Review Article - Interventional Cardiology (2022) Volume 14, Issue 2
Choice and timing of P2Y12 antiplatelet agents in non-STE acute coronary syndrome: A review
- Corresponding Author:
- J. Dawn Abbott
Division of Cardiology,
Warren Alpert Medical School of Brown University,
Lifespan Cardiovascular Institute,
Providence,
Rhode Island,
USA,
E-mail: jabbott@lifespan.org
Received date: 07-Feb-2022, Manuscript No. FMIC-22-53650; Editor assigned: 09-Feb-2022, PreQC No. FMIC-22-53650 (PQ); Reviewed date: 23-Feb-2022, QC No. FMIC-22-53650; Revised date: 02-Mar -2022, Manuscript No. FMIC-22-53650 (R); Published date: 09-Mar-2022, DOI: 10.37532/1755-5310.2022.14(2).488
Abstract
Acute coronary syndromes remain a leading cause of morbidity and mortality worldwide. Reducing both ischemic and bleeding complications after percutaneous coronary intervention remains of paramount importance. The use of dual antiplatelet therapy (aspirin and a P2Y12 inhibitor) in the setting of acute coronary syndrome significantly reduces ischemic outcomes including cardiovascular death, recurrent myocardial infarction, and target vessel revascularization. Contemporary data suggests that the use of a high potency P2Y12 inhibitor (ticagrelor or prasugrel) with aspirin is superior to dual antiplatelet therapy using the low potency P2Y12 inhibitor clopidogrel. Furthermore, downstream administration of a P2Y12 inhibitor in the setting of unstable angina or non-ST elevation MI lowers the risk of bleeding without an increase in ischemic outcomes among patients undergoing an early invasive strategy. Additionally, for patients at high bleeding risk including those on oral anticoagulants, data supports shorter durations of dual antiplatelet therapy. In this review, the pathophysiology of acute coronary syndrome and latest evidence and guideline updates on P2Y12 antiplatelet therapy are summarized.
Keywords
Acute coronary syndrome • Antiplatelet agents •Myocardial infarction • Cardiovascular death
Introduction
Coronary Heart Disease remains the leading cause of death in the United States [1]. Despite advancements in medical therapies and coronary revascularization techniques, the annual incidence of acute Myocardial Infarction (MI) is approximately 800,000 in those over age 35, with over 100,000 deaths [1,2]. Vulnerable atheromatous plaques, also known as thin-cap fibroatheroma, are composed of a necrotic core with an overlying thin-fibrous cap. High risk histomorphologic features of the vulnerable plaque include positive (outward) remodeling, a large lipid core with a high concentration of inflammatory cells, a thin fibrous cap, and increased neovascularity [3,4]. Advancements in cardiac imaging with computed tomography have phenotyped vulnerable plaques as having low attenuation with small focal calcifications and a ring of high attenuation surrounding an area of low attenuation (Napkin-ring sign) [5,6].
Literature Review
Mechanisms of plaque disruption in acute coronary syndromes
Acute Coronary Syndrome (ACS) typically results from plaque rupture or plaque erosion. Plaque rupture with local and systemic inflammation is the most common cause of ACS, resulting from overactivation of the innate and adaptive immune responses. Together, these changes lead to degradation and rupture of the fibrous cap, exposure of the thrombogenic lipid core to circulating blood products, and thrombus formation [3,7,8]. In contrast, plaque erosion results from non-laminar coronary flow at an area of inflamed endothelium resulting in endothelial detachment, neutrophil recruitment, platelet activation, fibrin formation, and thrombosis [7,9].
In addition to intrinsic activation of thrombosis in ACS, Percutaneous Coronary Intervention (PCI) triggers several vascular responses. Balloon angioplasty and stent implantation results in the combination of vascular elastic recoil, negative (inward) remodeling, and neointimal hyperplasia, placing patients at risk for vessel restenosis and Target Vessel Revascularization (TVR) [10]. Drug-Eluting Stents (DES) coated with polymers and antiproliferative agents have significantly reduced In- Stent Restenosis (ISR) and translated into lower rates of TVR compared with bare-metal stents [11-13]. Endothelial injury after angioplasty also results in platelet activation and microthrombosis, and in extreme cases contributes to distal embolization and stent thrombosis [14,15]. Antithrombotic and antiplatelet therapies, therefore, are a mandatory component in the treatment of patients with ACS or those undergoing PCI.
Antiplatelet agents currently used in ACS
The benefits of irreversible platelet inhibition in the setting of ACS are certain, with early studies showing significant reductions in death and non-fatal MI compared with placebo [16,17]. This review will therefore focus on P2Y12 inhibitors. P2Y12 antagonists inhibit the binding of ADP molecules to platelet P2Y12 surface receptors resulting in platelet inhibition [18]. Contemporary P2Y12 inhibitors include the oral agents clopidogrel, prasugrel, and ticagrelor, and the Intravenous (IV) agent cangrelor (Table 1). Over 20 years have passed since the CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) and PCI-CURE trials revealed that non-fatal MI and recurrent ischemia are reduced with the addition of clopidogrel to aspirin in patients with Non- ST Elevation MI (NSTEMI) [19,20]. Since that time, randomized control trials including TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction) and PLATO (Study of Platelet Inhibition and Patient Outcomes) have demonstrated that Dual Antiplatelet Therapy (DAPT) with a high-potency P2Y12 inhibitor such as prasugrel or ticagrelor reduces risk of non-fatal MI, urgent TVR, and stent thrombosis in patients with NSTEMI and ST-Elevation MI (STEMI) compared with clopidogrel [21,22]. Furthermore, the Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 trial observed lower rates of death, MI, or stroke, with similar rates of bleeding with the use of prasugrel compared to ticagrelor [23]. As a result, the 2021 American College of Cardiology/American Heart Association/ Society for Cardiovascular Angiography and Interventions (ACC/ AHA/SCAI) Guideline for Coronary Artery Revascularization has given Class 1b recommendations to administer a loading dose of aspirin and P2Y12 inhibitor followed by maintenance dosing in patients undergoing PCI to reduce ischemic outcomes. Furthermore, because of TRITON-TIMI 38 and PLATO, there is now a 2a recommendation to administer ticagrelor or prasugrel in preference to clopidogrel for further reduction of ischemic events including stent thrombosis [24].
| Clopidogrel (Plavix®) | Prasugrel (Effient®) | Ticagrelor (Brilinta®) | Cangrelor (Kengreal®) | |
|---|---|---|---|---|
| Formulation | Oral | Oral | Oral | Intravenous |
| Bioavailability | 50% | 80% | 36% | 100% |
| Onset of action | 2-6 hours | 30 minutes | 30 minutes | 2 minutes |
| Binding reversibility | Irreversible | Irreversible | Reversible | Reversible |
| Duration of effect | 3-10 days | 7-10 days | 3-5 days | 1-2 hours |
| Discontinuation before noncardiac surgery | At least 5 days | At least 7 days | At least 3 days | 1 hour |
| Loading dose | 300 or 600 mg | 60 mg | 180 mg | 30 mcg/kg |
| Maintenance dose | 75 mg/d | 10 mg/d | 90 mg BID | 4 mcg/kg/min |
| Resistance (% in population) | Yes (5%-44%) | No | No | No |
| FDA approval year | 1997 | 2009 | 2011 | 2015 |
| Clinical indication | PCI, ACS, ACS-PCI, SCAD | ACS-PCI | ACS, ACS-PCI | PCI, bridging in high ischemic risk patients undergoing noncardiac invasive surgery/procedure |
Abbreviations: ACS: Acute Coronary Syndrome; BID: Bis In Die, twice a day; d: day; FDA: Food and Drug Administration; kg: kilogram; mg: milligrams; min: minute; PCI: Percutaneous Coronary Intervention; SCAD: Spontaneous Coronary Artery Dissection
Table 1: Characteristics and properties of antiplatelet therapies.
Cangrelor, a short-acting IV P2Y12 inhibitor with rapid onset of action, should be considered in patients who have not been pretreated with oral P2Y12 inhibitor agents or are unable to take or absorb oral medications [24]. Few trials have compared cangrelor with oral P2Y12 agents. The Cangrelor vs. Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION PHOENIX) trial randomized over 11,000 patients who were undergoing either urgent or elective PCI to receive either IV cangrelor or 300 mg or 600 mg of clopidogrel, and found a significant reduction in the composite of death, MI, ischemia-driven revascularization or stent thrombosis, without an increase in severe bleeding at 48 hours [25]. A meta-analysis of the CHAMPION trials demonstrated a lower rate of composite death, MI, ischemia-driven revascularization, or stent thrombosis at 48 hours with cangrelor compared with clopidogrel. Minor bleeding was observed more frequently with the use of cangrelor [26]. In a pooled, patient-level analysis of the three CHAMPION trials, cangrelor was associated with reduced ischemic events and a reduced need for bailout/rescue glycoprotein IIb/IIIa use when compared with clopidogrel. Additionally, cangrelor was not observed to increase bleeding events [27]. There are no randomized trials comparing cangrelor to potent P2Y12 inhibitors and this is a knowledge gap that should be addressed since oral antiplatelet therapy may be delayed until the time of cardiac catheterization in patients with NSTEMI undergoing an early invasive strategy.
Timing of antiplatelet therapy
There is debate on the optimal timing of P2Y12 administration in patients presenting with NSTEMI. Pretreatment before coronary angiography may reduce periprocedural ischemic events, albeit with an increased risk for major bleeding and delay in surgical revascularization [28]. The ACCOAST (Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention (PCI) or as Pretreatment at the Time of Diagnosis in Patients with Non- ST Elevation Myocardial Infarction) trial revealed that pretreatment with prasugrel prior to angiography in the setting of NSTEMI did not reduce the composite of cardiovascular death, MI, stroke, urgent revascularization, or glycoprotein IIb/ IIIa inhibitor rescue therapy but was associated with increased risk of TIMI major bleeding and life-threatening bleeding [29]. More recently, the DUBIUS (Downstream vs. Upstream administration of P2Y12 receptor Blockers in Non-ST Elevated Acute Coronary Syndromes with Initial Invasive Indication) trial compared upstream (pretreatment with ticagrelor) versus downstream (no pretreatment) administration in patients with NSTEMI undergoing invasive treatment. Downstream P2Y12 administration after coronary angiography was not superior to upstream administration, with low ischemic and bleeding events in both groups. The study was terminated early due to futility, as there was unlikely be a benefit of one over the other had the study continued. In a large Swedish retrospective analysis of almost 65,000 patients presenting with NSTEMI with plan for PCI, pre-treatment with an oral P2Y12 inhibitor did not reduce observed rates of death or stent thrombosis, but was associated with increased rates of in-hospital bleeding [30]. As a result, the 2020 European Society of Cardiology (ESC) Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation provided a Class 3A recommendation to not administer routine pre-treatment with a P2Y12 inhibitor in patients with NSTEMI when an early invasive strategy is planned [31].
Discussion
Duration of antiplatelet therapy
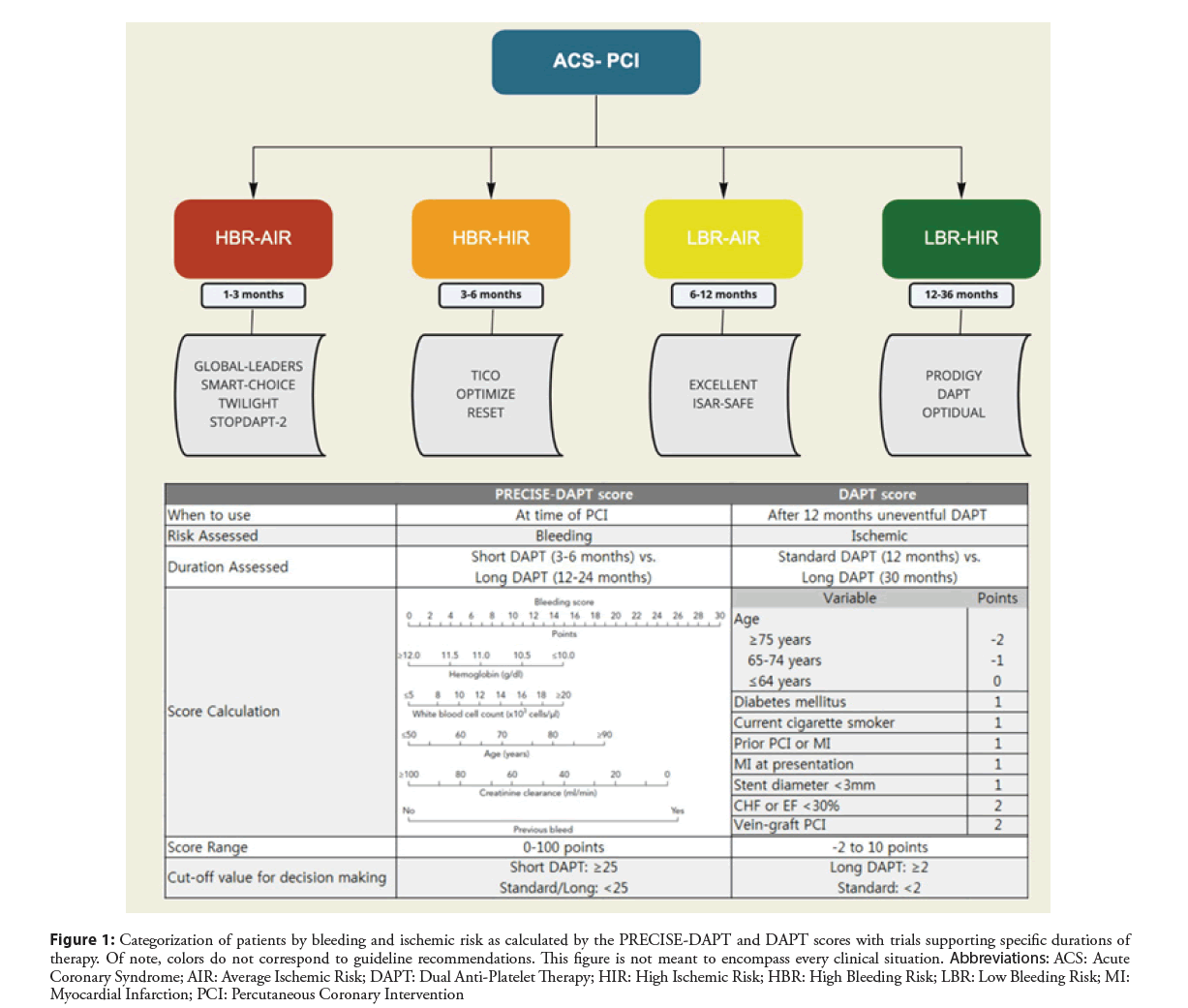
Determining the optimal duration of DAPT by weighing the risk of both ischemic and bleeding events is a critical step in post-PCI care (Figure 1) and risk calculators such as PRECISEDAPT, ARC-HBR, PARIS, and DAPT have been developed and validated to help predict these risks [32-35]. Randomized control trials evaluating various durations of DAPT therapy have been published (Table 2). As a result, the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease provided a Class I indication for 12 months of DAPT after an ACS treated with PCI, and a Class 2b recommendation suggesting discontinuation of the P2Y12 inhibitor after 6 months is reasonable in the setting of high bleeding risk or overt bleeding. Additionally, prolonged DAPT greater than 12 months in patients without a high bleeding risk received a Class 2b recommendation [36].
Figure 1: Categorization of patients by bleeding and ischemic risk as calculated by the PRECISE-DAPT and DAPT scores with trials supporting specific durations of therapy. Of note, colors do not correspond to guideline recommendations. This figure is not meant to encompass every clinical situation. Abbreviations: ACS: Acute Coronary Syndrome; AIR: Average Ischemic Risk; DAPT: Dual Anti-Platelet Therapy; HIR: High Ischemic Risk; HBR: High Bleeding Risk; LBR: Low Bleeding Risk; MI: Myocardial Infarction; PCI: Percutaneous Coronary Intervention
Trials have since focused on short-term (1-3 months) DAPT use as well as transitioning from DAPT to monotherapy with a P2Y12 inhibitor instead of aspirin use (Table 2). The STOPDAPT-2 (Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs. 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI) and GLOBAL LEADERS (Ticagrelor Plus Aspirin for 1 Month, Followed by Ticagrelor Monotherapy for 23 Months vs. Aspirin Plus Clopidogrel or Ticagrelor for 12 Months, Followed by Aspirin Monotherapy for 12 Months After Implantation of a Drug-eluting Stent: A Multicentre, Open-label, Randomised Superiority Trial) trials assessed DAPT for 1 month duration. In the STOPDAPT-2 trial, 1-month of DAPT followed by clopidogrel monotherapy resulted in a significantly lower rate of a composite of cardiovascular and bleeding events, showing both noninferiority and superiority to 12 months of DAPT [37]. In the GLOBAL LEADERS trial, in patients who underwent PCI with DES, 1 month of DAPT followed by ticagrelor monotherapy for 23 months was produced similar results to, but not superior to 12 months of DAPT followed by aspirin monotherapy. The composite of cardiovascular events and bleeding events was similar amongst the two groups [38].
| Trial (Year of Publication) | Patients analyzed (n) | Intervention vs. Control | Primary endpoint | Intervention vs. Control (%) | Risk (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Randomized trials evaluating duration of antiplatelet therapy: 1-3 months | ||||||
| STOPDAPT-2 (2019) [37] | 3,009 | Intervention: 1 month of DAPT (ASA/clopidogrel or prasugrel) ≥ clopidogrel monotherapy for 5 years. Control: 1 month of DAPT ≥ 12 months of DAPT | Composite of cardiovascular death, MI, definite stent thrombosis, ischemic or hemorrhagic stroke, or TIMI major or minor bleeding | 2.36% vs. 3.70% | HR 0.64 (0.42-0.98) | <0.001 |
| TWILIGHT (2019) [39] | 7,119 | Intervention: 3 months of DAPT (ASA/ticagrelor) ≥ ticagrelor monotherapy. Control: 12 additional months of DAPT | BARC type 2, 3, or 5 bleeding | 4.0% vs. 7.1% | HR 0.56 (0.45-0.68) | <0.001 |
| SMART-CHOICE (2019) [40] | 2,993 | Intervention: 3 months of DAPT (Aspirin/Clopidogrel or Ticagrelor or Prasugrel) followed by P2Y12 inhibitor alone. Control: 12 months of DAPT | Composite of all-cause death, MI, or stroke | 2.9% vs. 2.5% | ED 0.4 (- ∞ to 1.3) | 0.007 |
| GLOBAL-LEADERS (2018) [38] | 15,968 | Intervention: 1 month of DAPT (ASA/ticagrelor) ≥ 23 months of monotherapy with ticagrelor. Control: 12 months of DAPT (ASA/ clopidogrel or ticagrelor) | Composite of All-cause mortality or new Q-wave myocardial infarction | 3.81% vs. 4.37% | RR 0.87, (0.75-1.01) | 0.07 |
| Randomized trials evaluating duration of antiplatelet therapy: 3-6 months | ||||||
| TICO (2020) [41] | 2,978 | Intervention: Ticagrelor monotherapy following 3 months of DAPT (ASA, ticagrelor). Control: 12 months of DAPT (ASA, ticagrelor) | Composite of death, MI, stent thrombosis, stroke, target-vessel revascularization (MACCE) | 2.3% vs. 3.4% | HR 0.69 (0.45-1.06) | 0.09 |
| OPTIMIZE (2013) [50] | 3,119 | Intervention: 3 months of DAPT (ASA/clopidogrel). Control: 12 months of DAPT (ASA+clopidogrel) | Composite of all-cause death, MI, stroke, or major bleeding (MACCE) | 6.0% vs. 5.8% | RD 0.17 (-1.52–1.86) | 0.84 |
| RESET (2012) [51] | 2,117 | Intervention: 3 months of DAPT (ASA/clopidogrel). Control: 12 months of DAPT | Composite of cardiovascular death, MI, stent thrombosis, target/vessel revascularization, or bleeding | 4.7% vs. 4.7% | RD 0.0% (-2.5–2.5) | 0.84 |
| Randomized trials evaluating duration of antiplatelet therapy: 6-12 months | ||||||
| ISAR-SAFE (2015) [52] | 4,005 | Intervention: 6 months of DAPT (ASA/clopidogrel) followed by ASA monotherapy. Control: 12 months of DAPT | Composite of death, MI, definite/probable stent thrombosis, stroke or TIMI major bleeding | 1.50% vs. 1.60% | 0.001 | |
| EXCELLENT (2012) [53] | 1,443 | Intervention: 6 months of DAPT (ASA/clopidogrel). Control: 12 months of DAPT | Composite of cardiac death, MI, or ischemia-driven target vessel revascularization | 4.8% vs. 4.3% | HR 1.14 (0.70-1.86) | 0.6 |
| Randomized trials of prolonged DAPT therapy>12 months | ||||||
| OPTIDUAL (2015) [54] | 1,385 | Intervention: DAPT (aspirin/clopidogrel) for 48 months. Control: DAPT for 12 months followed by aspirin monotherapy | Composite of death, MI, stroke, and major bleeding | 5.8% vs. 7.5% | HR 0.75 (0.50–1.28) | 0.17 |
| DAPT (2014) [55] | 9,961 | Intervention: 12 additional 18 months of DAPT (aspirin/ clopidogrel or prasugrel). Control: DAPT for 12 months | Co-primary end points: Composite of definite/probable stent thrombosis | 0.4% vs. 0.4% | HR 0.29 (0.17-0.48) | <0.001 |
| Composite of death, MI, stroke | 4.3% vs. 0.9% | HR 0.71 (0.59–0.85) | <0.001 | |||
| PRODIGY (2012) [56] | 1970 | Intervention: 24 months of DAPT (ASA/clopidogrel). Control: 6 months of DAPT | Composite of death, MI, or stroke | 10.1% vs. 10.0% | HR 0.98 (0.74–1.29) | 0.91 |
Abbreviations: ASA: Aspirin; BARC: Bleeding Academic Research Consortium CI: Confidence Interval; DAPT: Dual Anti-Platelet Therapy; ED: Estimate of Difference; HR: Hazard Ratio; MACCE: Major Adverse Cardiovascular and Cerebrovascular Events; MI: Myocardial Infarction; RD: Risk Difference; RR: Risk Ratio; TIMI: Thrombolysis in Myocardial Infarction
Table 2: Major trials of antiplatelet agents in acute coronary syndrome, categorized by duration of antiplatelet therapy.
Three additional studies have evaluated the benefits of DAPT for a shortened duration of 3 months. The TWILIGHT trial randomized a population of high-risk patients who had undergone PCI and completed 3 months of DAPT, to ticagrelor monotherapy or continued DAPT. A lower incidence of bleeding without a higher risk of Major Adverse Cardiac and Cerebrovascular Events (MACCE) was noted with ticagrelor monotherapy) [39]. Similarly, the SMART-CHOICE trial assessed the safety and efficacy of 3 months of DAPT followed by P2Y12 monotherapy versus a standard 12-month regimen of DAPT, and concluded that shortduration therapy was produced similar results to longer-duration DAPT with regard to MACCE. Bleeding was also lower in the short-duration arm [40] Lastly, the TICO (Effect of Ticagrelor Monotherapy vs. Ticagrelor with Aspirin on Major Bleeding and Cardiovascular Events in Patients with Acute Coronary Syndrome) trial revealed that ticagrelor monotherapy after 3 months of DAPT was superior to DAPT for 12 months with regards to a primary composite outcome of death, MI, stent thrombosis, stroke, TVR or Thrombolysis in Myocardial Infarction (TIMI) major bleeding in patients with ACS who underwent PCI with an ultrathin biodegradable DES [41]. The composite of data supports that shorter duration of DAPT followed by P2Y12 monotherapy results in lower rates of bleeding events (40% reduction) with equivalent ischemic events.
Accordingly, the 2021 ACC/AHA/SCAI guidelines have been updated to reflect the above trials. While it remains a Class 1 indication to continue DAPT for 12 months following PCI, in selected patients undergoing PCI, discontinuation of aspirin after 1-3 months with continued P2Y12 monotherapy is now a 2a recommendation. In patients with high risk of bleeding or overt bleeding on DAPT, discontinuation of P2Y12 after 6 months may be reasonable (Class 2a recommendation) [24].
Switching P2Y12 inhibitor therapiesPatients commonly require switching between low and high potency P2Y12 inhibitors based on clinical and procedural characteristics. In 2017, the International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies was published to provide guidance on the various modalities of switching between P2Y12 inhibitor [42]. When switching, both the time between index PCI as well as escalation (low potency to high potency) or de-escalation (high potency to low potency) must be considered. When escalating from clopidogrel to either ticagrelor or prasugrel in the acute (<24 hours from index PCI) or early (1 day–30 days) phases, a loading dose of 180 mg or 60 mg respectively can be administered irrespective to the timing and dosing of clopidogrel. When de-escalating ticagrelor or prasugrel to clopidogrel in the acute or early phase, a 600 mg loading dose of clopidogrel should be administered 24 hours after the last dose of a high potency P2Y12 inhibitor. On the other hand, in the late (30 days–1 year) and very late (>1 year) phases, a 24 hours delay is warranted before switching between any two P2Y12 inhibitors. While a loading dose of clopidogrel is not required when deescalating from prasugrel to clopidogrel, a 600 mg load 24 hours after the last dose of ticagrelor is recommended. When escalating from clopidogrel to either prasugrel or ticagrelor, maintenance dosing can be initiated 24 hours after the last dose of clopidogrel [42].
When transitioning from cangrelor, a loading dose of the oral P2Y12 inhibitor is required. Clopidogrel and prasugrel loads should be administered immediately after discontinuation of cangrelor, while ticagrelor can be given at any point during the cangrelor infusion [42].
Triple therapy
For patients undergoing PCI who have or develop the need for oral anticoagulant therapy, including for atrial fibrillation, venous thromboembolism, and prosthetic heart valves, there is an important need to reduce ischemic events while reducing bleeding risks. The WOEST (Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial) trial evaluated treatment with clopidogrel alone vs. DAPT (aspirin and clopidogrel) among patients on oral anticoagulation who underwent PCI. The study found that among patients on oral anticoagulation undergoing PCI, the use of clopidogrel alone reduced the frequency of bleeding events without an increase in adverse ischemic events when compared to DAPT [43]. Secondly, the PIONEER AF-PCI trial evaluated three strategies of oral anticoagulation and antiplatelet agents in patients with atrial fibrillation undergoing PCI. Patients were randomized to three groups; group 1 with high-dose rivaroxaban 15 mg plus P2Y12 monotherapy for 12 months, group 2 with low-dose rivaroxaban 2.5 mg plus DAPT for 1, 6 or 12 months and group 3 warfarin plus DAPT for 1, 6 or 12 months. The study showed that a rivaroxaban-based strategy was associated with a lower rate of clinically significant bleeding in comparison to the warfarin/DAPT strategy. MACCE outcomes were similar within the three groups [44]. Lastly, the RE-DUAL PCI (Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation) evaluated dual therapy with dabigatran (at either a dose of 110 mg or 150 mg) and either clopidogrel or ticagrelor vs. triple therapy with aspirin, P2Y12 (clopidogrel or ticagrelor) and warfarin. Dual therapy was associated with reduced bleeding events and similar MACCE as the triple therapy group [45]. Given these findings, the 2019 focused update on atrial fibrillation guidelines gave a Class 2a recommendation for the use of P2Y12 inhibitor with a non-vitamin K oral anticoagulant (rivaroxaban or dabigatran) or a Vitamin-K Antagonist (VKA) over triple therapy with AC and DAPT [46].
Since the 2019 update, two additional trials have examined the benefits of dual therapy. The AUGUSTUS trial randomly assigned patients with atrial fibrillation who had undergone PCI and who were planning to take a P2Y12 inhibitor to receive either apixaban or VKA in addition aspirin or placebo for 6 months. The use of apixaban resulted in lower bleed compared with VKA when used in combination with P2Y12 inhibitor. Furthermore, the addition of aspirin led to an increase in bleeding risk without an increase in efficacy [47]. The ENTRUST-AF PCI trial compared the use of edoxaban with P2Y12 inhibitor to triple therapy with DAPT and VKA [48].
The 2020 ACC Expert Consensus Decision Pathway for Anticoagulant and Antiplatelet Therapy in Patients with Atrial Fibrillation or Venous Thromboembolism Undergoing Percutaneous Coronary Intervention with Atherosclerotic Cardiovascular Disease were thus updated, and provide a Class 1 indication for the discontinuation of aspirin 1 to 4 weeks after index PCI, while maintaining dual therapy with a P2Y12 inhibitor and oral anticoagulation to reduce the risk of bleeding. A Class 2a recommendation was also provided for the use of direct oral anticoagulants over VKA when either DAPT or P2Y12 inhibitor are required [49-56].
Conclusion
The study demonstrated that a combination of edoxaban and clopidogrel was produced similar results to VKA with DAPT for major or clinically relevant nonmajor bleeding, with similar ischemic outcomes. Dual antiplatelet therapy is the cornerstone of therapy in non-STE ACS, leading to decrease in ischemic events. The timing, duration, and combination of therapy should be based on the specific clinical setting to ensure the lowest incidence of ischemic and major bleeding events.
References
- Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: A report from the American heart association. Circulation. 143(8): e254-e743 (2021).
[CrossRef] [Google Scholar]) [PubMed]
- Van de Werf F. The history of coronary reperfusion. Eur Heart J. 35(37): 2510-2515 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Shah PK. Pathophysiology of plaque rupture and the concept of plaque stabilization. Cardiol Clin. 14(1): 17-29 (1996).
[CrossRef] [Google Scholar] [PubMed]
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 92(3): 657-671 (1995).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Shaw LJ, Blankstein R, Bax JJ, et al. Society of cardiovascular computed tomography/north American society of cardiovascular imaging-expert consensus document on coronary CT imaging of atherosclerotic plaque. J Cardiovasc Comput. 15(2): 93-109 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Dawson LP, Lum M, Nerleker N, et al. Coronary atherosclerotic plaque regression: JACC state-of-the-art review. J Am Coll Cardiol. 79(1): 66-82 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Crea F, Libby P. Acute coronary syndromes: The way forward from mechanisms to precision treatment. Circulation. 136(12): 1155-1166 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. New Engl J Medicine. 331(7): 417-424 (1994).
[CrossRef] [Google Scholar] [PubMed]
- Kolte D, Libby P, Jang I-K. New insights into plaque erosion as a mechanism of acute coronary syndromes. JAMA. 325(11): 1043-1044 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Chaabane C, Otsuka F, Virmani R, et al. Biological responses in stented arteries. Cardiovasc Res. 99(2): 353-363 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. New Engl J Medicine. 349(14): 1315-1323 (2003).
[CrossRef] [Google Scholar] [PubMed]
- Camenzind E, Wijns W, Mauri L, et al. Stent thrombosis and major clinical events at 3 years after zotarolimus-eluting or sirolimus-eluting coronary stent implantation: A randomised, multicentre, open-label, controlled trial. Lancet. 380(9851): 1396-1405 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: A collaborative network meta-analysis. Lancet. 370(9591): 937-948 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Dangas GD, Caixeta A, Mehran R, et al. Frequency and predictors of stent thrombosis after percutaneous coronary intervention in acute myocardial infarction. Circulation. 123(16): 1745-1756 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Rodgers GP, Minor ST, Robinson K, et al. The coronary artery response to implantation of a balloon-expandable flexible stent in the aspirin and non-aspirin-treated swine model. Am Heart J. 122(3): 640-647 (1991).
[CrossRef] [Google Scholar] [PubMed]
- Lewis HD, Davis JW, Archibald DG, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina-results of a veterans administration cooperative study. New Engl J Medicine. 309(7): 396-403 (1983).
[CrossRef] [Google Scholar] [PubMed]
- Group 1-2 (second ISOISC. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2 (Second International Study of Infarct Survival). Lancet. 332(8607): 349-360 (1988).
- Wijeyeratne YD, Heptinstall S. Anti-platelet therapy: ADP receptor antagonists: Anti-platelet therapy: ADP receptor antagonists. Brit J Clin Pharmaco. 72(4): 647-657 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. New Engl J Medicine. 345(7): 494-502 (2001).
[CrossRef] [Google Scholar] [PubMed]
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet. 358(9281): 527-533 (2001).
[CrossRef] [Google Scholar] [PubMed]
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel vs. clopidogrel in patients with acute coronary syndromes. New Engl J Medicine. 357(20): 2001-2015 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New Engl J Medicine. 361(11): 1045-1057 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Schüpke S, Neumann F-J, Menichelli M, et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. New Engl J Med. 381(16): 1524-1534 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Writing Committee Members, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. 79(2): e21-e129 (2021).
- Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. New Engl J Medicine. 368(14): 1303-1313 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Steg PG, Bhatt DL, Hamm CW, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: a pooled analysis of patient-level data. Lancet Lond Engl. 382(9909): 1981-92 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Vaduganathan M, Harrington RA, Stone GW, et al. Cangrelor with and without glycoprotein IIb/IIIa inhibitors in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 69(2): 176-185 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Capodanno D, Angiolillo DJ. Pre-Treatment with oral P2Y12 inhibitors in acute coronary syndromes without ST-segment elevation the saga continues. J Am Coll Cardiol. 73(8): 915-918 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Montalescot G, Bolognese L, Dudek D, et al. Pretreatment with prasugrel in non–ST-segment elevation acute coronary syndromes. New Engl J Medicine. 369(11): 999-1010 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Dworeck C, Redfors B, Angerås O, et al. Association of pretreatment with P2Y12 receptor antagonists preceding percutaneous coronary intervention in non–ST-segment elevation acute coronary syndromes with outcomes. JAMA Netw Open. 3(10): e2018735 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Collet J-P, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 42(14): 1289-1367 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet. 389(10073): 1025-1034 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the academic research consortium for high bleeding risk. Circulation. 140(3): 240-261 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Baber U, Mehran R, Giustino G, et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents risk scores from PARIS. J Am Coll Cardiol. 67(19): 2224-2234 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Yeh RW, Secemsky EA, Kereiakes DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 315(16): 1735-49 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease a report of the american college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 68(10): 1082-1115 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Watanabe H, Domei T, Morimoto T, et al. The STOPDAPT-2 randomized clinical trial. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs. 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI. JAMA. 321(24): 2414-2427 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Vranckx P, Valgimigli M, Jüni P, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: A multicentre, open-label, randomised superiority trial. Lancet. 392(10151): 940-949 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. New Engl J Med. 381(21): 2032-2042 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Hahn J-Y, Song YB, Oh J-H, et al. The SMART-CHOICE randomized clinical trial. Effect of P2Y12 Inhibitor monotherapy vs. dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention. JAMA. 321(24): 2428-2437 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Kim B-K, Hong S-J, Cho Y-H, et al. The TICO randomized clinical trial. Effect of ticagrelor monotherapy vs. ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome. JAMA. 323(23): 2407-2416 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Angiolillo DJ, Rollini F, Storey RF, et al. International expert consensus on switching platelet P2Y12 receptor–inhibiting therapies. Circulation. 136(20): 1955-1975 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet. 381(9872): 1107-1115 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. New Engl J Medicine. 375(25): 2423-2434 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. New Engl J Medicine. 377(16): 1513-1524 (2017).
[CrossRef] [Google Scholar] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 74(1): 104-132 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. New Engl J Med. 380(16): 1509-1524 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): A randomised, open-label, phase 3b trial. Lancet. 394(10206): 1335-1343 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Kumbhani DJ, Cannon CP, et al. 2020 ACC expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 77(5): 629-658 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Feres F, Costa RA, Abizaid A, et al. Three vs. twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: The OPTIMIZE randomized trial. JAMA. 310(23): 2510-2522 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Kim B-K, Hong M-K, Shin D-H, et al. A New strategy for discontinuation of dual antiplatelet therapy the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol. 60(15): 1340-1348 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Schulz-Schupke S, Byrne RA, Berg JM ten, et al. ISAR-SAFE: A randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J. 36(20): 1252-1263 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Gwon H-C, Hahn J-Y, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 125(3): 505-513 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Helft G, Steg PG, Feuvre CL, et al. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: The OPTIDUAL randomized trial. Eur Heart J. 37(4): 365-374 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. New Engl J Medicine. 371(23): 2155-2166 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Valgimigli M, Borghesi M, Tebaldi M, et al. Should duration of dual antiplatelet therapy depend on the type and/or potency of implanted stent? A pre-specified analysis from the PROlonging Dual antiplatelet treatment after grading stent-induced Intimal hyperplasia study (PRODIGY). Eur Heart J. 34(12): 909-919 (2013).
[CrossRef] [Google Scholar] [PubMed]