Research Article - Neuropsychiatry (2018) Volume 8, Issue 3
Changes of Growth Factor Family Genes Expressions in the Striatum Following the Dominant Hemisphere Ischemia in Rats
- Corresponding Authors:
- Jian-ying Tian
Department of Anatomy, College of Basic Medicine, Ningxia Medical University, Sheng Li Southern Street, Yinchuan 750004, Ningxia, China
Tel: +86-951-6980016
Huanmin Gao
Department of Neurology, People’s Hospital of Ningxia Hui Autonomous Region, Faculty of Medicine, Northwest University for Nationalities. 301 Zheng Yuan Northern Street, Yinchuan 750002, Ningxia, China
Tel: +86-951-5920076
Abstract
Abstract
Rationale
Handiness of the human implies the asymmetry in the brain. Asymmetry in the human brain denotes the dominant hemisphere has more elaborate and complicated functions, therefore, the ischemic injuries in the dominant hemisphere usually yield more serious clinical problems than that in the non-dominant hemisphere. The mechanism of the heavier ischemic injury in the dominant hemisphere has not been yet known thoroughly, especially at the genetic level; the specific gene expression pattern following the dominant hemisphere ischemia has not yet been reported.
Objectives
In this study it was to investigate the trend of gene expression in the striatum after the dominant hemisphere ischemia in the rats.
Methods
Behavioral tests were used to select 24 right-handed Wistar rats, and the dominant hemisphere ischemic model was archived by the left middle cerebral artery occlusion/ reperfusion (MCAO). 8000 dots gene chip was used to analyze the changes of gene expression profiles in the striatum. In situ hybridization was used to confirm the gene expression and immunohistochemistry stain to find the downstream protein expression of target gene.
Results
There was a lot of lower Expressed Sequence Tag (EST) both in the ischemia group and in the sham. A total of 4286 EST differentially expressed with the density value more than 800 and the ratio of Cy3/Cy5 >2.0. There were 368 EST with density value more than 800 and the ratio of Cy3/Cy5 <0.5 revealed higher expression and 79 EST lower expression in the ischemia group. Growth factors family genes lower expressed but individual genes higher expressed. Gene expressions in the striatum in the dominant hemisphere ischemia group were lower than that of the non-dominant hemisphere ischemia group significantly (p<0.05).
Conclusion
The results mentioned above suggested that growth factors family gene expression in the dominant hemisphere ischemic striatum were lower than that of non-dominant hemisphere, cerebral ischemia in the dominant hemisphere produced sever injury at gene expression level.
Keywords
Dominant hemisphere, Reperfusion injury, Growth factor, Gene chip, Rat
Introduction
Now the Cerebrovascular diseases are serious impacts on human health worldly. Handiness of the human implies the asymmetry in the human brain, that is, human brain has the dominant hemisphere and the non-dominant hemisphere. Asymmetry in the human brain denotes the dominant hemisphere has more elaborate and complicated functions including language control; therefore, the ischemic injuries in the dominant hemisphere usually yield more serious clinical problems than that in the nondominant hemisphere such as dysphasia [1-5]. The mechanism of the heavier ischemic injury in the dominant hemisphere has not been yet known thoroughly, especially at the genetic level, the specific gene expression pattern following the dominant hemisphere ischemia has not yet been reported at the initiating stage of the field.
Gene expression is the first step of functional protein synthesis. However, laboratory study usually detects gene expression using in situ hybrzadation to observe one gene in one experiment. But the individual gene expression was in the different conditions and difficult to compare. cDNA microarray technology known as Gene chip provides a platform of high flow gene detection at the same conditions and easy to compare.
To begin to address these limitations of gene expression research, our initial objectives were to: a) determine gene expression patterns of candidate growth factors family (specifically, insulin-like growth factor-1) in the striatum on the dominant hemisphere ischemia rat model which previously established by our laboratory [5,6]; b) evaluate IGF-1 gene expression and protein expression to confirm the gene expression characteristic; c) to investigate the trend of gene expression in the striatum after the dominant hemisphere ischemia in the rat.
Materials and Methods
▪ The right paw preferred rats and group
35 male Wistar rats, body weighing 260~280 g, adapted to the experimental environment for 7 d, and fasted 2 d before the experiment. On the day of experiment, the rat was put in an individual cage with the front of the cage having a small open window of 1 cm × 1 cm apart, that size can only permit rat’s paw in cautiously, not to put the whole mouth out of the cage to fetch the food.
Paw preferred rats were determined by the method described by Tang and Verstynen [7] and modified in our laboratory [5,6]. The probability of the left and the right paw touching rat’s cake per minute was calculated. Based on the binomial probability distribution principle, determine the “right preference”, “left preference” or “Bilateral preference” [6,7]. Probability greater than 29 was regarded as right paw preference; probability less than 21 as the left paw preference; the probability between 22 ~ 28 as non-preference or bilateral preference, and expelled from this study. On this basis of the observed data and calculation, 24 right paw preferred rats were selected from 35 rats in this experiment.
24 right paw preferred rats were randomly selected into two groups according with the random numbers table, 12 into the left middle cerebral artery ischemia/reperfusion (MCAO) group as the dominant hemisphere ischemia group for the “right-handed” rats, 12 into the right MCAO group as the non-dominant hemisphere ischemia group for the “right-handed” rats. In each group, 9 rats in the ischemia group and 3 in the Sham. The protocol has been approved by Ningxia People’s Hospital of Ningxia Hui Autonomous Region and Northwest University for Nationalities Ethics Committee.
▪ MCAO model in rats
The middle cerebral artery ischemia/reperfusion (MCAO) model in rat by Longa and colleagues [8] was carefully followed and modified in our previously rat model of the dominant hemisphere ischemia study [5,6].
Preoperative fasting overnight rats received 8% chloral hydrate (300 mg/kg) intraperitoneal injection of anesthesia, near the midline incision in the neck; carefully separating common carotid artery, internal carotid artery and external carotid artery. The external carotid artery was tied knot loosely and then snipped for later use to insert the blood vessel blocker in. Single 4-0 nylon surgical thread tip baked obtuse by lit mosquitorepellent incense in windless environment, then with poly lysine on the tip to increase the degree of smooth. The surgical thread was inserted into the residual end of the external carotid artery mentioned above, slightly tighten the spare live line around the external carotid artery, then into 18~20 mm when the distal has slight resistance, then stop inserting, at last fully tighten the spare line junction. 2 h later pulled out the surgical thread for reperfusion.
Sham operation was exactly the same procedures, but not blocked the middle cerebral artery by inserting the surgical thread less than 15 mm.
Anal temperature was monitored while a heating pad maintains its anus temperature at about 37.5℃, 25℃ in room temperature control.
▪ Modified neurological defections assessment
Modified Neurological defections assessment [8,9] which focus on: a) status of consciousness, b) the paws movement ability and c) the ability to feel, was performed 4 times: before ischemia, 24, 48, 72 h reperfusion, respectively.
Tail suspension tested the posture of the rat: no nerve injury symptoms indicated 0 point; Tail suspension test cannot be fully extended on front side indicated 1point; Fore resistance ability of lateral reduced indicated 2 points; to the contralateral circle indicated 3 points.
Suspension of rat tail, rats upper body posture maintain time: when the contra-lateral front or back claws into the body side, according to the time needed for each claw to straighten: 0 (< 1 s), 1 (< 5 s), 2 (> 5 s).
In horizontal posture reflex test, the rat body was put to one side: 0 point (fully resistance to push in the opposite direction), 1 point (little resistance initially then quickly recovery), 2 points (obviously decreased resistance), 3 points down to the contralateral.
In the rat circling tests, the movement of the scoring: 0 point (linear motion), 1 point (to the right movement), 2 (circling movement), 3 points (cannot move at all).
This study adopted the modified neurological defect assessments with a total of 0 ~ 11 points. 0 point indicated normal and 11 points indicated serious neurological defection.
▪ Gene chip detection
Bilateral striatum were removed from the rat brain in the ischemia group and in the sham operation control, and put into liquid nitrogen tank immediately, 2 h later transferred into -80℃ low temperature refrigerator.
▪ Total RNA extraction
cryopreservation striatum was hammered and grinded into fine powder in the liquid nitrogen to keep low temperature. Air dried RNA for 5~10 min. No RNase water suspension RNA incubated for 10 min at 55~ 60℃, ultraviolet spectrophotometer measured RNA content, adjusted the concentration to 1 μg/μL, -70℃ preserved for later use.
▪ Gene chip detection
mRNA purification: using oligotex kit (QIAGEN) according to the manual operation. Samples Retroviruses labeled: Cy3- duTP and Cy5 - duTP.8,000 dots chips were provided by Shanghai Boxing Company. Hybridization in the chips after reverse transcribed into cDNA and then hybrid with the brain tissue cDNA: using Cy3 labeled tissue 1, Cy5 labeled tissue 2, mixed probes hybridization, in two wavelengths of laser scanning.
▪ Gene chip Data analysis
Selected the absolute value of screening of Cy3 >800 and Cy5 >800 respectively, the ratio of Cy3/Cy5 (R/G) > 2.0, or <0.5. No differentially expressed genes within R/G values ranged 0.5~2.0. Outside of the R/G range existed significant differential expression of genes.
In situ hybridization to confirm IGF-1 gene expression: Removed the paraffin by dipping sections in xylene and ethanol. Rehydrated and post-fixed for 30 min in the same fixative. Treated with Proteinse K for 15 min at 37℃. Digitalis-labeled probe for rat IGF-1 mRNA was:
5’-AAGGTGAGCAGGCACAGCGCCAGGTAGA
AG AGATG-3’
And
5’-CTCCCTCTACTTGCGTTCTTCAAATGTACT
TCCTT-3’.
The probe was applied on tissue sections and covered with a sliconized coverlip. Incubated overnight at 40~42℃. Washed the slides successively in 2 × SSC (standard saline citrate: NaCl+C6H8O7Na3.2H2O), 5 min×2 at 37℃, 0.5×SSC, 15 min×1, 0.2×SSC, 15 min × 1; Added the block solution for 30 min at 37℃. Added biotin-mouse-anti-digitalis for 60 min at 37℃, washed with 0.5 M PBS for 5 min × 4; Incubated with SABC for 20 min at 37℃, washed with 0.5 M PBS for 5 min×3; Incubated with biotin-peroxidase for 20 min at 37℃, washed with 0.5 M PBS for 5 min × 4; DAB (diaminobenzidine, from ABC kit) for 20 min, washed with water.
Negative control sections received the same treatment as mentioned above except for exposure to the digitalis-labeled probe.
▪ Immunohistochemistry to confirm IGF-1 protein
After 9 h reperfusion the rats were anesthetized with 8% Chloral hydrate and perfused transcardially with normal saline followed by 4% paraformaldehyde. After decapitation, the brain was immediately removed, post-fixed for 2 d in 4% buffered paraformaldehyde+20% sucrose at 4℃, in 30% sucrose perfusion fixative for 2 d at 4℃. After gradient desiccation and paraffin coating the brain was serially sectioned in a coronal plane into 30 micron sections with microtomer (Shouda, Okayama, Japan).
Immunochemstaining against IGF-1 in the rat brain was performed by avidin-biotin-peroxidase (ABC) method. The brain sections were fixed in the ice cold 4% paraformaldehyde for 30 min at room temperature. Washed with 0.01 M PBS 3 times, 5 min each, 400 ml 8% albumin in 0.01 M PBS for 30 min at room temperature. Washed with 0.01 M PBS 3 times, 5 min each, at room temperature. Incubated with 10 µg/ml anti-IGF-1 in PBS overnight at 4℃. Washed the sections with 0.01M PBS 4 times, 15 min each, at room temperature. Incubated with 1:100 dilution of goat anti-mouse fluorescenin conjugated secondary antibody in 1% BSA (bovine serum) 2 h at the room temperature. Washed with 0.01M PBS 3 times, 15 min each, at room temperature.
Also, run a negative control (no primary antibody) to check for non-specific staining. Examine the sections under the fluorescent microscope.
Statistical analysis
Data are summarized using standard descriptive statistics: frequency and percentage for categorical variables; and mean, standard deviation (SD) for continuous variables. All calculated p-values were two-sided and p-values less than 0.05 were considered statistically significant. Statistical analyses were performed using the SAS version 9.2 software package (SAS Institute, Inc., Cary, NC).
Results
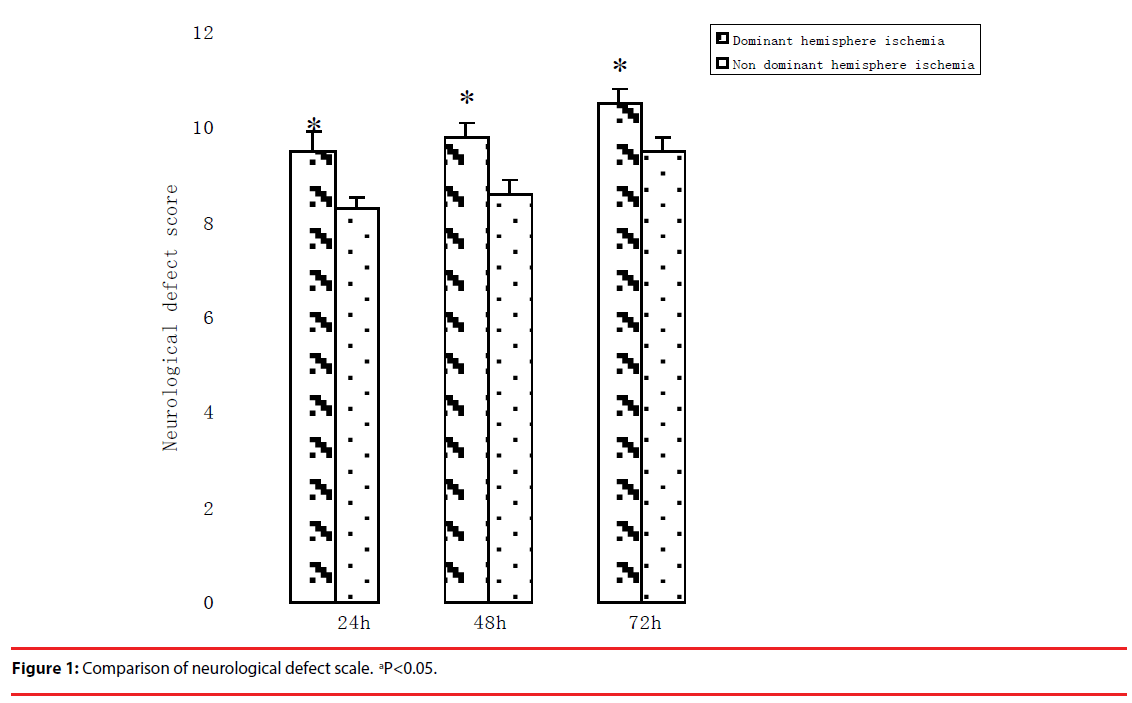
▪ Neurological defect assessments comparison
Neurological function defect existed in all rats after MCA occlusion/reperfusion compared with Sham operation.
Neurological defect score in the dominant hemisphere ischemia group was lower than that in non-dominant hemisphere ischemia group significantly (p<0.05, Figure 1). This result implied that the dominant hemisphere ischemia produced more brain injuries than the nondominant hemisphere ischemia in rats.
▪ Changes of gene expression in the striatum after ischemia in rats
Total RNA Extracted from the striatum had no gDNA pollution by PCR examination after DNase I digestion, A260/A280 values between 1.9~2.0, electrophoresis examination without degradation with 28s, 18s, 5s clearly.
The cDNA of the striatum mRNA hydrated within 8,000 gene chip microarrayed by software analysis, yield the absolute value of gene expression at each dot of the gene chip.
Microarray showed that there were a lot of lower expressions of expression sequence target (EST) both in the cerebral ischemia group and in Sham group. A total of 4286 genes differentially expressed. There were 368 EST, defined in the gene bank, the density value more than 800 and the ratio of Cy3/Cy5>2.0.;There were 79 EST with density value more than 800 and the ratio of Cy3/Cy5 <0.5. Results showed that 368 high expression and 79 lower expressions in the ischemic striatum.
Growth factor family genes individual highly expressed, but most low expressed in the ischemic striatum as shown in Table 1.
| Group | X06562 | M64347 | X57025 | M96995 | U28811 |
|---|---|---|---|---|---|
| Dominant hemisphere ischemia | 3.75 ± 0.85a,b | 0.43 ± 0.13a,b | 0.37 ± 0.78a,b | 0.43 ± 0.04a,b | 0.33 ± 0.06a,b |
| Nondominant hemisphere ischemia | 5.50 ± 0.75 a | 0.59 ± 0.10a | 1.75 ± 0.12a | 0.65 ± 0.03a | 0.75 ± 0.15a |
| t | 9.732 | 8.674 | 7.982 | 8.547 | 6.347 |
| P | 0.034 | 0.041 | 0.047 | 0.039 | 0.028 |
Dominant hemisphere ischemia group compared with the non-dominant hemisphere ischemia group, bP<0.05
Note: X06562 growth hormone receptor; M64347 new growth factor receptor; X57025 insulin-like growth factor-1; M96995 epidermal growth factor receptor protein GRB2; U28811 rich in cysteine fibroblast growth factor receptor (CFR-1).
Table 1: Comparison of growth factor related gene expression in striatum after the dominant hemisphere ischemia in rats.
The genomic response to cerebral ischemia has different pattern. Among the nearly 8,000 genes arrayed nearly 8% of the total numbers of genes examined were changed after cerebral ischemia. These altered expressed genes may be classified into 3 groups: a) known genes previously documented in the pathogenesis of ischemia, b) known genes barely previously involved in the ischemia, and c) cDNAs representing yet uncharacterized genes.
Many pathways including membranes protein, protein kinases, and transcription factors were changed after ischemia. Major ischemic altered genes were down-regulated.
Most growth factor family genes lower expressed in the ischemic striatum, and the IGF-1 mRNA could be the example from this study.
▪ IGF-1 mRNA expression confirmed by in situ hybridization
The DNA chip study should be at relative earlier stage of cerebral ischemia considering the detect sensitivity and the efficacy, the ischemic brain tissue might not be used at 24 h reperfusion according our previous study [5]. Therefore the present study for in situ hybridization was designed 9h reperfusion on rat MCAO model.
The baseline expression of IGF-1 mRNA in the normal striatum was relatively low (the positive cell numbers: 15.0±0.74). And the sham operation group showed similar pattern (the positive cell numbers: 14.77 ± 0.60, p>0.05, compared with the normal striatum baseline expression).
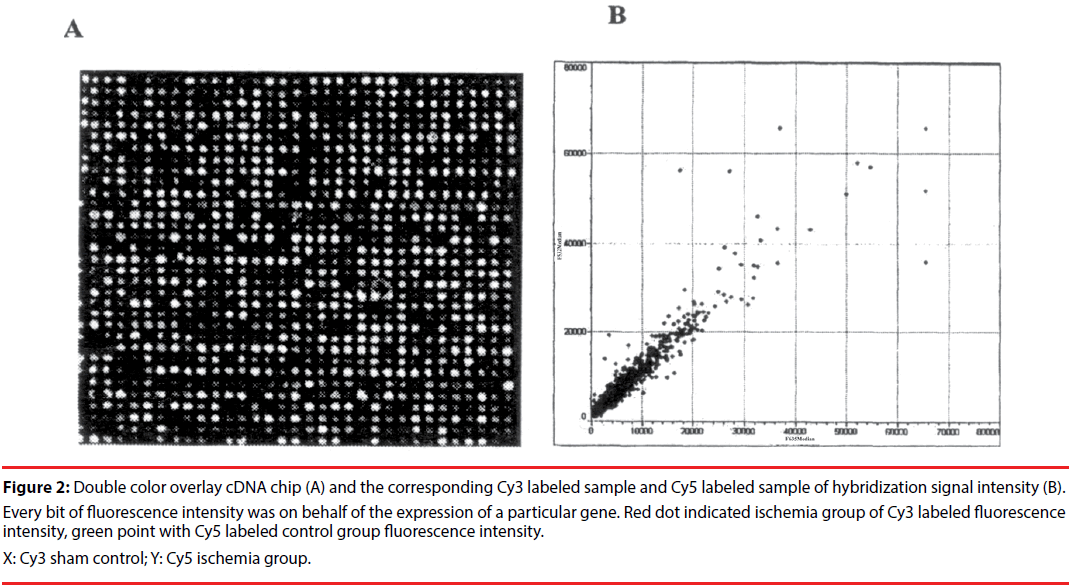
But the expressions decreased in the ischemia group at 9h reperfusion ( the numbers of positive cells: 9.72 ± 1.18 vs. 14.77 ± 0.60, p<0.01, Figure 2).
Figure 2: Double color overlay cDNA chip (A) and the corresponding Cy3 labeled sample and Cy5 labeled sample of hybridization signal intensity (B). Every bit of fluorescence intensity was on behalf of the expression of a particular gene. Red dot indicated ischemia group of Cy3 labeled fluorescence intensity, green point with Cy5 labeled control group fluorescence intensity.
X: Cy3 sham control; Y: Cy5 ischemia group.
The expression in the dominant hemisphere ischemia group was lower than that in the nondominant hemisphere ischemia group.
▪ IGF-1 protein expression confirmed by immunohistochemistry
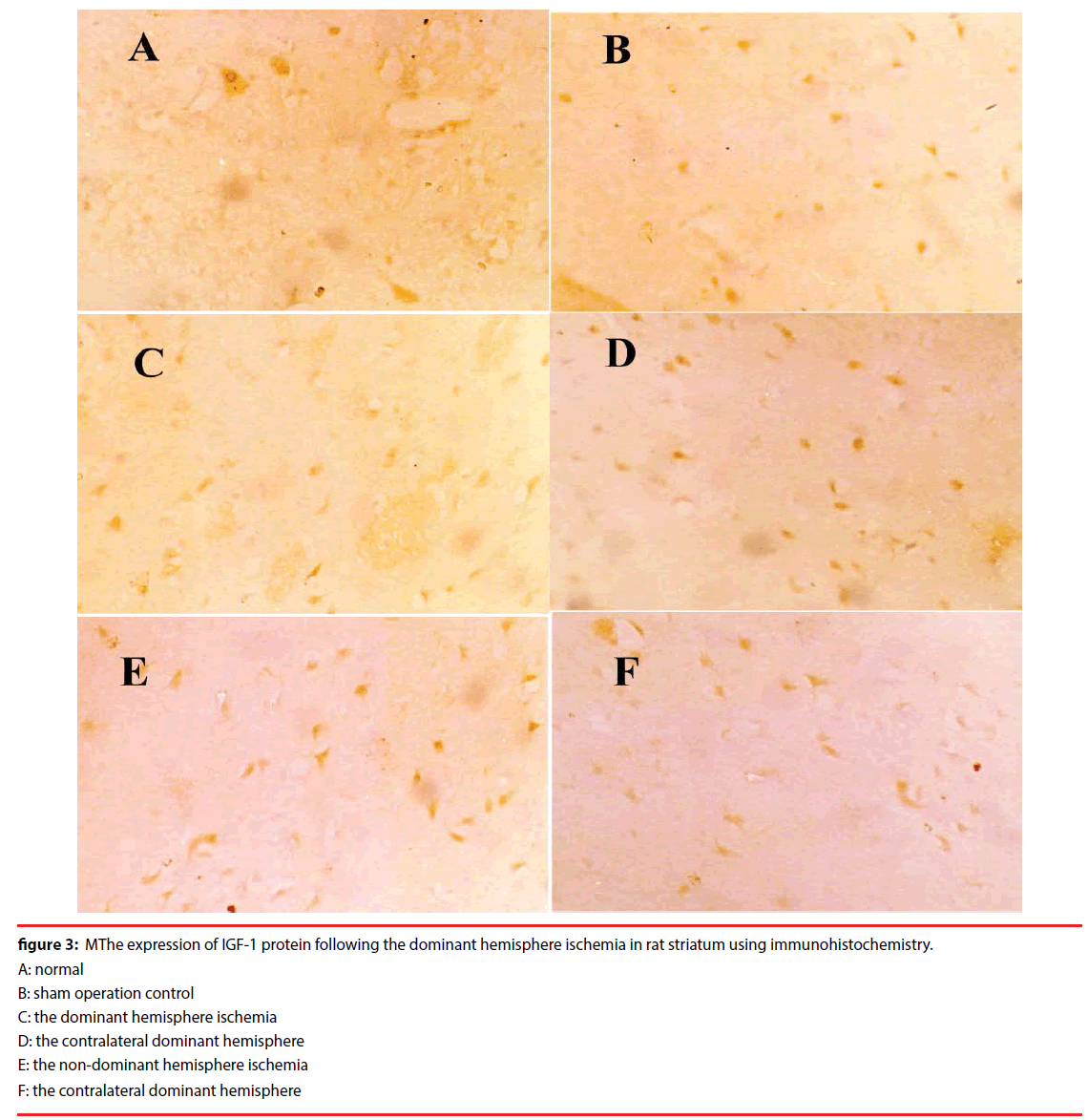
The baseline expression of IGF-1 protein in the normal striatum was relatively low (the immunoreactive positive cell numbers: 33.62 ± 0.99). And the sham had the same way, the positive cell numbers: 44.71 ± 0.76, compared with the normal striatum baseline expression, p>0.05, Figure 3.
Figure 3: MThe expression of IGF-1 protein following the dominant hemisphere ischemia in rat striatum using immunohistochemistry.
A: normal
B: sham operation control
C: the dominant hemisphere ischemia
D: the contralateral dominant hemisphere
E: the non-dominant hemisphere ischemia
F: the contralateral dominant hemisphere
But the IGF-1 protein expressions decreased in the ischemia group after 9h reperfusion.
The IGF-1 protein expression in the dominant hemisphere ischemia group was lower than that in the non-dominant hemisphere ischemia group.
This result showed that IGF-1 protein expression decreased in the ischemic striatum of the dominant hemisphere than that in the nondominant hemisphere ischemia in the rat.
Discussion
Human hands preference means asymmetry in the brain. Nearly 90% individuals are right handed [1]. Most of the right-handed has the dominant hemisphere at left hemisphere of the brain, where the language centre and the other complicated function canters located. Dominant hemispheres of the human brain have more function in regulating complex behavior, senior psychological activities than that of the non-dominant hemisphere. Modern neuropsychological researches [2-4] have shown that the left hemisphere of the human brain majors in controlling functions such as language, logical thinking, analytical ability, figures skill and calculation; while the right hemisphere skill for space orientation, shape recognition, music, art, comprehensive ability and short visual memory.
Ischemic injury in the dominant hemisphere of the human brain interfere with important functions such as language, is one of the heavier type stroke clinically without specific treatment now. Possible reason is no dominant hemisphere ischemia animal model available [5,6].
The middle cerebral artery ischemia/reperfusion model in rats has been used widely, for this model is close to the mankind stroke, but the cerebral infarction volume size, infarction distribution vary largely between different individual laboratory, thus cause problems for statistical analysis sometimes. The reason might be, in addition to rat species differences and occlusion depth of the middle cerebral artery, some researcher occlude of left middle cerebral artery, the others occlude the right middle cerebral artery, no any guideline, only according to the researchers’ habit [6].
In fact, the preference of rat paw is similar to the human’s handiness. Asymmetry in the brain is a common phenomenon, not only exists in the human brain, but also exists in the animals such as frog, rat. Rat front paw has handiness is decided by existing the dominant hemisphere in the brain [11].
Robinson reported [12] the biochemical and behavior changes after the left or the right hemispheres ischemia in the rat respectively. Anatomical and physiological differences mean asymmetry in the rat brain.
Middle cerebral arteries are susceptible artery to the cerebrovascular disease. The earliest report of the MCAO model by Bannister and Chapman [13] is proposed to occlude the right middle cerebral artery, and then followed by Longa and colleagues [7], Kuge et al. [14]. Why chose the right middle cerebral artery? No explanation.
Through the neck surgery made the middle cerebral artery ischemia/reperfusion rat model, would inevitably damage to the vagus nerves in some degree, and the left vagus nerve distributed directly in the heart, therefore has influence on the heart regulation directly, and often can cause blood pressure fluctuations. We often see rat struggle, phlegm and irregular breathing when separation the carotid sheath with the vagus nerve. Another important factor of success for this rat model is smaller outside brain factors as little as possible. Attention should be paid to the asymmetry of the rat brain and reducing the system influence outside of the brain, such as breath and blood pressure, according to the purpose of the study to determine the occlusion side, and do not choose right MCAO or left MCAO at liberty.
Gene chip (cDNA microarray) has tens of thousands dots on the glass, silicon or nylon membrane, represents different genes of oligonucleotide probes, labeled with 32P, 33P, 35S, or fluorescent for DNA hybridization, con-focal laser scanning, computer analysis of fluorescence optical density, obtain the hybridization signal brightness and distribution, and then determine the gene expression profiles of the sample. Because it is in the same condition to detect genes, gene chip has a characteristic of high sensitivity, quantitative and qualitative.
In this study, 8,000 gene chip microarray was used; there were significant differences between the dominant hemisphere ischemia compared with control expression of non-dominant hemisphere ischemia. In our dominant hemisphere MCAO model, there was lower gene expression in the striatum of dominant hemisphere ischemia, suggested heavier energy metabolism disorders.
In summary, our study contributes to the research on gene altered by the dominant hemisphere ischemia in rats. While cerebral ischemia induces gene expression inhibition, the degree of gene expression for example of the growth factors family inhibition is greater in the dominant hemisphere ischemic striatum, suggesting cerebral ischemia in the dominant hemisphere might produce more severe injury in gene expression level. Previous work suggests dominant hemisphere ischemia model in the rat exist differences between the left MCAO and the right MCAO: this rat model at gene expression level would provide platform for high-flux genes study other than individual gene expression. Future studies should be guided by gene expression pattern of this cDNA microarray.
Conflict of Interest
The authors declare there are no financial or nonfinancial competing interests.
Acknowledgement
Grant support was provided by the Ningxia Natural Science Funding (NZ11159).
References
- Guranski K, Podemski R. Emotional prosody expression in acoustic analysis in patients with right hemisphere ischemic stroke. Neurol. Neurochir. Pol 49(2), 113-20 (2015).
- Rymarczyk K, Grabowska A. Sex differences in brain control of prosody. Neuropsychologia 45(5), 921-930 (2007).
- Ochfeld E, Newhart M, Molitoris J, et al. Ischemia in broca area is associated with broca aphasia more reliably in acute than in chronic stroke. Stroke 41(2), 325-30 (2010).
- Wildgruber D,Ackermann H, Kreifelts B, et al. Cerebral processing of linguistic and emotional prosody: fMRI studies. Prog. Brain. Res 156(1), 249-68 (2006).
- Gao H, Zhang M. Asymmetry in the brain influenced the neurological deficits and infarction volume following the middle cerebral artery occlusion in rats. Behav. Brain. Funct 4(1), 57 (2008).
- Zhang H, Shen Y, Wang W, et al. Rat Model of Focal Cerebral Ischemia in the Dominant Hemisphere. Int. J. Clin. Exp. Med 8(1), 504-511 (2015).
- Tang AC, Verstynen T. Early life environment modulates 'handedness' in rats. Behav. Brain. Res.; 131(1-2), 1-7 (2002).
- Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1), 84-91 (1989).
- Calvey C, Zhou W, Stakleff KS, et al. Short-term electrical stimulation to promote nerve repair and functional recovery in a rat model. J. Hand. Surg. Am 40(2), 314-322 (2015).
- Chen L, Zhao Y, Zhang T, et al. Protective effect of Sheng-Nao-Kang decoction on focal cerebral ischemia-reperfusion injury in rats. J. Ethnopharmacol 151(1), 228-36 (2014).
- Hami J, Sadr-Nabavi A, Sankian M, et al. Sex differences and left-right asymmetries in expression of insulin and insulin-like growth factor-1 receptors in developing rat hippocampus. Brain. Struct Funct 217(2), 293-302 (2012).
- Robinson RG. Differential behavioral and biochemical effects of right and left hemispheric cerebral infarction in the rat. Science 205(4407), 707-710 (1979).
- Bannister CM, Chapman SA. Ischemia and revascularization of the middle cerebral territory of the rat brain by manipulation of the blood vessels in the neck. Surg. Neurol 21(4), 351-257 (1984).
- Kuge Y, Minematsu K, Yamaguchi T, et al. Nylon monofilament for intraluminal middle cerebral artery occlusion in rats. Stroke 26(9), 1655-1657 (1995).