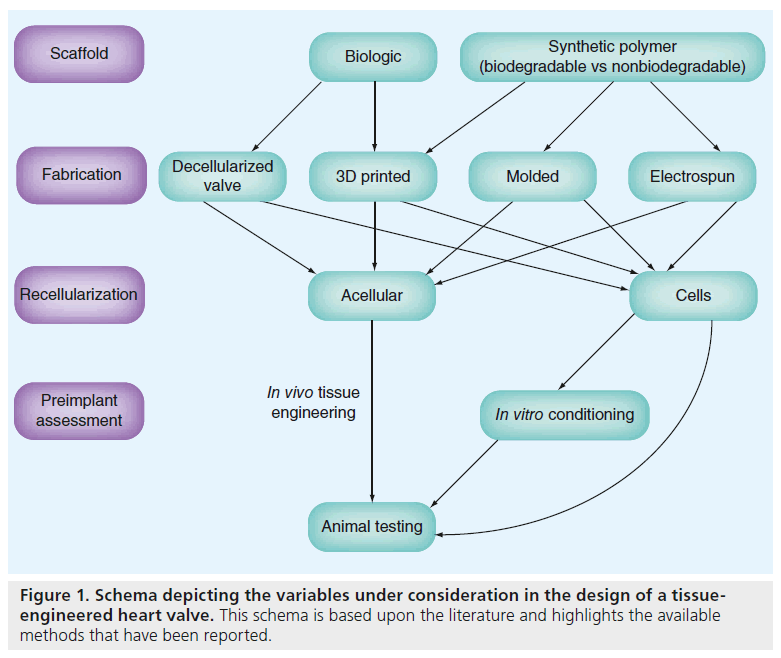
Owing to the limitations of the available prosthetic valves, there are a variety of strategies currently under investigation to develop a TEHV. At this point, there is not one clear pathway to success. The variables in play with regards to TEHV development include the material used as a scaffold (biological vs synthetic), the methods used to fabricate these materials into a valve construct and the type of cells used to seed the construct.
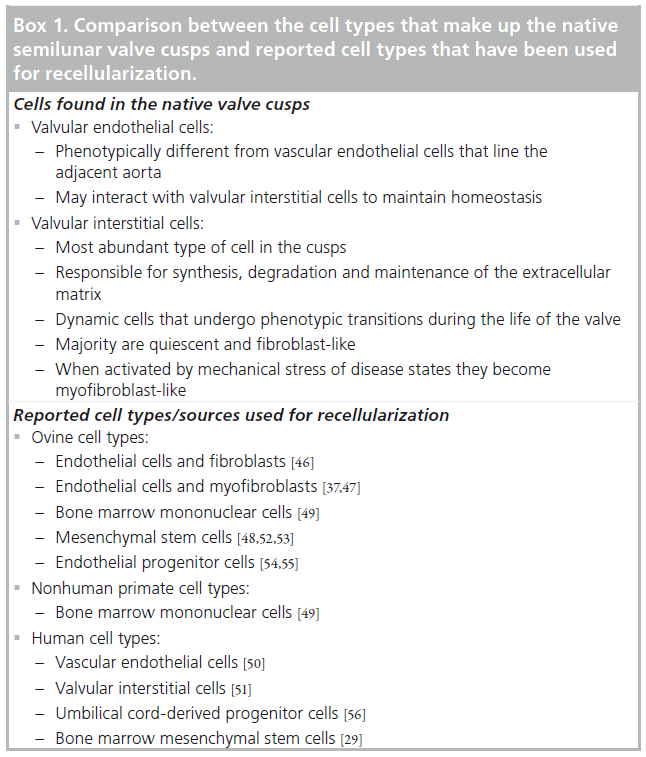
When deciding among the various cell types and scaffold materials available for the development of a TEHV, a thorough understanding of the anatomy and histology of the native valve is essential. The architecture of a native heart valve consists of three layers: the ventricularis on the inflow side, the spongiosa in the middle and the fibrosa on the outflow side. Valvular interstitial cells (VICs) are found throughout all three layers; however, they are more concentrated in the spongiosa. Valvular endothelial cells (VECs) line the surfaces of the valve cusps and are phenotypically distinct on the inflow and outflow surfaces. The fibrosa contains a dense matrix of collagen aligned circumferentially for mechanical strength during diastole. The spongiosa contains glycosaminoglycans (GAGs) for lubrication during flexure. The ventricularis contains collagen and elastin for efficient coaptation during valve closure and distensibility during diastole.
The complex structure of the native semilunar valves leads to complex biomechanical functionality. Achieving similar functionality with a TEHV requires careful cell and scaffold selection, as well as a strategy for directing those components into a functional tissue. Two major paradigms of TEHV development have been described (Figure 1). The first pathway of TEHV design involves an in vitro stage of cell seeding, sometimes followed by a conditioning phase in a bioreactor, prior to the in vivo phase, which is implantation in a large animal model. The second pathway consists of an unseeded scaffold that is placed directly into a large animal model with the expectation that the scaffold contains biological signals necessary to attract the appropriate cells in vivo. The following section will review some strategies that have been previously attempted taking into account the different approaches with regards to scaffold materials, fabrication methods and recellularization, including cell types and timing of cell introduction.
Scaffolds
The role of the scaffold is to provide an initial architecture for cellular attachment. Ideally, the scaffold would provide the appropriate biomechanical and biochemical signals to allow for cell attachment and migration, setting in motion the process of remodeling, repair and growth. Scaffolds can be made of synthetic or biologic materials.
▪ Synthetic scaffolds
Synthetic scaffolds can be made of either biodegradable polymers or nonbiodegradable polymers. Synthetic scaffolds ideally should have a highly porous microstructure and a surface that allows for cellular attachment. Ideally, the scaffold would completely bioresorb without producing toxic byproducts as the cells construct their own matrix. The advantages to synthetic scaffolds are that they are easily produced and allow for control of the material structure/properties such as pore size and degradation rate. Disadvantages to using synthetic scaffolds include limited perfusion of nutrients to cells, premature degradation, lack of biocompatibility and difficulty controlling cell adhesion. The most common synthetic scaffold materials in use today are the polymers polyglycolic acid, polylactic acid and poly(-lactic-co-glycolic) acid. In addition, poly (e-caprolactone), polyhydroxyalkaneoate, poly- 4-hydroxybutyrate (P4HB) and polyurethane have been used to fabricate TEHV.
Nonbiodegradable scaffolds persist and, therefore, need to interact favorably with the recipient over an extended period of time. Polyhedral oligomeric silsesquioxane-poly(carbonate-urea) urethane (POSS-PCU) is a new class of nonbiodegradable polymer, and it offers several advantages over previous materials for synthetic heart valve leaflet fabrication [14]. In addition to having suitable mechanical properties, POSSPCU has been shown to exhibit superior biocompatibility, hemocompatibility, antithrombogenicity, resistance to calcification and resistance to inflammation [15,16]. Biocompatibility and hemocompatibility can be further enhanced by the formation of a viable endothelium on the surface of the leaflets. POSS-PCU has demonstrated the potential for in situ self-endothelialization, which can be harnessed by in vivo tissue-engineering strategies [16].
▪ Biologic scaffolds
Biologic materials include collagen, fibrin or decellularized aortic/pulmonic valve scaffolds, which are a combination of collagen and elastin. In addition, alginate [17,18] and haluronan [19,20] have also been investigated as a possible biologic scaffold material. The potential advantages to biologic scaffolds are that they maintain the architecture of the native tissue and they can maintain biological signaling cues (GAGs and growth factors, among others) that can help guide cellular recruitment, adhesion, migration and differentiation. Some disadvantages to biologic scaffolds include the difficulty of getting cells to migrate into the interior and possible immunogenicity associated with xenogenic transplants [21,22].
One common biological scaffold option is the decellularized porcine xenograft. Ideally, human homografts would be used as the source of scaffolding; however, owing to the limited supply of human valves, porcine valves have been used due to their availability and similar anatomy to human valves. A potential benefit to using a decellularized scaffold is that there is no additional fabrication necessary and intrinsic molecular cues directing cellular migration and differentiation may be retained. Following the decellularization process, the product should be an intact valve with retained mechanical properties. Studies have shown the ability of recellularization, as well as matrix remodeling and growth potential of decellularized xenografts, in juvenile sheep models [23,24]. Decellularization has been attempted by numerous groups using a variety of protocols. The major agents for decellularization can be categorized in the following groups: chemical agents that include acids/bases, hypotonic/hypertonic solutions, ionic detergents, nonionic detergents, zwitterionic detergents and solvents; biologic agents that include enzymes and chelating agents; and physical and miscellaneous agents including temperature, application of force and pressure. Some common agents used in the decellularization of heart valve tissue include sodium dodecyl sulfate [25–28], triton X-100 [28–30] and the combination of triton X-100 and sodium cholate [31– 33]. During the decellularization process, care must be taken to minimize disruption to the extracellular matrix (ECM; i.e., preserve matrix integrity) and remove all remaining decellularization solution. It has been demonstrated by solid phase extraction and HPLC that intensive washing of the scaffold following decellularization can lower the residual decellularization solution to noncytotoxic levels (<50 mg/l) where cellular repopulation is achievable [27]. Despite removal of all cellular material, it is possible that some residual immunogenicity may be present due to collagen and elastin [21]. In addition to the removal of cellular material, ensuring the removal of the major porcine antigen, the a-Gal epitope, has been demonstrated to be important in avoiding hyperacute rejection of untreated porcine valves [34].
▪ Methods of scaffold fabrication
The methods used to fabricate the scaffold materials into a valve construct differ depending on the material used. Reported methods of fabrication include molding, electrospinning and bioprinting. Molded valves have been made using fibrin [35–38], polyglycolic acid [39,40], polyhydroxyalkaneoate [41] and P4HB [42]. Molding offers precise morphology; however, it is limited by the fact that the material must be homogenous, which does not allow for multiple cell types in different regions.
Electrospinning is a technique that can be used to produce polymeric fibers with diameters ranging from nano- to micro-meters that can be intertwined in a meshlike structure. TEHV design has been attempted using electrospinning with poly(e-caprolactone) [43,44] and poly (ester urethane) ureas [45] and have shown promise in producing the scaffold of a TEHV. These electrospun scaffolds offer the advantage of high porosity and anisotropic mechanical properties closely resembling that of native heart valve cusps.
Butcher and colleagues have demonstrated the potential effectiveness of bioprinting using a 3D printer and alginate/gelatin hydrogel [17,18]. With this process they have reported the ability to print an anatomical architecture with the direct incorporation of two cell types in a regionally constrained manner, which would not be possible with traditional molding. The results of their work demonstrate that anatomically complex heterogeneously encapsulated valve conduits can be produced with 3D bioprinting.
Cells
Native valve cusps consist of two cell types: VECs and VICs (Box 1). VICs are the most abundant cell type and are responsible for synthesis, degradation and maintenance of the ECM [46,47]. VICs are a dynamic population of cells that undergo phenotypic transition during the life of the individual depending on the physiological or pathological conditions to which the valve is exposed [48]. The vast majority of VICs in the healthy adult valve are quiescent and fibroblast- like; however, when activated by changes in mechanical stress or disease states, VICs become myofibroblast-like. Recapitulation of a VIC-like cell population is central to the production and maintenance of a functional valve with the ability to grow and adapt. Theoretically, the ideal tissue-engineered valve would be constructed from autologous and easily obtained cell sources that could perform functions of the native fibroblast/myofibroblast VICs.
Differentiated cell types, as well as progenitor/ stem cell types, from both humans and other animal sources have been used for the recellularization of TEHV scaffolds (Box 1). The differentiated cell types that have been used include: ovine endothelial cells and fibroblasts [49], ovine endothelial cells and myofibroblasts [40,50], ovine bone marrow mononuclear cells [51], nonhuman primate bone marrow mononuclear cells [52], human VECs [53] and human VICs [54]. Progenitor/stem cell types that have been used include: ovine mesenchymal stem cells [51,55,56], ovine peripheral blood endothelial progenitor cells [57,58], human umbilical cord-derived progenitor cells [59] and human bone marrow mesenchymal stem cells [32].
One of the major considerations with autologous cell harvest is determining a site of harvest that is easily accessible. Recent work from the SCIPIO and CADUCEUS trials have laid the ground work for the generation and delivery of autologously-derived cardiac cells and demonstrated the feasibility of this approach in trials of cardiac regeneration [60,61]. Thus, one could consider using cells generated from cardiac biopsies for in vitro seeding of TEHVs.
Some groups have seeded cells on scaffolds and then conditioned those constructs in bioreactors prior to implantation in large animal models. Sodian et al. reported early attempts to seed polyhydroxyalkaneoate scaffolds with ovine carotid artery vascular cells followed by up to 8 days of bioreactor conditioning [41]. They report cell proliferation and migration, as well as collagen and GAG production. From the same group, Hoerstrup et al. improved upon that approach by seeding ovine carotid artery endothelial cells and myofibroblasts onto P4HB-coated polyglycolic acid scaffolds followed by up to 28 days of bioreactor conditioning [40]. They report collagen, DNA and GAG content to be approximately 85, 60 and 60%, respectively, compared with native tissue. Elastin production was not detected. These valves functioned well in a sheep model for up to 20 weeks. Since these pioneering studies, several groups have taken the approach of seeding cells onto a scaffold and conditioning the cell-seeded constructs in a bioreactor. Examples include ovine carotid artery endothelial cells and myofibroblasts seeded onto decellularized porcine pulmonary valves [62], human umbilical cord cells seeded onto P4HB [42], ovine carotid artery vascular cells seeded onto fibrin [63], human dermal fibroblasts seeded onto fibrin [64], ovine bone marrow-derived mesenchymal stem cells seeded onto poly(-lactic-co-glycolic) acid [65], and human VECs and fibroblasts seeded onto polyurethane [66].
Other groups have taken the approach of eliminating the bioreactor and moving directly from a recellularized valve into large animal models. Hoerstrup et al. have recently reported success using synthetic scaffolds seeded with autologous cells percutaneously implanted in large animals (ovine and nonhuman primates) without the additional conditioning in a bioreactor [52,67]. Their work has demonstrated the potential feasibility of a transcatheter, stem cell-based TEHV implantation into both the pulmonic- and aortic-valve positions within a one-step intervention.
Still others have taken the approach eliminating all in vitro work and placing an acellular valve in a large animal model and allowing the recipient to recellularize the graft after implantation an approach that has been termed in vivo tissue engineering. A number of studies have reported success with this approach [23,68–72]. A possible advantage to the in vivo tissue engineering approach is that it is less time consuming and has the potential for off-the-shelf availability. A possible concern with the in vivo tissue engineering approach of allowing an acellular graft to become recellularized by the host is that pathologic cells may be the cells that attach and repopulate speeding up the eventual degeneration or calcification of the scaffold. The success of this method could theoretically differ on a patient specific basis. For example, one could imagine that the cells repopulating the valves could differ in young versus old patients and in patients with multiple comorbidities (diabetes, hypertension and hyperlipidemia, among others) versus healthy individuals.
Yoo et al. has tried to overcome this potential limitation of in vivo tissue engineering by attempting to attract a specific cell population through antibody labeling of their decellularized constructs [73]. They have shown early success using CD133 antibody labeling of decellularized porcine pulmonic valves in an attempt to attract an endothelial progenitor cell type following implantation. Their results demonstrated that the decellularized CD133 antibody labeled valves had improved recellularization with endothelial cells when compared with unconjugated valves and valves that were unconjugated and statically seeded with endothelial cells prior to implantation. In addition, the CD133 antibody conjugated valves showed increased interstitial cell and structural protein content after 1 and 3 months.
Finally, a unique approach undertaken by Tranquillo et al. has used a combination of the two above methods [38]. They initially use cells in a fibrin mold and allow these cells to produce their own matrix in vitro. After a period of time when enough matrix has been deposited the scaffold is then decellurized and implanted in a large animal model and allowed to be recellularized by the animal. Their early results have shown success with this method and they have produced engineered leaflets with similar tensile properties and collagen content compared with native leaflets.
In vitro conditioning & testing
▪ Purpose of a bioreactor
A cell-seeded scaffold provides a starting point for the generation of a TEHV. Additional in vitro tissue remodeling may be necessary before the valve is ready for successful in vivo function, remodeling and maintenance. A bioreactor can be used to provide biomechanical and biochemical stimuli to a TEHV in a controlled environment in order to direct in vitro tissue formation [74,75].
One goal of a bioreactor is to develop proper tissue architecture. This requires positioning the proper cells and generating the proper ECM components in the various regions of the tissue. When subjected to appropriate stimuli, cells may proliferate, migrate, differentiate and align in a manner that distributes the desired cell types throughout the tissue. In addition, cells may resorb, synthesize and align ECM in a manner that distributes and properly orientates the desired ECM constituents throughout the tissue. The ECM, in turn, provides signaling cues to the cells, which further direct tissue formation. Furthermore, turnover of the original scaffold material and replacement with autologous ECM may be critical to the acceptance of the TEHV upon implantation.
Another goal of a bioreactor is to achieve proper tissue function. The functionality of a TEHV depends upon its biomechanics, which in turn depends upon the tissue’s underlying structure. Thus, it is critically important for a TEHV to achieve and maintain proper morphology and ECM architecture throughout its entire service life. Degeneration, thickening and calcification of the cusps alter their biomechanics and can lead to catastrophic valve failure in vivo.
▪ Functions of a bioreactor
Bioreactors can expose TEHV to a variety of biomechanical stimuli, including stretch, flexure, shear and pressure. Cyclic stretch has been shown to increase cell proliferation [76], collagen synthesis [76–79], stiffness [78] and GAG content [80]. Cyclic flexure has been shown to increase collagen content [81,82], stiffness [81,82] and cell migration [81]. Oscillating fluid shear stress has been shown to increase strength [83], alignment [84,85], inflammation resistance [84,85], protection from calcification [84,85], GAG content [86] and protein content [86]. Pressure has been shown to increase collagen synthesis [87–89] and increase GAG synthesis [88,89].
Bioreactors can also expose TEHV to a variety of biochemical stimuli, including growth factors, nutrients and dissolved gasses. These factors can stimulate cells to proliferate, migrate, differentiate and synthesize ECM. For example, FHGF was shown to increase endothelial cell proliferation [90], TGF-b1 and insulin were shown to increase elastin and collagen production [91], HGF was shown to promote cell adhesion [92], bFGF was shown to increase cell proliferation [93,94] and migration [94], as well as collagen production [65], ascorbate was shown to increase collagen synthesis [93], VEGF was shown to promote endothelial cell proliferation [95,96] and TGF-b1 was shown to induce endothelial cell differentiation [95].
An additional benefit of bioreactors is the improved nutrient diffusion created by convection. Many bioreactor designs incorporate perfusion or mixing of culture medium, which helps to evenly distribute nutrients around and within a tissue. This makes it possible for cells deep within the tissue to survive owing to nutrient diffusion alone.
▪ Current bioreactor designs
Various cardiac-valve bioreactor designs have been described in the literature and they tend to fall into three main categories based on their objective: flow-based whole-valve conditioning (Table 1); strain-based whole-valve conditioning (Table 2); and isolated cusp stimulation (Table 3). The first bioreactor type seeks to condition a TEHV by simulating conditions similar to physiological systole and diastole. This is performed by pulsing media through the valve lumen in a manner that opens and closes the cusps with the proper pressure gradients. The second bioreactor type seeks to condition a TEHV by simulating conditions similar to physiological diastole. This is performed by cyclically pressurizing media around the valve, such that the tissue strains but the cusps remain closed and there is no flow through the valve. The third bioreactor type allows isolated cusps or cusp segments to be studied for their response to specific biomechanical stimuli.
| Study (year) | Pump | Res | Cap | Mount | Materials | Sterilization | Tissue | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Konig et al. (2012) | Pneumatic | No | No | Suture | Acrylic | Formaldehyde | Valve | Endoscopic visualization | [108] |
| Kaasi et al. (2011) | Pneumatic | Yes | Yes | Suture | n/s | Autoclave | Valve | Ventricular assist device | [101] |
| Sierad et al. (2010) | Pneumatic | Yes | Yes | Suture | Acrylic | Autoclave | Valve | Can accommodate various | [100] |
| valve types | |||||||||
| Ruel and Lachance (2010) | n/a | Yes | Yes | n/a | n/a | n/a | n/a | Windkessel model, RRC | [97] |
| configuration is best | |||||||||
| Ziegelmueller et al. | Pneumatic | No | No | n/s | n/s | etOH | Valve | Optical monitoring | [107] |
| (2010) | |||||||||
| Durst and Grande-Allen | None | No | No | Suture | n/s | Autoclave | Valve | Valves mounted to | [128] |
| (2010) | actuating pistons | ||||||||
| Lee et al. (2009) | Gear | Yes | Yes | Suture | Polycarb | n/s | Valve | Laminar flow | [58] |
| through valve | |||||||||
| Ruel and Lachance | Pneumatic | Yes | Yes | O-ring | n/s | n/s | Valve | Windkessel RC model | [129] |
| (2009) | |||||||||
| Migneco et al. (2008) | Peristaltic | Yes | No | Clamp | n/s | n/s | Valve | Heated reservoir | [130] |
| Flanagan et al. (2007) | Pneumatic | No | No | Suture | Plexiglass | Gas plasma | Valve | Column of fluid for | [63] |
| afterload | |||||||||
| Morsi (2007) | Pneumatic | No | No | n/s | PMMA | n/s | Valve | Multiple valve types, laser | [131] |
| Doppler validation | |||||||||
| Lichtenberg et al. (2006) | Piston | No | Yes | n/s | n/s | n/s | Valve | Cell seeding inlets | [98] |
| Karim et al. (2006) | n/s | No | No | n/s | Glass | n/s | Valve | For decell and recell | [132] |
| Warnock et al. (2005) | Pneumatic | No | Yes | n/s | n/s | Autoclave | Valve | Silicon tubes for gas | [133] |
| exchange | |||||||||
| Hildebrand et al. (2004) | Pneumatic | Yes | Yes | Suture | Polycarb | etOH | Valve | First sophisticated system | [99] |
| Narita et al. (2004) | Pneumatic | Yes | Yes | n/s | Acrylic | etOH | Valve | Digital camera | [106] |
| Schenke-Layland et al. | Pneumatic | No | No | n/s | n/s | Autoclave | Valve | Similar to Zeltinger except | [62] |
| (2003) | valve below bladder | ||||||||
| Dumont et al. (2002) | Pneumatic | Yes | Yes | n/s | PMMA | etOH | Valve | First resistor and capacitor | [111] |
| Zeltinger et al. (2001) | Pneumatic | No | No | Staple | n/s | Electron- | Valve | More pressure control | [134] |
| irradiation | |||||||||
| Hoerstrup et al. (2000) | Pneumatic | No | No | Suture | PMMA | etOH | Valve | First system | [110] |
Table 1. Flow-based whole-valve bioreactor designs reported in the literature.
| Study (year) | Pump | Res | Cap | Mount | Materials | Sterilization | Tissue | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Vismara et al. (2010) | Peristaltic | No | No | n/s | PMMA | etOH | Valve | Strain-based, compliance | [103] |
| monitoring, DPS | |||||||||
| Syedain and | Piston | No | No | n/s | n/s | n/s | Valve | Radial cyclic stretch | [105] |
| Tranquillo (2009) | |||||||||
| Kortsmit et al. | Pneumatic | Yes | No | n/s | n/s | n/s | Valve | Strain-based, deformation | [102] |
| (2009) | feedback control | ||||||||
| Kortsmit et al. | Pneumatic | Yes | No | n/s | n/s | n/s | Valve | Strain-based, volumetric | [104] |
| (2009) | deformation measurement | ||||||||
| Mol et al. (2005) | Pneumatic | No | Yes | n/s | Polycarb | etOH | Valve | Strain-based, DPD | [39] |
Table 2. Strain-based whole-valve bioreactor designs reported in the literature.
| Study (year) | Pump | Res | Cap | Mount | Materials | Sterilization | Tissue | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Metzler et al. (2012) | None | No | No | n/s | Polycarb | Autoclave | Cusp | Cyclic stretch, | [109] |
| confocal imaging | |||||||||
| Sun et al. (2011) | Peristaltic | No | No | Suture | Polycarb | n/s | Cusp | Side-specific shear | [118] |
| stress | |||||||||
| Schipke et al. (2011) | Pneumatic | No | No | None | n/s | n/s | Cusp | Cyclic pressure | [119] |
| Barzilla et al. (2010) | None | No | No | n/s | Polycarb | etOH, UV | Cusp | Splashing rotating | [117] |
| system | |||||||||
| Engelmayr et al. (2008) | Paddle | No | No | Spiral | Polycarb | etOH | Strip | Flex, stretch, flow | [120] |
| Balachandran et al. (2006) | None | No | No | Suture | Polysulfon | Gas plasma | Cusp | Cyclic stretch | [79] |
| Engelmayr et al. (2003) | None | No | No | Pins | PMMA | etOH | Strip | Flex bioreactor | [114] |
| Weston and Yoganathan | Peristaltic | No | No | Suture | Polycarb | n/s | Cusp | Parallel plate flow | [116] |
| (2001) | chamber |
Table 3. Isolated cusp stimulation bioreactor designs reported in the literature.
Flow-based whole-valve conditioning bioreactors typically achieve desired flows and pressures by utilizing a computer-controlled pump, a capacitance element and a resistance element [97]. Other common elements include a valve chamber, a media reservoir and a gas exchanger (Table 1). More advanced systems include sensors for flow, pressure, temperature, pH, pO2, pCO2, glucose, lactate and valve deformation. These sensors are used for system monitoring [63,98–101], feedback control [99,102,103] and tissue monitoring [102–106]. Videos or photographs of the valve may also be captured using a digital camera [100,101,105,107], endoscope [108] or microscope [109].
The first TEHV bioreactor design was described by Hoerstrup et al. [110]. Their design utilized a diaphragm pump to generate 50–2000 ml/min of flow through the valve. The resulting pressures ranged from 10 to 240 mmHg, but no independent pressure control was possible. The group pioneered key concepts including flow control, sterilizability, a media reservoir and housing the system within an incubator. They were able to generate functional TEHVs using this system in their subsequent studies [40–42].
Independent pressure and flow control were achieved by Dumont et al. by utilizing a variable capacitance element and a variable resistance element [111]. The capacitance is varied by pumping air in or out of a chamber that is filled partially with circulating culture medium. The resistance is varied by tightening or loosening a clamp on a segment of tubing that carries circulating culture medium. Together, these two elements create an afterload on the valve by mimicking the compliance of the large arteries and the resistance of the arterioles and capillaries. A flow probe and a pressure transducer were used to demonstrate a wide range of physiological flows and pressures.
A very sophisticated system capable of achieving a range of flow and pressure conditions, including both pulmonary and systemic conditions, was described by Hildebrand et al. [99]. Their design included a computer-controlled resistance element, a capacitance element and sensors for pressure and flow. A feedback control loop was employed to adjust the driving pressure and the resistance in order to automatically maintain the mean pressure and mean flow. Proportional pressure regulators were used to generate highly detailed driving pressure waveforms at prescribed stroke volumes and beat frequencies.
As examples of more recent designs, Kaasi et al. [101] and Sierad et al. [100] introduced systems utilizing pneumatic pumps, capacitance elements and resistance elements to achieve various flow and pressure conditions. Both designs require the valves to be mounted by suturing, which is tedious and increases the possibility for contamination; however, a more simplistic and reliable method has yet to be described. In addition, both designs are capable of achieving only pulmonary circulation conditions, underscoring the difficulty of achieving systemic circulation conditions even with modern designs.
The second category of bioreactor designs is the strain-based whole-valve conditioning approach (Table 2). This approach was pioneered by Mol et al. with their diastolic pulse duplicator [39]. The valve is cyclically pressurized in its closed state in a manner mimicking diastole. This approach is hypothesized to accelerate tissue growth and development since the valve experiences the largest strain during diastole [112]. This group was able to demonstrate successful tissue conditioning using the diastolic pulse duplicator approach [39,113].
Precise control of strain is important for the success of the strain-based approach; however, this is made difficult by the changing biomechanical properties of the developing tissue. One solution is to design feedback control algorithms based on measured estimates of the strain in order to consistently achieve the desired strain value. One such feedback control system was described by Kortsmit et al., who estimated strain based on the volume of fluid pushed into the valve during each cycle [102,104]. Another feedback control system was developed by Vismara et al., who utilized pressure as the control variable [103].
The complexities of a feedback control algorithm were avoided by Syedain and Tranquillo who described a strain-based approach that generates consistent cyclic radial distension by mounting a TEHV inside a latex tube [105]. Consistent strain is achieved, since the stiffer latex tube dominates the mechanical response. Distention is achieved by means of a syringe pump that cyclically pressurizes culture media within the tube. This approach also introduces the possibility of simulating somatic growth by increasing the radial strain.
The third category of bioreactor designs focuses on exposing isolated cusps or cusp segments to specific mechanical cues (Table 3). Rather than conditioning a whole valve for the purposes of tissue engineering, these bioreactors enable researchers to study the effects of biomechanical stimulation on isolated tissue specimens. These bioreactors have been designed to expose tissue specimens to strain [79,109,114,115], flow [116–118], pressure [119] or a combination thereof [120].
▪ Benefits of bioreactor conditioning
Both the biomechanical and the biochemical stimuli are thought to contribute to the conditioning of a TEHV in a bioreactor. The benefits of conditioning a TEHV in a bioreactor have been demonstrated in numerous studies. For example, some groups have demonstrated improved cell distribution throughout the matrix due to increased proliferation and migration [39,41,42,62,63,66,82,106,113,117]. Other groups have demonstrated improved ECM composition and alignment [39,41,42,62–66,79,106,113,117]. In addition, other groups have demonstrated improved mechanical properties, such as stiffness and strength [39,62,113]. These benefits can be realized after days or weeks of conditioning within a bioreactor.
Nevertheless, some groups have reported success implanting their TEHVs into animal models without any bioreactor conditioning [52,67,73], and it remains unclear how necessary bioreactor conditioning is to the success of certain types of TEHVs. Potential benefits need to be weighed against drawbacks of bioreactor conditioning, such as the additional development time, increased costs, added procedural complexity and the risk of contamination.
Independent of their role in conditioning TEHVs, bioreactors may also be used for in vitro testing of function and durability prior to testing in an animal model. This requires a bioreactor capable of achieving physiological flow and pressure conditions, as well as other key physiological parameters. It may even be possible to test the valve’s response to simulated somatic growth [105].
Preclinical testing
Regardless of the roles that bioreactors will ultimately play in valvular tissue engineering programs, there will come a point in which the tissueengineered construct will need to undergo further evaluation in a large animal model. The most common model for the testing of valves is the ovine model [121]. This has long been considered the gold standard by which prosthetic valves are studied owing to the exuberant fibrotic response and rapid calcification that it produces [121,122]. The theory has been that if a valve can withstand the more demanding conditions of the ovine model, then it will be able to withstand the less harsh environment of the human cardiac cycle. This exaggerated response does, however, raise some questions with regards to the translatability of the results into humans. A particular case that highlights this concern was with the preclinical testing of the Sulzer Carbomedics PhotoFix®-a pericardial valve. This valve performed well in the ovine model but then developed severe abrasions to the leaflets when implanted in humans [122]. Eventually the leaflet abrasion problem was attributed to a design flaw that was not noticed in animal testing owing to the exuberant fibrotic response. Owing to the differences seen in the ovine model, some groups have begun to use nonhuman primates for more extensive testing following the ovine experiments. A rigorously validated animal model that is known to correlate with human outcomes will be essential to demonstrating safety and efficacy prior to future human studies [123].
Another question regarding animal models is where anatomically (aortic vs pulmonic position) is the best site to test the tissue-engineered construct? This may depend on the ultimate planned site of human implantation. Many groups have chosen to use the pulmonic position owing to milder hemodynamic conditions.
Finally, what is the required duration of large animal experiments that will give us enough information about the long-term durability? To date, studies have used a variety of time points from days, months to over a year. The optimal time point likely depends on what is specifically being evaluated, but to truly understand the long-term durability, the optimal time point has likely not been established.
Challenges for the translation of engineered tissue valves: preclinical to clinical studies
Despite the limitations, the function and durability of the current generation of prosthetic heart valves have set the bar high for the durability and performance requirements of a TEHV. A major question for the translation of TEHVs is when should a construct be taken from the preclinical testing in large animals and evaluated in humans? Two clinical trials that resulted in very poor outcomes have highlighted the importance of this question.
▪ Clinical trials using TEHVs
The initial trial used the Synergraft™ valve (Cryolife Inc., USA) that was described as the “first tissue-engineered decellularized porcine heart valve” [124]. It was approved and received the CE mark in Europe in 2000 and was introduced as an alternative to conventional biological valves. The decellularized porcine constructs were either aortic composite grafts or whole pulmonary roots and were supposedly rendered cell free by a proprietary process. In 2001, four valves were implanted in children (aged 2.5–11 years) in the right ventricular outflow tract as a root. The results of these implantations was that three children died, two suddenly with severely degenerated valves (6 weeks postoperation and 1 year postoperation), and the third child died on the seventh postoperative day owing to an acutely ruptured valve. Owing to the poor results the forth graft was explanted prophylactically 2 days after implantation. Upon histological evaluation, all grafts showed severe inflammation starting on the outside (day-2 explant) leading to structural failure seen in the day-7 explant and severe degeneration of the leaflets and wall seen in the 6-week and 1-year explants. Significant calcific deposits were seen at all stages of valve harvest and no cell repopulation of the porcine matrix occurred even at the 1-year explant time point. Tragically, preimplant samples revealed incomplete decellularization and calcific deposits.
The second clinical trial also involved the implantation of xenogenic decellularized tissue- engineered pulmonary valve conduits in patients undergoing reconstruction of the right ventricular outflow tract [125]. Between 2006 and 2010, 93 patients underwent right ventricular outflow tract reconstruction using Matrix P™ and Matrix P Plus™ valves (AutoTissue GmbH, Berlin, Germany). A total of 33 patients (35.5%) experienced conduit failure, and conduit dysfunction occurred in 27 (29%) of the patients. The most common reason for conduit failure was stenosis in 20 cases (60%). Histological examination showed inflammatory giant-type cells and poor autologous recellularization in all explanted valves.
These two trials highlight the importance of rigorous in vitro and preclinical animal studies prior to human trials. They also suggest that when using a decellularized construct self repopulation by the recipient with circulating cells, without any preimplant, recellularization is not likely to be effective in humans. While ensuring the quality control of constructs seems simple, there has not been a universally accepted definition of what a safe tissue-engineered valve should consist of or how it should function prior to implantation in humans. In addition, individual patients may respond differently to a tissue-engineered valve, which may make predicting the outcome of a replacement valve more difficult then with the currently available prosthesis. It is possible that we may see dramatic differences in how the valve is recellularized or integrated into the host depending on individual factors associated with the recipient. One could imagine the pediatric population having a more exuberant response to the valve, which could be either beneficial or detrimental to its ultimate function. Older patients with multiple comorbidities could theoretically have a more difficult time repopulating the valves with healthy cells that could recapitulate the function of the native VICs. Because of these factors, the patient population that the valve is going into may determine the type of cells and source of cells used for recellularization. Thus, the field may not focus on creating one perfect tissue-engineered valve to be used in all patients, but multiple valves with different design strategies that could be individualized for different patient populations. In order to predict the success of a TEHV, a means of identifying important patient-specific factors (i.e., genetic characteristics or biomarkers) that could reliably predict patient-specific outcomes are essential. In addition, the development and validation of in vivo imaging/monitoring to ensure appropriate valve development and function would be useful tools for helping physicians predict outcomes and tailor therapy to optimize valve function [123,126].
At this point, it is clear that an optimal translational approach has not been identified to help move from preclinical studies into clinical trials using TEHVs. Owing to the high standards set by the currently available prosthesis, particularly in adult patients, it is important that these challenges be clearly delineated and solutions be outlined prior to subjecting patients to unnecessary risks. In addition, the surgical community has suggested that for a tissue-engineered valve to see routine clinical use, particularly in the adult population, it must show that the 15-year lifetime of conventional prosthetic valves can be greatly exceeded [127].
Conclusion
Tissue engineering is an exciting field with the potential to make major advances in the treatment of a variety of diseases. Great progress has been made over the past decade in TEHV development, and from this progress the challenges that lie ahead continue to be highlighted. As of today, it is evident that there has not been one clear path to success identified. Each type of scaffold material and fabrication type has it own inherent benefits and limitations and the ideal cell type has yet to be defined. The potential role for bioreactors in the development and conditioning of TEHV constructs has been demonstrated, but the optimal use has yet to be established.
Future perspective
While it is clear there is a need for superior prosthetic valves, particularly for the pediatric population, it is important that this is done in a systematic way with clearly defined objectives/end points and methods by which to measure that these objectives/ end points have been met prior to human implantation. The field has two examples of what can happen when this does not occur in a clearly defined fashion. While it is clear that there is a lot at stake for the person/group that develops the ‘perfect TEHV’, this field would be wise not to treat the development of an ideal TEHV as a so-called ‘arms race’. Collaboration and scientific rigor are paramount in order to ensure that the needs of the patient come first. Important lessons have been gleaned from the previous human implantations and the field is moving toward successful clinical trials in the future of TEHV development. Over the next 10 years, further advances in the design, testing and clinical performance/durability of TEHVs will be made.
Financial & competing interests disclosure
The authors wish to gratefully recognize the support of SH bin Z Al Nahyan, the Grainger Foundation, the Asper Foundation and the Center for Regenerative Medicine at the Mayo Clinic. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Current valve replacement options & unmet needs
▪ Current replacement options include mechanical and bioprosthetic valves.
▪ Mechanical valves are limited by the need for anticoagulation.
▪ Bioprosthetic valves are limited by their durability.
▪ The most pressing unmet need is for improved valve replacement options in the pediatric population.
Strategies of biological valve development
▪ Multiple strategies of valve development are underway involving different scaffolds, fabrication methods and cell types.
▪ There is not one clear pathway to success.
In vitro conditioning & testing
▪ Bioreactors have been developed to provide biomechanical and biochemical stimuli to direct in vitro tissue formation.
▪ It remains to be determined how necessary bioreactor conditioning is to the success of tissue-engineered heart valves.
Preclinical testing
▪ The ovine model has been considered the gold standard to test tissue-engineered heart valves.
▪ Questions remain as to the translatability of the results seen in the ovine model to humans.
Challenges for the translation of tissue-engineered valves from preclinical to clinical studies
▪ Previous implantation of decellularized scaffolds into humans without recellularization have had very poor results.
▪ The poor results of the previous human trials highlight the importance of rigorous in vitro and preclinical animal studies.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation 118(18), 1864–1880 (2008).
- Aikawa E, Whittaker P, Farber M et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation 113(10), 1344–1352 (2006).
- Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Ann. Rev. Physiol. 73, 29–46 (2011).
- Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the veterans affairs randomized trial. J. Am. Coll. Cardiol. 36(4), 1152–1158 (2000).
- Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363(17), 1597–1607 (2010).
- Bloomf eld P, Wheatley DJ, Prescott RJ, Miller HC. Twelve-year comparison of a Bjork–Shiley mechanical heart valve with porcine bioprostheses. N. Engl. J. Med.324(9), 573–579 (1991).
- Rao PS, Solymar L, Mardini MK, Fawzy ME, Guinn G. Anticoagulant therapy in children with prosthetic valves. Ann. Thorac. Surg. 47(4), 589–592 (1989).
- Ross D, Jackson M, Davies J. Pulmonary autograft aortic valve replacement: long-term results. J. Cardiac Surg. 6(Suppl. 4), S29–S33 (1991).
- Ross D, Jackson M, Davies J. The pulmonary autograft – a permanent aortic valve. Eur. J. Cardiothorac. Surg. 6(3), 113–116;discussion 117 (1992).
- Stewart RD, Backer CL, Hillman ND, Lundt C, Mavroudis C. The Ross operation in children: effects of aortic annuloplasty.Ann. Thorac. Surg. 84(4), 1326–1330 (2007).
- Takkenberg JJ, Van Herwerden LA, Galema TW et al. Serial echocardiographic assessment of neo-aortic regurgitation and root dimensions after the modified Ross procedure. J. Heart Valve Dis. 15(1), 100–106; discussion 106–107 (2006).
- Go AS, Mozaffarian D, Roger VL et al. Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation 127(1), e6–e245 (2013).
- Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database.Thorac. Cardiovasc. Surg. 137(1), 82–90(2009).
- Kidane AG, Burriesci G, Edirisinghe M, Ghanbari H, Bonhoeffer P, Seifalian AM. novel nanocomposite polymer for development of synthetic heart valve leaflets. Acta Biomater. 5(7), 2409–2417 (2009).
- Ghanbari H, Kidane AG, Burriesci G, Ramesh B, Darbyshire A, Seifalian AM. The anti-calcification potential of a silsesquioxane nanocomposite polymer under in vitro conditions: potential material forsynthetic leaflet heart valve. Acta Biomater. 6(11), 4249–4260 (2010).
- Ghanbari H, De Mel A, Seifalian AM. Cardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: a glimpse into prospective horizons. Int.J. Nanomed. 6, 775–786 (2011).
- Duan B, Hockaday LA, Kang KH, Butcher JT. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 101(5), 1255–1264 (2012).
- Hockaday LA, Kang KH, Colangelo NW et al. Rapid 3D printing of anatomicallyaccurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 4(3), 035005 (2012).
- Masters KS, Shah DN, Leinwand LA, Anseth KS. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials 26(15), 2517–2525 (2005).
- Ramamurthi A, Vesely I. Evaluation of the matrix-synthesis potential of crosslinked hyaluronan gels for tissue engineering of aortic heart valves. Biomaterials 26(9), 999–1010 (2005).
- Van Nooten G, Somers P, Cornelissen M et al. Acellular porcine and kangaroo aorticvalve scaffolds show more intense immune-mediated calcification than cross-linked Toronto SPV valves in the sheep model. Interact. Cardiovasc. Thorac. Surg. 5(5), 544–549 (2006).
- Kasimir MT, Rieder E, Seebacher G et al. Decellularization does not eliminate thrombogenicity and inflammatory stimulation in tissue-engineered porcine heart valves. J. Heart Valve Dis. 15(2), 278–286 (2006).
- Erdbrugger W, Konertz W, Dohmen PM et al. Decellularized xenogenic heart valves reveal remodeling and growth potential in vivo. Tissue Eng. 12(8), 2059–2068 (2006).
- Dohmen PM, Da Costa F, Holinski S et al. Is there a possibility for a glutaraldehyde-free porcine heart valve to grow? Eur. Surg. Res. 38(1), 54–61 (2006).
- Ott HC, Matthiesen TS, Goh SK et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 14(2), 213–221 (2008).
- Tudorache I, Cebotari S, Sturz G et al. Tissue engineering of heart valves: biomechanical and morphological properties of decellularized heart valves. J. Heart Valve Dis. 16(5), 567–573; discussion 574 (2007).
- Cebotari S, Tudorache I, Jaekel T et al. Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells. Artif. Organs 34(3), 206–210 (2010).
- Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials 29(8), 1065–1074 (2008).
- Yang M, Chen CZ, Wang XN, Zhu YB, Gu YJ. Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves. J. Biomed. Mater. Res. Part B Appl. Biomater. 91(1),354–361 (2009).
- Rieder E, Kasimir MT, Silberhumer G et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells.Thorac. Cardiovasc. Surg. 127(2), 399–405(2004).
- Gallo M, Naso F, Poser H et al. Physiological performance of a detergent decellularized heart valve implanted for 15 months in Vietnamese pigs: surgical procedure, follow-up, and explant inspection. Artif. Organs 36(6), E138–E150 (2012).
- Iop L, Renier V, Naso F et al. The influence of heart valve leaflet matrix characteristics on the interaction between human mesenchymal stem cells and decellularized scaffolds. Biomaterials 30(25), 4104–4116 (2009).
- Naso F, Gandaglia A, Formato M et al. Differential distribution of structural components and hydration in aortic and pulmonary heart valve conduits: impact of detergent-based cell removal. Acta Biomater. 6(12), 4675–4688 (2010).
- Naso F, Gandaglia A, Iop L, Spina M, Gerosa First quantitative assay of a-Gal in soft tissues: presence and distribution of the epitope before and after cell removal from xenogeneic heart valves. Acta Biomater. 7(4), 1728–1734 (2011).
- Lai VK, Frey CR, Kerandi AM, Lake SP, Tranquillo RT, Barocas VH. Microstructural and mechanical differences between digested collagen-fibrin co-gels and pure collagen and fibrin gels. Acta Biomater. 8(11), 4031–4042 (2012).
- Lai VK, Lake SP, Frey CR, Tranquillo RT, Barocas VH. Mechanical behavior of collagen-fibrin co-gels reflects transition from series to parallel interactions with increasing collagen content. J. Biomech. Eng. 134(1), 011004 (2012).
- Bjork JW, Meier LA, Johnson SL, Syedain ZH, Tranquillo RT. Hypoxic culture and insulin yield improvements to fibrin-based engineered tissue. Tissue Eng. Part A 18(7–8), 785–795 (2012).
- Syedain ZH, Bradee AR, Kren S, Taylor DA, Tranquillo RT. Decellularized tissue-engineered heart valve leaflets with recellularization potential. Tissue Eng. Part A 19(5–6), 759–769 (2012).
- Mol A, Driessen NJ, Rutten MC, Hoerstrup SP, Bouten CV, Baaijens FP. Tissue engineering of human heart valve leaflets: a novel bioreactor for a strain-based conditioning approach. Ann. Biomed. Eng. 33(12), 1778–1788 (2005).
- Hoerstrup SP, Sodian R, Daebritz S et al. Functional living trileaflet heart valves grown in vitro. Circulation 102(19 Suppl. 3),III44–III49 (2000).
- Sodian R, Hoerstrup SP, Sperling JS et al. Tissue engineering of heart valves: in vitro experiences. Ann. Thorac. Surg. 70(1), 140–144 (2000).
- Sodian R, Lueders C, Kraemer L et al. Tissue engineering of autologous human heart valves using cryopreserved vascular umbilical cord cells. Ann. Thorac. Surg. 81(6), 2207–2216 (2006).
- Del Gaudio C, Bianco A, Grigioni M. Electrospun bioresorbable trileaflet heart valve prosthesis for tissue engineering: in vitro functional assessment of a pulmonary cardiac valve design. Ann. Ist. Super. Sanita 44(2), 178–186 (2008).
- Del Gaudio C, Grigioni M, Bianco A, De Angelis G. Electrospun bioresorbable heart valve scaffold for tissue engineering. Int. Artif. Organs 31(1), 68–75 (2008).
- Courtney T, Sacks MS, Stankus J, Guan J, Wagner WR. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials 27(19), 3631–3638 (2006).
- Mulholland DL, Gotlieb AI. Cell biology of valvular interstitial cells. Can. J. Cardiol. 12(3), 231–236 (1996).
- Dreger SA, Taylor PM, Allen SP, Yacoub MH. Prof le and localization of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in human heart valves. Heart Valve Dis. 11(6), 875–880;discussion 880 (2002).
- Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology.Am. J. Pathol. 171(5), 1407–1418 (2007).
- Shinoka T, Breuer CK, Tanel RE et al. Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann. Thorac. Surg. 60(Suppl. 6), S513–S516(1995).
- Steinhoff G, Stock U, Karim N et al. Tissue engineering of pulmonary heart valves on allogenic acellular matrix conduits: in vivo restoration of valve tissue. Circulation 102(19 Suppl. 3), III50–III55 (2000).
- Vincentelli A, Wautot F, Juthier F et al. In vivo autologous recellularization of a tissue-engineered heart valve: are bone marrow mesenchymal stem cells the best candidates? J. Thorac. Cardiovasc. Surg. 134(2), 424–432(2007).
- Weber B, Scherman J, Emmert MY et al. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur. Heart J. 32(22), 2830–2840 (2011).
- Dohmen PM, Lembcke A, Hotz H, Kivelitz D, Konertz WF. Ross operation with a tissue-engineered heart valve. Ann. Thorac. Surg. 74(5), 1438–1442 (2002).
- Frank BS, Toth PB, Wells WK et al. Determining cell seeding dosages for tissue engineering human pulmonary valves. J. Surg. Res. 174(1), 39–47 (2012).
- Perry TE, Kaushal S, Sutherland FW et al. Thoracic Surgery Directors Association Award. Bone marrow as a cell source for tissue engineering heart valves. Ann. Thorac. Surg. 75(3), 761–767; discussion 767 (2003).
- Sutherland FW, Perry TE, Yu Y et al. From stem cells to viable autologous semilunar heart valve. Circulation 111(21), 2783–2791 (2005).
- Sales VL, Mettler BA, Engelmayr GC Jr et al. Endothelial progenitor cells as a sole source for ex vivo seeding of tissue-engineered heartvalves. Tissue Eng. Part A 16(1), 257–267 (2010).
- Lee DJ, Steen J, Jordan JE et al. Endothelialization of heart valve matrix using a computer-assisted pulsatile bioreactor. Tissue Eng. Part A 15(4), 807–814 (2009).
- Schmidt D, Mol A, Odermatt B et al. Engineering of biologically active living heart valve leaflets using human umbilical cord-derived progenitor cells. Tissue Eng. 12(11), 3223–3232 (2006).
- Bolli R, Chugh AR, D’Amario D et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised Phase 1 trial. Lancet 378(9806), 1847–1857 (2011).
- Makkar RR, Smith RR, Cheng K et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised Phase 1 trial. Lancet 379(9819), 895–904 (2012).
- Schenke-Layland K, Opitz F, Gross M et al. Complete dynamic repopulation of decellularized heart valves by application of defined physical signals – an in vitro study. Cardiovasc. Res. 60(3), 497–509 (2003).
- Flanagan TC, Cornelissen C, Koch S et al. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials 28(23), 3388–3397 (2007).
- Robinson PS, Johnson SL, Evans MC, Barocas VH, Tranquillo RT. Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Eng. Part A 14(1), 83–95 (2008).
- Ramaswamy S, Gottlieb D, Engelmayr GC Jr et al. The role of organ level conditioning onthe promotion of engineered heart valve tissue development in-vitro using mesenchymal stem cells. Biomaterials 31(6), 1114–1125 (2010).
- Aleksieva G, Hollweck T, Thierfelder N et al. Use of a special bioreactor for the cultivation of a new flexible polyurethane scaffold for aortic valve tissue engineering. Biomed. Eng. Online 11, 92 (2012).
- Emmert MY, Weber B, Wolint P et al. Stem cell-based transcatheter aortic valve implantation: first experiences in a pre-clinical model. JACC Cardiovasc. Interv. 5(8), 874–883 (2012).
- Goldstein S, Clarke DR, Walsh SP, Black KS, O’Brien MF. Transpecies heart valve transplant: advanced studies of a bioengineered xeno-autograft. Ann. Thorac. Surg. 70(6), 1962–1969 (2000).
- Elkins RC, Goldstein S, Hewitt CW et al. Recellularization of heart valve grafts by a process of adaptive remodeling. Semin.Thorac. Cardiovasc. Surg. 13(4 Suppl. 1),S87–S92 (2001).
- Leyh RG, Wilhelmi M, Rebe P et al. In vivo repopulation of xenogeneic and allogeneic acellular valve matrix conduits in the pulmonary circulation. Ann. Thorac. Surg. 75(5), 1457–1463; discussion 1463 (2003).
- Takagi K, Fukunaga S, Nishi A et al. In vivo recellularization of plain decellularized xenografts with specific cell characterization in the systemic circulation: histological and immunohistochemical study. Artif. Organs 30(4), 233–241 (2006).
- Iwai S, Torikai K, Coppin CM, Sawa Y. Minimally immunogenic decellularized porcine valve provides in situ recellularization as a stentless bioprosthetic valve. J. Artif. Organs 10(1), 29–35 (2007).
- Jordan JE, Williams JK, Lee SJ, Raghavan D, Atala A, Yoo JJ. Bioengineered self-seeding heart valves. J. Thorac. Cardiovasc. Surg. 143(1), 201–208 (2012).
- Berry JL, Steen JA, Koudy Williams J, Jordan JE, Atala A, Yoo JJ. Bioreactors for development of tissue engineered heart valves. Ann. Biomed. Eng. 38(11), 3272–3279 (2010).
- Gandaglia A, Bagno A, Naso F, Spina M, Gerosa G. Cells, scaffolds and bioreactors for tissue-engineered heart valves: a journey from basic concepts to contemporary developmental innovations.Eur. J. Cardiothorac. Surg. 39(4), 523–531(2011).
- Balachandran K, Bakay MA, Connolly JM, Zhang X, Yoganathan AP, Levy RJ. Aortic valve cyclic stretch causes increased remodeling activity and enhanced serotonin receptor responsiveness. Ann. Thorac. Surg. 92(1), 147–153 (2011).
- Ku CH, Johnson PH, Batten P et al. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc. Res. 71(3), 548–556 (2006).
- Merryman WD, Lukoff HD, Long RA, Engelmayr GC Jr, Hopkins RA, Sacks MS. Synergistic effects of cyclic tension and transforming growth factor-b1 on the aortic valve myofibroblast. Cardiovasc. Pathol. 16(5), 268–276 (2007).
- Balachandran K, Konduri S, Sucosky P, Jo H, Yoganathan AP. An ex vivo study of the biological properties of porcine aortic valves in response to circumferential cyclic stretch. Ann. Biomed. Eng. 34(11), 1655–1665 (2006).
- Gupta V, Werdenberg JA, Lawrence BD, Mendez JS, Stephens EH, Grande-Allen KJ. Reversible secretion of glycosaminoglycans and proteoglycans by cyclically stretched valvular cells in 3D culture. Ann. Biomed. Eng. 36(7), 1092–1103 (2008).
- Engelmayr GC Jr, Rabkin E, Sutherland FW, Schoen FJ, Mayer JE Jr, Sacks MS. The independent role of cyclic flexure in the early in vitro development of an engineered heart valve tissue. Biomaterials 26(2), 175–187 (2005).
- Engelmayr GC Jr, Sales VL, Mayer JE Jr, Sacks MS. Cyclic flexure and laminar flow synergistically accelerate mesenchymal stem cell-mediated engineered tissue formation: implications for engineered heart valve tissues. Biomaterials 27(36), 6083–6095 (2006).
- Jockenhoevel S, Zund G, Hoerstrup SP, Schnell A, Turina M. Cardiovascular tissue engineering: a new laminar flow chamber for in vitro improvement of mechanical tissueproperties. ASAIO J. 48(1), 8–11 (2002).
- Butcher JT, Penrod AM, Garcia AJ, Nerem RM. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler. Thromb. Vasc. Biol. 24(8),1429–1434 (2004).
- Butcher JT, Tressel S, Johnson T et al. Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences: influence of shear stress. Arterioscler. Thromb. Vasc. Biol. 26(1), 69–77 (2006).
- Butcher JT, Nerem RM. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress.Tissue Eng. 12(4), 905–915 (2006).
- Xing Y, He Z, Warnock JN, Hilbert SL, Yoganathan AP. Effects of constant static pressure on the biological properties of porcine aortic valve leaflets. Ann. Biomed. Eng. 32(4), 555–562 (2004).
- Xing Y, Warnock JN, He Z, Hilbert SL, Yoganathan AP. Cyclic pressure affects the biological properties of porcine aortic valve leaflets in a magnitude and frequency dependent manner. Ann. Biomed. Eng. 32(11), 1461–1470 (2004).
- Ikhumetse JD, Konduri S, Warnock JN, Xing Y, Yoganathand AP. Cyclic aortic pressure affects the biological properties of porcine pulmonary valve leaflets. J. Heart Valve Dis. 15(2), 295–302 (2006).
- Ota T, Sawa Y, Iwai S et al. Fibronectin-hepatocyte growth factor enhances reendothelialization in tissue-engineered heart valve. Ann. Thorac. Surg. 80(5), 1794–1801 (2005).
- Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 22(4), 339–350 (2003).
- Huang SD, Liu XH, Bai CG et al. Synergistic effect of fibronectin and hepatocyte growth factor on stable cell-matrix adhesion, re-endothelialization, and reconstitution in developing tissue-engineered heart valves. Heart Vessels 22(2), 116–122 (2007).
- Hoerstrup SP, Zund G, Schnell AM et al. Optimized growth conditions for tissue engineering of human cardiovascular structures. Int. J. Artif. Organs 23(12), 817–823 (2000).
- Narine K, De Wever O, Van Valckenborgh D et al. Growth factor modulation of fibroblastproliferation, differentiation, and invasion: implications for tissue valve engineering. Tissue Eng. 12(10), 2707–2716 (2006).
- Dvorin EL, Wylie-Sears J, Kaushal S, Martin DP, Bischoff J. Quantitative evaluation of endothelial progenitors and cardiac valve endothelial cells: proliferation and differentiation on poly-glycolic acid/ poly-4-hydroxybutyrate scaffold in response to vascular endothelial growth factor and transforming growth factor b1. Tissue Eng. 9(3), 487–493 (2003).
- Johnson EN, Lee YM, Sander TL et al. NFATc1 mediates vascular endothelial growth factor-induced proliferation of human pulmonary valve endothelial cells. J. Biol. Chem. 278(3), 1686–1692 (2003).
- Ruel J, Lachance G. Mathematical modeling and experimental testing of three bioreactor configurations based on windkessel models. Heart Int. 5(1), e1 (2010).
- Lichtenberg A, Tudorache I, Cebotari S et al. In vitro re-endothelialization of detergentdecellularized heart valves under simulated physiological dynamic conditions. Biomaterials 27(23), 4221–4229 (2006).
- Hildebrand DK, Wu ZJ, Mayer JE Jr, Sacks MS. Design and hydrodynamic evaluation of a novel pulsatile bioreactor for biologically active heart valves. Ann. Biomed. Eng. 32(8), 1039–1049 (2004).
- Sierad LN, Simionescu A, Albers C et al. Design and testing of a pulsatile conditioning system for dynamic endothelialization of polyphenol-stabilized tissue engineered heart valves. Cardiovasc. Eng. Technol. 1(2), 138–153 (2010).
- Kaasi A, Cestari IA, Stolf NA, Leirner AA, Hassager O, Cestari IN. A new approach to heart valve tissue engineering: mimicking the heart ventricle with a ventricular assist device in a novel bioreactor. J. Tissue Eng. Regen. Med. 5(4), 292–300 (2011).
- Kortsmit J, Rutten MC, Wijlaars MW, Baaijens FP. Deformation-controlled load application in heart valve tissue engineering. Tissue Eng.Part C Methods 15(4), 707–716 (2009).
- Vismara R, Soncini M, Talo G et al. A bioreactor with compliance monitoring for heart valve grafts. Ann. Biomed. Eng. 38(1), 100–108 (2010).
- Kortsmit J, Driessen NJ, Rutten MC, Baaijens FP. Real time, non-invasive assessment of leaflet deformation in heart valve tissue engineering. Ann. Biomed. Eng. 37(3), 532–541 (2009).
- Syedain ZH, Tranquillo RT. Controlled cyclic stretch bioreactor for tissue-engineered heart valves. Biomaterials 30(25), 4078–4084 (2009).
- Narita Y, Hata K, Kagami H, Usui A, Ueda M, Ueda Y. Novel pulse duplicating bioreactor system for tissue-engineered vascular construct. Tissue Eng. 10(7–8), 1224–1233 (2004).
- Ziegelmueller JA, Zaenkert EK, Schams R et al. Optical monitoring during bioreactorconditioning of tissue-engineered heart valves. ASAIO J. 56(3), 228–231 (2010).
- Konig F, Hollweck T, Pfeifer S et al. A pulsatile bioreactor for conditioning of tissue-engineered cardiovascular constructs under endoscopic visualization. J. Funct. Biomater. 3, 480–496 (2012).
- Metzler SA, Digesu CS, Howard JI, Filip To SD, Warnock JN. Live en face imaging of aortic valve leaflets under mechanical stress. Biomech. Model Mechanobiol. 11(3-4),355–361 (2012).
- Hoerstrup SP, Sodian R, Sperling JS, Vacanti JP, Mayer JE Jr. New pulsatile bioreactor for in vitro formation of tissue engineered heartvalves. Tissue Eng. 6(1), 75–79 (2000).
- Dumont K, Yperman J, Verbeken E et al. Design of a new pulsatile bioreactor for tissue engineered aortic heart valve formation. Artif. Organs 26(8), 710–714 (2002).
- Mol A, Bouten CV, Zund G et al. The relevance of large strains in functional tissue engineering of heart valves. Thorac. Cardiovasc. Surg. 51(2), 78–83 (2003).
- Mol A, Rutten MC, Driessen NJ et al. Autologous human tissue-engineered heart valves: prospects for systemic application. Circulation 114(Suppl. 1), I152–I158 (2006).
- Engelmayr GC Jr, Hildebrand DK, Sutherland FW, Mayer JE Jr, Sacks MS. A novel bioreactor for the dynamic flexural stimulation of tissue engineered heart valve biomaterials. Biomaterials 24(14), 2523–2532 (2003).
- Gould RA, Chin K, Santisakultarm TP et al. Cyclic strain anisotropy regulates valvular interstitial cell phenotype and tissue remodeling in three-dimensional culture. Acta Biomater. 8(5), 1710–1719 (2012).
- Weston MW, Yoganathan AP. Biosynthetic activity in heart valve leaflets in response to in vitro flow environments. Ann. Biomed. Eng. 29(9), 752–763 (2001).
- Barzilla JE, Mckenney AS, Cowan AE, Durst CA, Grande-Allen KJ. Design and validation of a novel splashing bioreactor system for use in mitral valve organ culture. Ann. Biomed.Eng. 38(11), 3280–3294 (2010).
- Sun L, Rajamannan NM, Sucosky P. Design and validation of a novel bioreactor to subject aortic valve leaflets to side-specific shear stress. Ann. Biomed. Eng. 39(8), 2174–2185 (2011).
- Schipke KJ, To SD, Warnock JN. Design of a cyclic pressure bioreactor for the ex vivo study of aortic heart valves. J. Vis. Exp. (54), e3316 (2011).
- Engelmayr GC Jr, Soletti L, Vigmostad SC et al. A novel flex-stretch-flow bioreactor forthe study of engineered heart valve tissue mechanobiology. Ann. Biomed. Eng. 36(5), 700–712 (2008).
- Gallegos RP, Nockel PJ, Rivard AL, Bianco RW. The current state of in-vivo pre-clinical animal models for heart valve evaluation.J. Heart Valve Dis. 14(3), 423–432 (2005).
- Schoen FJ. Pathologic findings in explanted clinical bioprosthetic valves fabricated from photooxidized bovine pericardium. J. Heart Valve Dis. 7(2), 174–179 (1998).
- Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann. Biomed. Eng. 34(12), 1799–1819 (2006).
- Simon P, Kasimir MT, Seebacher G et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur. J. Cardiothorac. Surg. 23(6), 1002–1006; discussion 1006 (2003).
- Perri G, Polito A, Esposito C et al. Early and late failure of tissue-engineered pulmonary valve conduits used for right ventricular outflow tract reconstruction in patients with congenital heart disease. Eur.J. Cardiothorac. Surg. 41(6), 1320–1325(2012).
- Hjortnaes J, Bouten CV, Van Herwerden LA, Grundeman PF, Kluin J. Translating autologous heart valve tissue engineering from bench to bed. Tissue Eng. Part B Rev. 15(3), 307–317 (2009).
- Rahimtoola SH. The next generation of prosthetic heart valves needs a proven track record of patient outcomes at > or =15 to 20 years. J. Am. Coll. Cardiol. 42(10), 1720–1721 (2003).
- Durst CA, Grande-Allen KJ. Design and physical characterization of a synchronous multivalve aortic valve culture system. Ann. Biomed. Eng. 38(2), 319–325 (2010).
- Ruel J, Lachance G. A new bioreactor for the development of tissue-engineered heart valves. Ann. Biomed. Eng. 37(4), 674–681 (2009).
- Migneco F, Hollister SJ, Birla RK. Tissue-engineered heart valve prostheses: ‘state of the heart’. Regen. Med. 3(3), 399–419 (2008).
- Morsi YS, Yang WW, Owida A, Wong CS. Development of a novel pulsatile bioreactor for tissue culture. J. Artif. Organs 10(2), 109–114 (2007).
- Karim N, Golz K, Bader A. The cardiovascular tissue-reactor: a novel device for the engineering of heart valves. Artif. Organs 30(10), 809–814 (2006).
- Warnock JN, Konduri S, He Z, Yoganathan AP. Design of a sterile organ culture system for the ex vivo study of aortic heart valves.J. Biomech. Eng. 127(5), 857–861 (2005).
- Zeltinger J, Landeen LK, Alexander HG, Kidd ID, Sibanda B. Development and characterization of tissue-engineered aortic valves. Tissue Eng. 7(1), 9–22 (2001).
▪ Excellent review that includes a thorough discussion of the function, mechanics and pathology of native cardiac valves.
▪ Pioneering study into the exciting new area of 3D tissue valve printing.
▪ Unique tissue engineering approach involving a fibrin mold that is decellularized after the cells create the appropriate structure.
▪ Novel approach to heart valve tissue engineering based on a minimally invasive technique for both cell harvest and valve delivery, which demonstrates feasibility in nonhuman primates.
▪▪ State-of-the-art bioreactor exhibiting many innovative solutions to the challenging engineering problems that exist when creating physiological flow.
▪▪ Excellent review focusing on translational issues within the field of heart valve tissue engineering.