Mini Review - Interventional Cardiology (2022) Volume 14, Issue 2
Cholesterol crystal embolism as complication of percutaneous coronary intervention
- Corresponding Author:
- Kotaro Takahashi
Department of Cardiovascular Medicine,
Kurashiki Central Hospital,
1-1-1 Miwa, Kurashiki 710-8602,
Japan,
E-mail: kotaka331@gmail.com
Received date: 25-Feb-2022, Manuscript No. FMIC-22-55475; Editor assigned: 28-Feb-2022, PreQC No. FMIC-22-55475 (PQ); Reviewed date: 14-Mar-2022, QC No. FMIC-22-55475; Revised date: 18-Mar-2022, Manuscript No. FMIC-22-55475 (R); Published date: 25-Mar-2022, DOI: 10.37532/1755-5310.2022.14(2).468
Abstract
Percutaneous Coronary Intervention (PCI) has become common treatment of coronary artery disease especially for acute coronary syndrome nowadays, while several complications cannot be completely prevented. Cholesterol Crystal Embolism (CCE) is one of the rare but serious complication of PCI, still occur even now that the PCI devices have been dramatically improved. CCE is a systemic disease caused by small atherosclerotic particles, often described as cholesterol crystals shower embolizing small vessels of each organs, leading to ischemic and inflammatory damage to single or multiple organs. This syndrome could occur spontaneously, while endovascular procedures, for example coronary angiography and PCI are the most affected iatrogenic causes. CCE as complication of PCI is recognized in cardiac interventionalists, while the data describing risk factors of CCE after PCI are scarce. This review will mainly discuss about the risk factors of CCE to consider how to reduce the risk of causing CCE, and whom to be aware of causing CCE in PCI performed patients.
Keywords
Cholesterol crystal embolism • Percutaneous coronary intervention • Complications • Inner sheath
Introduction
Cholesterol Crystal Embolism (CCE) is a syndrome occurred by embolization of cholesterol crystals thought to be released from atherosclerotic plaques of the aorta. This often causes end-organ damage and leads to several clinical presentation depending on the embolized organs. The syndrome is first described in 1862 by the Danish pathophysiologist Peter Ludvig Panum as autopsy findings [1], and blue toe syndrome was then described in 1976 as the specific manifestation of this syndrome [2]. Since then, several literatures have described the clinical findings of this syndrome, but cause of the disease are not always recognized. It can occur spontaneously or sometimes as a complication of vascular or endovascular maneuvers [3-5]. There has been significant variablity among studies discussing the incidence of CCE. This is since the patients eligible for the studies varies, while studies in autopsy series states frequency of CCE as 0.31%-2.4% [4,6], they were 0.6%-1.4% in patients undergoing cardiac catheterization [7-9]. Other reasons could be due to the difficulty of diagnosing the syndrome. As is often the case, this syndrome has variety of clinical presentation upon the different vessel involved in shower embolism, and with nonspecific acute inflammatory response as the initial symptoms, such as fever, fatigue, weight loss, and anorexia [10-12].
CCE after PCI becomes evident when contents of atherosclerotic plaque are showered from the aorta or other major arteries and micro embolism causes damage to embolized end organs. Clinical presentation varies by the organ which has been embolized. The onset of symptoms from the procedure and severality could also vary by each case, maybe by the amount of cholesterol crystals released from atherosclerotic plaques, and the vascular bed of the embolized organs. This makes the diagnosis of this syndrome difficult, but there are some diagnostic points. Skin and kidney are the most affected organs, and specific clinical manifestation and some nonspecific laboratory findings are expressed after some periods from procedures [1,13-16]. The definite diagnosis of the syndrome is made by histological features such as cholesterol clefts revealed by biopsy of affected organs, often is a skin or kidney [13,15,17,18]. Probable diagnosis can be made by combinations of specific signs in skin and laboratory findings. Livedo reticularis and blue toe syndrome are frequent cutaneous signs in early phase, which triggers suspicion of the syndrome [19,20]. Hypocomplementemia, renal impairment and eosinophilia after endovascular procedures are some of the laboratory findings of the syndrome. Renal impairment can also be caused by contrast, but it often occurs within 48-72 hours after procedures, while the one with CCE often occur after 1 to 2 weeks after the procedure [13,21]. Other difference between Contrast-Induced Nephropathy (CIN) and cholesterol crystal embolism is that there are other systemic peripheral manifestations with CCE while not with CIN, and urine is often bland with proteinuria with CCE, and these could help diagnosis. Other symptoms by other organ embolization, such as abdominal frank pain, diarrhea, gastrointestinal bleeding as result of involving gastrointestinal tract, hollenhorst plaque seen in retina can also be present after PCI with CCE.
Literature Review
Risk factors of CCE
There are not many literatures describing the risk factors of CCE associated with PCI. Risk factors of CCE could be distinguished to factors associated with aortic atherosclerosis, and factors for embolization. Generally considered risk factors of CCE in patient base are those with similar to that of risk factors for atherosclerosis [22-24]. The patients often are older ages, smokers, are obese, and have a medical history of hypertension, dyslipidemia, diabetes mellitus, cerebrovascular disease, chronic kidney disease. When these risk factors are present and process of atherosclerosis progresses, aortic wall especially the intima-media layer accelerate to store lipids. One study comparing high blood pressure and atherosclerosis of the thoracic arota showed that age (for age <75 per 10-y increase, OR 3.44, 2.52-4.70), smoking history (OR: 2.00, 1.32-3.03), and pulse pressure (per 10 mm Hg increase, OR: 1.31, 1.07-1.60) independently associated with aortic atherosclerosis assessed by transesophageal echocardiography [25]. Furthermore, transesophageal echocardiography evaluated protruding plaques, especially ≥ 4 mm in thickness, ulceration, and or mobile thrombi are recognized as risk of CCE in several studies [26-29]. Chronic inflammation is also considered to associate with occurrence of CCE, from the fact that Fukumoto, et al. showed the relation with plasma C-reactive protein >1.0 mg/dl and CCE occurrence in patients after cardiac catheterization [19]. Use of anticoagulation has also been considered as a risk factor of CCE by causing plaque hemorrhage, while recent randomized study assessing patients with aortic plaque proven by transesophageal echocardiography showed anticoagulation had no relations with incidence of CCE, making the conclusion uncertain[19,30,31].
The other important risk factors of CCE are factors associated with embolization of the aortic plaques. This process could occur spontaneously, but often iatrogenic associated with vascular related procedures, such as catheter manipulations and surgeries [9,16,32]. With the recent rise in the number of cardiac catheter procedures and the widespread awareness of CCE, up to three times more iatrogenic causes than spontaneous causes have been reported [32]. Aortic aneurysms are known to be a source of CCE. Fukumoto, et al. reported that there were no significant differences in the prevalence of femoral approach usage rate between CCE and non-CCE patients, and concluded that the ascending thoracic aorta may be the main embolic source, not abdominal aorta, but it is controversial [19]. Actually, Carroccio, et al. reported in their propective study of following 660 patients with abdominal aortic aneurysm after aortic stent grafts implantation that 2.9% of patients were diagnosed as having CCE with the mean follow up of 15 months [33]. From the previous reports, it is obvious to consider that both abdominal and thoracic aorta atherosclerosis and/or aneurysm with intravascular procedures are a risk factor of causing CCE.
Association of PCI procedures and CCE
We all recognize that catheter procedures especially PCI that needs large size of catheter, and require long, dynamic manipulation is associated with occurrence of CCE, but the true incidence and risk factors of CCE after PCI is not well known. Tanaka, et al. collected national data from the Japanese Diagnosis Procedure Combination database, and showed the incidence of CCE after Coronary Angiography (CAG) and PCI to be 0.02% and 0.05 with odds ratio of 3.2 with PCI in reference of CAG incidence [34]. They also showed the use of mechanical circulatory support, especially intra-aortic balloon pumping that consist with large size catheter was also associated with increase in risk of CCE. (Odds ratio of 9.80, 7.43-12.9 CAG with reference) [34]. PCI may have higher incidence of causing CCE as complication compared with CAG, since its larger size of catheter used and use of mechanical circulatory support in the selected procedures.
Other important factor is the route of coronary intervention. Since abdominal aorta is often involved with atherosclerotic plaques, femoral approach may be the risk factor of CCE compared with other approach. Kooiman, et al. has showed significantly lower incidence of acute kidney injury after PCI procedures with transradial intervention compared to trans femoral intervention, which some of the renal impairment are considered to be due to CCE [35]. Ando, et al. has also shown the similar results that transradial approach had 3-fold less prevalent of acute kidney injury including CCE than transfemoral approach for patients performed PCI for acute coronary syndrome [36]. Although, there are some studies showing no significant difference in prevalence of CCE with femoral and non-femoral approach, and concluded that ascending aorta may be the main embolic source of CCE, so that femoral approach may be a risk but controversial. There were no study showing relations of equipment used in PCI and CCE.
Discussion
We aim to assess the risk factors of CCE in PCI performed patients in order to improve our understanding, so that we know when and how to prevent this complication. In our recently published paper, 23,184 patients undergoing PCI in our institution were assessed and evaluated the incidence, risk factors, and prognosis of CCE occurred patients [37]. There were 88 patients (0.38%) whom diagnosed as CCE in 20 years, and the incidence of CCE seemed to decline through the investigated 20 years. We concluded that use of inner-sheath and radial approach which increased in later years may have associated with decrease in prevalence of CCE. As described previously, femoral approach could be one of the risk factors of CCE after PCI, and our study also showed femoral approach as a positive predictor of CCE. (70.5% vs. 44.8%, p<0.001) [37]. The report from Fukumoto, et al. stated femoral approach as not an independent risk factor for CCE, although the complications were seen in 1.59% of 1258 patients treated with a femoral approach and 0.95% of 528 patients treated with other approaches, suggesting that a femoral approach is associated with a not significantly but numerically higher incidence of CCE than other approaches and may had a significant difference with larger number of patients [19].
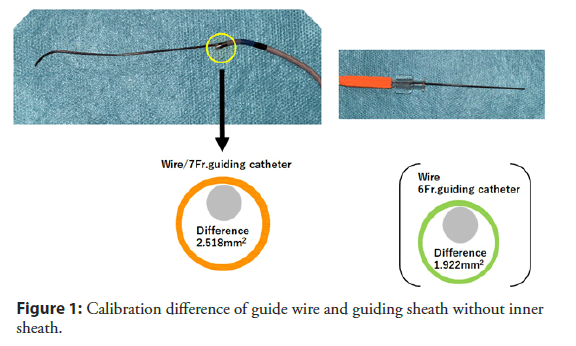
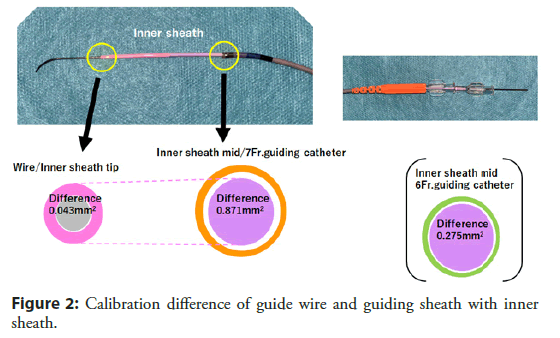
One important finding from our study was that the use of inner sheath (Outlook 5 Fr Straight; Terumo, Tokyo, Japan) was a negative predictor of CCE in our study population. (62.5% vs. 77.3%, p<0.001) [37]. The inner sheath has inner and outer diameters of 0.92 and 1.68 mm with 115 cm in length and used in combination with a conventional guiding catheter to reduce the caliber difference between the guiding catheter and the guide wire (Figures 1 and 2). Differences in areas with guide wires were 1.92 mm2 for 6 Fr guiding catheters and 2.52 mm2 for 7 Fr guiding catheters, while the difference in the use of an inner sheath was as small as 0.04 mm2 for both sizes of guiding catheters, and the decrease in caliber difference may have contributed in prevention of CCE by avoiding catheter to shave off aortic wall while delivering into coronary artery. This idea is also supported by previous report from Keeley, et al. that they prospectively analyzed 1,000 PCI and showed 51% of overall cases had some degree of aortic debris scraped by the guiding catheter [38]. We believe this is an important fact that not only using radial for approach, we may have chance of preventing this disastrous complications by using one nice catheter as combination of guiding catheter.
Conclusion
Since the study was retrospective, and single center analysis, further discussion should be made for confirmation. CCE is a rare but harmful complication of PCI, still we experience nowadays. Patient characteristics for example older ages, smoking habit, history of dyslipidemia are important for us to be aware of causing the syndrome, while we may have chance of preventing them by using radial approach and inner sheath in combination of guiding catheter especially for high risk patients listed above.
Conflicts of Interest
The authors have no conflicts of interest directly relevant to the content of this article.
References
- Scolari F, Ravani P. Atheroembolic renal disease. Lancet. 375(9726): 1650-60 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Karmody AM, Powers SR, Monaco VJ, et al. "Blue toe" syndrome: An indication for limb salvage surgery. Arch Surg. 111(11): 1263-8 (1976).
[CrossRef] [Google Scholar] [PubMed]
- Kealy WF. Atheroembolism. J Clin Pathol. 31(10): 984-9 (1978).
[CrossRef] [Google Scholar] [PubMed]
- Cross SS. How common is cholesterol embolism? J Clin Pathol. 44(10): 859-61 (1991).
[CrossRef] [Google Scholar] [PubMed]
- Karalis DG, Quinn V, Victor MF, et al. Risk of catheter-related emboli in patients with atherosclerotic debris in the thoracic aorta. Am Heart J. 131(6): 1149-55 (1996).
[CrossRef] [Google Scholar] [PubMed]
- Drost H, Buis B, Haan D, et al. Cholesterol embolism as a complication of left heart catheterisation. Report of seven cases. Br Heart J. 52(3): 339-42 (1984).
[CrossRef] [Google Scholar] [PubMed]
- Lin PH, Bush RL, Conklin BS, et al. Late complication of aortoiliac stent placement-atheroembolization of the lower extremities. J Surg Res. 103(2): 153-9 (2002).
[CrossRef] [Google Scholar] [PubMed]
- Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 122(6): 631-41 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Saklayen MG, Gupta S, Suryaprasad A, et al. Incidence of atheroembolic renal failure after coronary angiography. A prospective study. Angiology. 48(7): 609-13 (1997).
[CrossRef] [Google Scholar] [PubMed]
- Fine MJ, Kapoor W, Falanga V. Cholesterol crystal embolization: A review of 221 cases in the English literature. Angiology. 38(10): 769-84 (1987).
[CrossRef] [Google Scholar] [PubMed]
- Falanga V, Fine MJ, Kapoor WN. The cutaneous manifestations of cholesterol crystal embolization. Arch Dermatol. 122(10): 1194-8 (1986).
[Google Scholar] [PubMed]
- Donohue KG, Saap L, Falanga V. Cholesterol crystal embolization: An atherosclerotic disease with frequent and varied cutaneous manifestations. J Eur Acad Dermatol Venereol. 17(5): 504-11 (2003).
[CrossRef] [Google Scholar] [PubMed]
- Thadhani RI, Camargo CA Jr., Xavier RJ, et al. Atheroembolic renal failure after invasive procedures. Natural history based on 52 histologically proven cases. Medicine (Baltimore). 74(6): 350-8 (1995).
[CrossRef] [Google Scholar] [PubMed]
- Mayo RR, Swartz RD. Redefining the incidence of clinically detectable atheroembolism. Am J Med. 100(5): 524-9 (1996).
[CrossRef] [Google Scholar] [PubMed]
- Belenfant X, Meyrier A, Jacquot C. Supportive treatment improves survival in multivisceral cholesterol crystal embolism. Am J Kidney Dis. 33: 840-50 (1999).
[CrossRef] [Google Scholar] [PubMed]
- Scolari F, Ravani P, Pola A, et al. Predictors of renal and patient outcomes in atheroembolic renal disease: A prospective study. J Am Soc Nephrol. 14(6): 1584-90 (2003).
[CrossRef] [Google Scholar] [PubMed]
- Haqqie SS, Urizar RE, Singh J. Nephrotic-range proteinuria in renal atheroembolic disease: Report of four cases. Am J Kidney Dis. 28(4): 493-501 (1996).
[CrossRef] [Google Scholar] [PubMed]
- Scolari F, Tardanico R, Zani R, et al. Cholesterol crystal embolism: A recognizable cause of renal disease. Am J Kidney Dis. 36(6): 1089-109 (2000).
[CrossRef] [Google Scholar] [PubMed]
- Fukumoto Y, Tsutsui H, Tsuchihashi M, et al. The incidence and risk factors of cholesterol embolization syndrome: A complication of cardiac catheterization: A prospective study. J Am Coll Cardiol. 42(2): 211-6 (2003).
[CrossRef] [Google Scholar] [PubMed]
- Saric M, Kronzon I. Cholesterol embolization syndrome. Curr Opin Cardiol. 26(6): 472-9 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Rudnick MR, Berns JS, Cohen RM, et al. Nephrotoxic risks of renal angiography: Contrast media-associated nephrotoxicity and atheroembolism: A critical review. Am J Kidney Dis. 24(4): 713-27 (1994).
[CrossRef] [Google Scholar] [PubMed]
- Quinones A, Saric M. The cholesterol emboli syndrome in atherosclerosis. Curr Atheroscler Rep. 15(4): 315 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Tunick PA, Rosenzweig BP, Katz ES, et al. High risk for vascular events in patients with protruding aortic atheromas: A prospective study. J Am Coll Cardiol. 23(5): 1085-90 (1994).
[CrossRef] [Google Scholar] [PubMed]
- Ozkok A. Cholesterol-embolization syndrome: Current perspectives. Vasc Health Risk Manag. 15: 209-20 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Agmon Y, Khandheria BK, Meissner I, et al. Independent association of high blood pressure and aortic atherosclerosis: A population-based study. Circulation. 102(17): 2087-93 (2000).
[CrossRef] [Google Scholar] [PubMed]
- Karalis DG, Chandrasekaran K, Victor MF, et al. Recognition and embolic potential of intraaortic atherosclerotic debris. J Am Coll Cardiol. 17: 73-8 (1991).
[CrossRef] [Google Scholar] [PubMed]
- Amarenco P, Cohen A, Tzourio C, et al. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 331(22): 1474-9 (1994).
[CrossRef] [Google Scholar] [PubMed]
- Coy KM, Maurer G, Goodman D, et al. Transesophageal echocardiographic detection of aortic atheromatosis may provide clues to occult renal dysfunction in the elderly. Am Heart J. 123(6): 1684-6 (1992).
[CrossRef] [Google Scholar] [PubMed]
- Nickol J, Richards T, Mullins J. Cholesterol embolization syndrome from penetrating aortic ulcer. Cureus. 12(6): e8670 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Hyman BT, Landas SK, Ashman RF, et al. Warfarin-related purple toes syndrome and cholesterol microembolization. Am J Med. 82(6): 1233-7 (1987).
[CrossRef] [Google Scholar] [PubMed]
- Blackshear JL, Zabalgoitia M, Pennock G, et al. Warfarin safety and efficacy in patients with thoracic aortic plaque and atrial fibrillation. SPAF TEE Investigators. Stroke Prevention and Atrial Fibrillation. Transesophageal echocardiography. Am J Cardiol. 83: 453-5, A9 (1999).
[CrossRef] [Google Scholar] [PubMed]
- Scolari F, Ravani P, Gaggi R, et al. The challenge of diagnosing atheroembolic renal disease: Clinical features and prognostic factors. Circulation. 116: 298-304 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Carroccio A, Olin JW, Ellozy SH, et al. The role of aortic stent grafting in the treatment of atheromatous embolization syndrome: Results after a mean of 15 months follow-up. J Vasc Surg. 40(3): 424-9 (2004).
[CrossRef] [Google Scholar] [PubMed]
- Tanaka H, Yamana H, Matsui H, et al. Proportion and risk factors of cholesterol crystal embolization after cardiovascular procedures: A retrospective national database study. Heart Vessels. 35(9): 1250-5 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Kooiman J, Seth M, Dixon S, et al. Risk of acute kidney injury after percutaneous coronary interventions using radial versus femoral vascular access: Insights from the blue cross blue shield of Michigan cardiovascular consortium. Circ Cardiovasc Interv. 7(3): 190-8 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Ando G, Cortese B, Russo F, et al. Acute kidney injury after radial or femoral access for invasive acute coronary syndrome management: AKI-MATRIX. J Am Coll Cardiol. S0735-1097(17): 36897-3 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Takahashi K, Omuro A, Ohya M, et al. Incidence, Risk factors, and prognosis of cholesterol crystal embolism because of percutaneous coronary intervention. Am J Cardiol. 167: 15-19 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Keeley EC, Grines CL. Scraping of aortic debris by coronary guiding catheters: A prospective evaluation of 1,000 cases. J Am Coll Cardiol. 32(7): 1861-5 (1998).
[CrossRef] [Google Scholar] [PubMed]