Review Article - Interventional Cardiology (2011) Volume 3, Issue 3
Choosing the correct therapeutic option for acute limb ischemia
- Corresponding Author:
- Gregory Mishkel
Prairie Cardiovascular Consultants, PO Box 19420, Springfield, IL 62794-9420, USA
Tel: +1 217 788 0708
Fax: +1 217 788 8794
E-mail: gmishkel@prairieheart.com
Abstract
Keywords
acute limb ischemia, arterial embolus, arterial thrombosis, catheter‑directed thrombolysis, limb salvage, peripheral arterial disease, thrombectomy, thrombolysis
Present day cardiology has evolved from exclusively directing the cardiologic health of patients, to additionally managing their vascular health. Increasingly, both general and particularly interventional cardiologists are called upon to manage the care of the patients in their practice when they present with acute vascular emergencies. Unfortunately, subsequent to their subspecialty training, many cardiologists have lost the ability to manage limb ischemia, forfeiting their patients to vascular surgeons or interventional radiologists.
This article will provide a basis for the diagnosis and treatment of patients who present with acute limb ischemia (ALI), so that at the very least, appropriate medical management can be instituted and a rationale treatment plan can be implemented.
Incidence and etiology
Acute limb ischemia occurs due to abrupt interruption of arterial blood flow to an extremity. This results in profound limb ischemia leading to rest pain, ischemic ulcerations and gangrene. The true incidence worldwide is not reliably known, but a few national registries and regional surveys estimate an annual incidence of 140 million cases per year and occurs at a rate of 14 per 100,000 US hospital admissions [1,2]. If treated promptly, restoration of perfusion may lead to limb salvage. However, delay is likely to result in limb loss and possibly death. ALI is associated with limb loss rates of 5–30% and mortality rates of 11–18% [3–5].
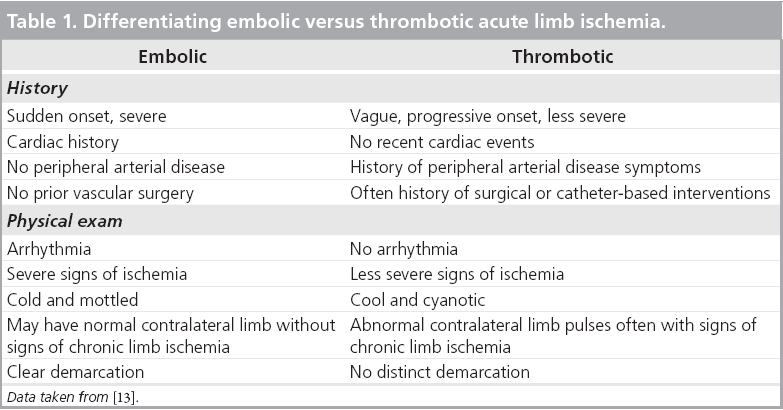
Acute limb ischemia is either due to embolic or in situ thrombotic arterial occlusion. A total of 15–20% of patients with peripheral arterial disease (PAD) may eventually develop ALI [3]. The majority of ALI cases (85%) are due to in situ thrombosis, with the remaining 15% of cases due to embolic arterial occlusion. The vast majority of emboli, upwards of 90%, are cardiac in etiology [6] and are usually due to either atrial fibrillation or acute myocardial infarction. Emboli tend to lodge at arterial bifurcations such as the aortoiliac, femoral and the distal popliteal artery [6]. Thrombotic occlusions usually occur at the site of pre-existing atherosclerosis, so patients with thrombotic ALI can be expected to have pre-existing symptomatic PAD with associated collateralization. By contrast, embolic ALI patients may have a relatively healthy arterial tree without preconditions necessary for collateral formation, and so when the embolus occurs, symptoms are more abrupt and severe. Thrombotic occlusions of peripheral arterial bypass grafts are currently the most common cause of ALI [6].
Less frequent causes of ALI include entities such as endocarditis, air and fat embolism, trauma and dissection, and hypercoagulable states. Of these less frequent etiologies, embolism from a proximal aneurysm such as the popliteal artery is an important cause. Thrombosis of, or embolization from a popliteal aneurysm is indeed a more frequent complication than rupture [7]. ALI occurs in 17–46% of symptomatic popliteal artery aneurysms [7–10]. ALI due to popliteal artery aneurysms has a 20–60% incidence of limb loss and 12% mortality [10]. Massive deep venous thrombosis can lead to a condition known as phlegmasia cerulea dolens, which due to the associated soft-tissue swelling can result in local pressure sufficient to impede arterial flow to the extremity resulting in ALI. A more complete listing of the causes of embolism and thrombosis is presented in Box 1.
Presentation
Limb salvage is dependent on timely clinical evaluation, identification of the etiology and early intervention. Delay in treatment results from a failure of diagnosis, and results in a high risk of morbidity and mortality. When treatment begins within 12 h of onset of ischemia, the mortality and limb salvage rate is 19 and 93%, respectively. With delay of treatment longer than 12 h, these rates worsen to 31 and 78%, respectively [11,12].
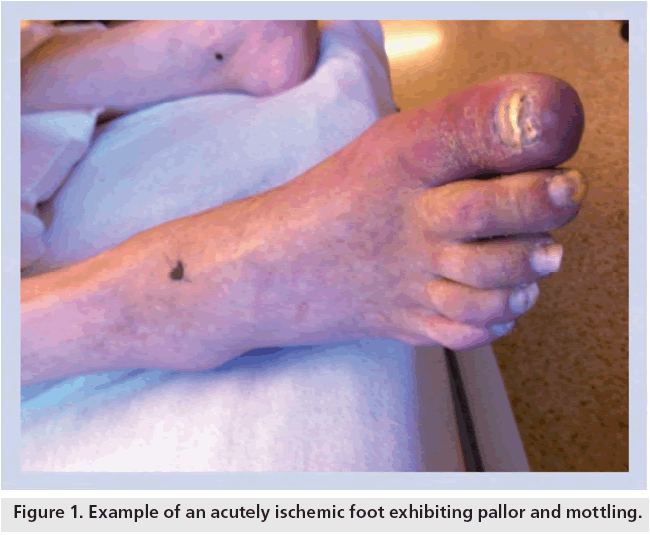
ALI is confirmed by the presence of the six ‘P’s’: pain, pallor, polar (cold), pulselessness, paresthesia and, ultimately, paralysis [13]. Pain, pallor and a cool extremity (polar) are early indications. Pain generally progresses with the duration of ischemia, but may diminish over time if collaterals are recruited or if ischemia progresses to ischemic sensory loss. Pallor develops owing to emptying and vasospasm of arteries and is an early manifestation. As ischemia progresses, mottling appears owing to stagnation in capillary beds. Blanching typically represents reversibility of ischemia (Figure 1). An early physical finding that establishes the level of disease is pulselessness in the affected extremity. The more proximal the occlusion, the more significant the findings of ischemia will be. Signs of ischemia are usually most pronounced one joint distal to the level of occlusion. For example, if there is occlusion at the distal popliteal or tibial trifurcation, classically the calf will appear relatively well perfused and the foot will appear ischemic. If the occlusion is at the femoral bifurcation, the foot and lower leg will often be ischemic. Paresthesia and the subsequent development of paralysis occur later in the course of ALI and represent urgency to avoid limb loss. Sensory deficits typically occur in the order of the diameter of the nerve fibers, beginning with light touch, vibration and proprioception and then much later deep pain and pressure sense. Paralysis is the last to occur, beginning with the intrinsic foot muscles followed by the leg muscles. Detecting early muscle weakness is difficult because toe movements (other than abduction/adduction) are produced mainly by the larger leg muscles. Once paralysis occurs, limb salvage is much less likely and reperfusion injuries more likely.
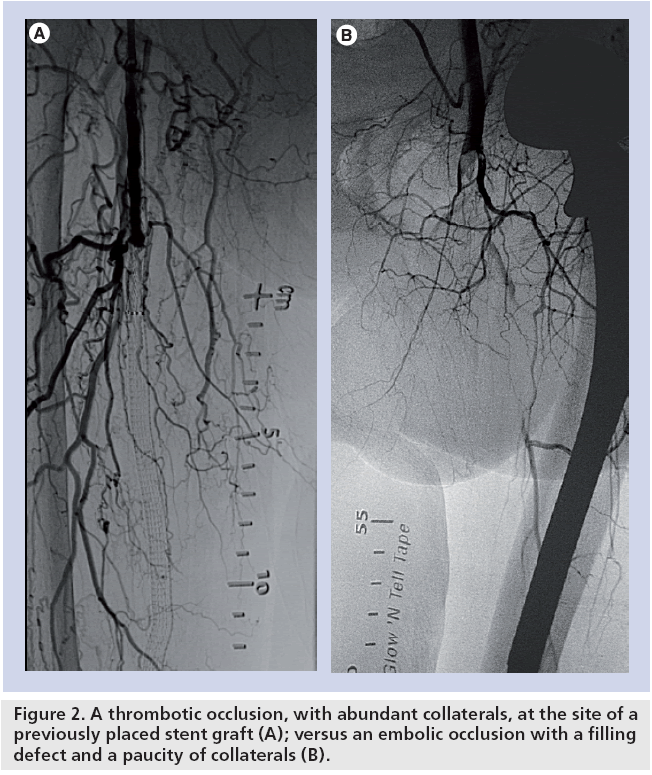
Identification of the underlying cause can often direct management strategies. Acute arterial embolism can often be treated directly with a surgical embolectomy without prior diagnostic testing. In addition, because an embolus often occurs in the setting of a previously normal arterial circulation without collaterals, emboli tend to produce more acute and limb-threatening ischemia. Patients with embolic occlusions tend to remember the moment of onset. In arterial thrombosis, the onset of symptoms and ischemic manifestation tends to be more gradual (Table 1). Just as the history and physical exam are helpful in identifying patients with ALI and distinguishing between thrombosis versus embolism, peripheral angiography will also provide clues to the etiology. Significant disease proximal to the level of the occlusion along with a plethora of collateral vessels suggests thrombosis. Conversely, a clean proximal vasculature and the absence of collaterals in association with an arterial cutoff or meniscus suggests embolism (Figure 2).
The management options available for the treatment of ALI all depend on the appropriate categorization of the patient’s limb ischemia. Typically, ankle brachial indices are not of much clinical utility other than confirming the clinical exam. Of the greatest utility is the use of a bedside Doppler to document both arterial and venous signals in addition to an assessment of the patient’s sensory and motor function. The presence of pedal signals usually indicates that there is time for conventional arteriography and proper patient preparation. Other clinical parameters may assist in this regard. Clinical risk factors, prior vascular surgery, skin mottling, sensory/motor loss and muscle tenderness may all increase the risk of amputation, as does elevations of creatinine phosphokinase and white blood count.
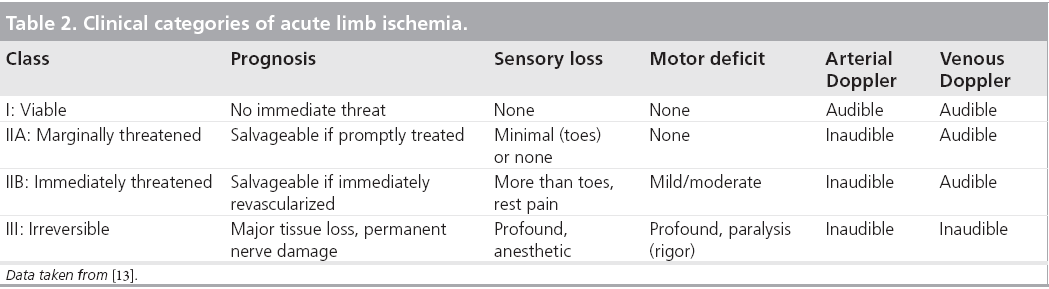
With the pedal Doppler signals in hand, the ischemic limb is classified as viable (class I), marginally threatened (class IIA), immediately threatened (class IIB) and irreversible (class III). The clinical categorization of ALI determines the urgency and potentially the type of revascularization, as well as the prognosis of the affected limb (Table 2) [13].
Treatment
The goal of therapy is reperfusion of the ischemic limb. These patients should be promptly anticoagulated with heparin and started on aspirin on presentation even before the diagnosis of limb ischemia is fully established. This can prevent propagation of the thrombus or embolus and maintain patency of any collateral vessels. After immediate initialization of anticoagulation, the patient should be evaluated with a history and physical examination including Doppler evaluation of the pulses.
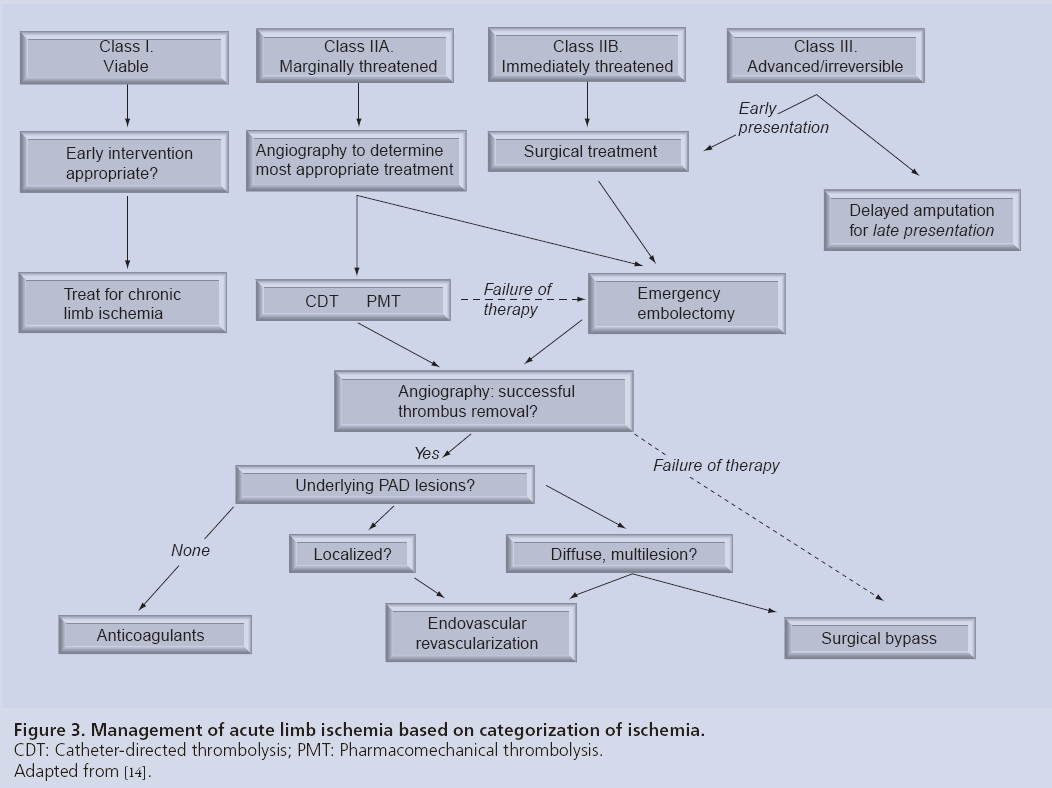
Figure 3 is an adaptation of the widely published Rutherford management strategy [14] incorporating angiography, endovascular techniques and surgical options. A class I limb faces no immediate threat and can often be treated as any chronically ischemic limb would be, pursuant to a full vascular work-up. Many of these patients may in fact have significant comorbidities and conservative measures may be more appropriate. Sedentary patients may actually end up ultimately asymptomatic after a course of anticoagulant therapy. Functional class I patients deserve revascularization and should be treated similarly to class IIA patients, who are defined by having a threatened limb that is salvageable if treated promptly. Therefore, prompt angiography is an appropriate first step, and more often than not, the limb can be salvaged with endovascular techniques such as catheterdirected thrombolysis (CDT) or percutaneousmechanical thrombectomy (PMT). The option for bail out to surgery without compromising the patient’s limb exists if the procedure is not successful. An immediately threatened limb, class IIB, is still salvageable if immediate revascularization occurs. Surgery, most often an embolectomy, is the default treatment of choice in order to reperfuse the limb in adequate time to maintain viability. Pulse examination often guides the surgical procedure, but in the majority of cases surgical revascularization is approached from the common femoral artery. From here balloon catheter embolectomy can be performed from the aorta to the ankle. Embolic occlusions may only require embolectomy where as thrombotic occlusions may require endarterectomy or more likely bypass. Like endovascular intervention, completion arteriography is generally recommended unless embolectomy results in complete restoration of normal distal pulses. Distal clot may be treated by a popliteal cut down with embolectomy or with CDT.
Figure 3: Management of acute limb ischemia based on categorization of ischemia.
CDT: Catheter-directed thrombolysis; PMT: Pharmacomechanical thrombolysis.
Adapted from [14].
Class III ischemia is considered to be irreversible. In addition to inaudible arterial and venous Doppler signals, these patients exhibit profound anesthesia and paralysis, along with muscle rigor and fixed mottling. The duration of ischemia is critical in these cases. If early after onset of ischemia (within 2 h), emergency embolectomy may still salvage the limb. Revascularization attempts in later presenting cases are unlikely to result in limb salvage but may result in renal failure due to rhabdomyolysis as well as a nonfunctional contracted limb, which may compromise future amputations. Compartment syndrome is a frequent accompaniment of surgical reperfusion strategies in patients with severe ischemia. Compartment pressures should be measured unless fasciotomies are performed prophylactically [15].
Orthodox teaching strictly limited endovascular techniques to class I and IIA patients, as it was felt that surgery offered the most prompt reperfusion potential for patients presenting with class IIB and III ischemia. In the past, CDT was associated with prolonged treatment times with the infusion of lytics for potentially days to achieve any degree of recanalization. It has become increasingly apparent that the choice of intervention is often not that clear cut. Advances in technology, such as accelerated thrombolytic regimens and the use of mechanical thrombectomy, have resulted in a considerable decrease in the time to reperfusion. Although thrombolysis may take longer to restore full patency, it must be kept in mind that improvement in limb perfusion, during thrombolysis, occurs before complete thrombus removal. Thrombolysis may also have the added benefit of a gradual return of arterial flow, which may result in a reduction in reperfusion injury. Overall, there has been considerable blurring between the choice of techniques, with many patients with class IIB and III ischemia having some endovascular treatment during their hospital course. Indeed, although the clinical relevance may be disputed, it must be kept in mind that in 36% of cases after a surgical embolectomy, a thrombus remains within the artery [16].
Ultimately, the choice of technique incorporates many additional factors such as the etiology of the ischemia, presence of underlying medical conditions, local capabilities and expertise, and even prosaic decisions such as how long it takes to mobilize a surgical team versus an angiographic suite, where some flow can be promptly established.
The advantages of an endovascular approach relative to surgery are that it is minimally invasive, performed under local anesthesia, may result in improved thrombus resolution in smaller vessels, and it allows for subsequent endovascular and indeed surgical procedures.
The site of arterial access is determined by the suspected site of arterial occlusion and any previous surgical or endovascular interventions. A contralateral femoral artery approach has the distinct advantage of allowing for angiographic evaluation of the aorta and iliac inflow arteries, as well as generally being safer, particularly if lytics are infused over time. An antegrade ipsilateral femoral artery approach is useful in situations where there is severe calcification or tortuosity of the iliac vessels or in the situation where there is significant distal tibial disease and particularly occlusions.
After angiography has been performed, the choice of CDT versus PMT depends on operator preference and other institutional factors. It is our practice to initially utilize CDT and reserve other forms of PMT for cases of incomplete thrombolysis. In our institution, we have experienced good success with the EkoSonic® Endovascular System (Ekos Corporation, Bothell, WA, USA [101]). This catheter combines an infusion catheter with multiple high-frequency, low-energy ultrasonic energy transducers along its length. By using ultrasound energy to loosen the fibrin strands within the thrombus and by exposing a greater surface area of thrombus to the drug, the dose of administered lytic is reduced by as much as 50–70% and the duration of infusion considerably shortened. A success rate of 92% compared with 68.8% in the TOPAS trial has been demonstrated with this catheter [17]. The duration of infusion was likewise shorter, 16.9 versus 24.4 h.
Initially, a guidewire, in combination with a catheter, is advanced across the occlusion. Distal intra-luminal catheter placement is confirmed angiographically. The catheter is then exchanged for the EkoSonic infusion catheter. The catheter of the appropriate length is placed so that the side holes span the length of the occlusion. A bolus of 5–10 mg tissue plasminogen activator (tPA) is followed by a continuous infusion of 0.5–2.0 mg tPA/h. Although not US FDA-approved for this indication, tPA is now the most widely used thrombolytic in part due to its short half-life of 5–7 min. Heparin is administered through a peripheral intravenous line at 400–800 U/h to maintain a partial thromboplastin time <60 s. Despite its fibrin specificity, fibrinogen reductions of 16–36% have been observed with tPA [6]. Fibrinogen levels are monitored periodically during thrombolytic infusion. If fibrinogen levels fall below 120–150 mg/dl the dose of tPA is decreased. Typically, relook angiography is performed within 12 h. This may vary owing to signs of reperfusion, deterioration of the limb or hemorrhagic complications. In the event of worsening ischemia or hemorrhagic complication, surgical intervention is indicated. The potential for hemorrhagic complications with CDT is 6–12% [18]. The most serious complication of thrombolytic therapy is intracranial hemorrhage, which may be fatal. If suspected, the lytic should be stopped and an emergent head CT obtained. Following successful thrombolysis, angiography may reveal underlying lesions, which are treated with either endovascular techniques or surgical bypass as appropriate.
PMT techniques have the potential advantage of rapid thrombus debulking, limiting the duration of ischemia and simplifying treatment by allowing for treatment of the underlying disease in one treatment session. Typically, there is less resource utilization and reduced risk of lytic complications. The simplest example of this technique is percutaneous aspiration thrombectomy (PAT). PAT is a technique that uses thin-wall large lumen catheters with applied suction from a 50 or 60 cc syringe to remove the embolus or thrombus. It is a simple and safe technique and may be used as a standalone procedure or in conjunction with more sophisticated techniques. A more sophisticated PMT system is the AngioJet® Ultra Thrombectomy System (MedRad Interventional, PA, USA), which can deliver pulse spray focally as well as aspirate thrombus [102]. The Trellis® peripheral infusion system (Covidien, MA, USA [103]) is approved for the management of DVT but also has utility in the arterial vasculature. The catheter has two occluding balloons with drug infusion holes between, along with mechanical drug dispersion capabilities with an oscillating wire within the isolated vessel segment. Lytic drug can be applied directly without systemic effect and the thrombus and drug aspirated. A success rate of 92% without hemorrhagic complications has been demonstrated [19].
Surgery may not represent the best first choice for many patients, but similarly there are those patients where an endovascular approach is left wanting and dependant on the age of the thrombus; once organized, both CDT and PMT are less effective. Embolic disease can be particularly problematic. The onset of limb-threatening ischemia can be swift, and surgery so simple in many cases that an endovascular approach is not really appropriate. In addition, emboli usually consist of organized thrombus and therefore may be more resistant to thrombolytic therapy. Furthermore, many of these patients are elderly with significant co-morbidities, which can present relative or absolute contraindications to prolonged thrombolysis. Finally, with all these techniques there is a significant potential for distal embolization, which may jeopardize the microcirculation and worsen the ischemia.
Post-revascularization treatment
After revascularization and reperfusion of an ischemic limb, reperfusion syndrome may develop. When reperfusion leukocytes and platelets become activated, inflammatory mediators are generated, cellular membrane ion pumps are disrupted, free radicals are generated and cell death can occur [20]. As a result, myoglobin, potassium, lactate and microthrombi enter the systemic circulation. This may cause renal failure, arrhythmias or even death. This syndrome is associated with morbidity and mortality rates as high as 41% [21].
Biotoxins accumulate within ischemic tissue, distal to an occlusion, resulting in tissue edema. This edema may be worsened by abrupt reperfusion. In general, the more profound the ischemia the more likely the limb is to develop reperfusion compartment syndrome. With short ischemic times and no evidence of elevated compartment pressures, clinical observation is warranted. Swelling or compartment pressures exceeding 30 mmHg following revascularization are indications for fasciotomy [15].
The need for anticoagulation depends on the etiology of the limb ischemia. Patients with emboli of cardiac etiology or other sites that are not correctable should be placed on long-term anticoagulation. The rate of recurrent emboli ranges from 6 to 45%. In one study, the recurrence rate was 9% in anticoagulated patients and 31% in patients who were not anticoagulated [22]. Patients with thrombosis of bypass grafts may be considered for long-term anticoagulation especially in the absence of a correctable etiology, although randomized data for this approach is lacking.
Antiplatelet therapy with at least aspirin 81 mg daily is indicated in all patients with ALI. In patients with thrombosis who undergo endovascular revascularization it is our practice to initiate long-term dual antiplatelet therapy with both aspirin and a P2Y12 inhibitor, particularly if they have received an intravascular stent.
Randomized trials
Since 1994 to date, three prospective randomized trials have been published comparing thrombolytic therapy to surgical intervention in patients with ALI. All of these studies preceded the routine use of tPA as well as the more sophisticated CDT and PMT devices previously discussed. Regardless of this, they provide a positive backdrop to the continued used of endovascular therapy as it appears to yield equivalent long-term results to surgery with less associated mortality.
The results of the Rochester trial were published in 1994 [23]. In this study, 114 patients with class IIA or IIB ALI of at least 7 days duration were either randomized to treatment with urokinase or surgery. The 1-year limb salvage rate was 82% in both groups. However, the survival rate was significantly higher with thrombolysis – 84 versus 58%.
The same year, the multicenter Surgery versus Thrombolysis for Ischemia of the Lower Extremity trial results were published [5]. A total of 393 patients with class I and II symptoms of less than 6‑months duration were randomized to CDT or surgery. The amputation- free survival in the subgroup of patients admitted with ALI (symptoms <14 days) was 84.7% in the CDT group and 63.5% in the surgery group. This positive outcome can mostly be attributed to the good results observed with acute graft occlusion. In these patients, the 1-year freedom from amputation rates were 52 versus 20%.
The Thrombolysis or Peripheral Arterial Surgery study only enrolled patients with symptoms of less than 14 days [18,24]. ALI patients again were randomized to urokinase or surgery. The amputation-free survival rate at 12 months was similar in both groups – 61.2 and 71.4% for urokinase and surgery, respectively (p = 0.10). In patients with an occluded bypass graft, the amputation-free survival rate was 68.2 and 68.8%, respectively (p = 0.91). Patients randomized to urokinase had more bleeding complications, but a lesser need for surgical procedures during follow-up without a resultant increase in the risk of amputation or death.
The comparative outcomes of CDT versus surgical thrombectomy were undoubtedly influenced by the subsequent disease management. As PTA was usually associated with CDT and bypass with thrombectomy, to an extent these results reflect the improved durability associated with bypass compared with PTA. Therefore, the advantage of CDT may be underrepresented in these trials. Indeed we are already seeing the results of these advances in endovascular techniques nationwide. Rowe and colleagues analyzed the Nationwide Inpatient Sample 1996–2005 database and found that utilization of open procedures for the treatment of acute PAD admissions decreased from 34.5 to 26.3%, whereas the likelihood of undergoing an endovascular procedure increased from 12.7 to 28.3% [25]. Reassuringly, the likelihood of undergoing an amputation decreased significantly from 30.2 to 21.8%.
Conclusion
Acute limb ischemia is a vascular emergency that requires prompt evaluation of the severity and etiology of the ischemia. Treatment options include anticoagulation, endovascular strategies of CDT/PMT and surgical thrombectomy. The choice depends on the severity of ischemia. With newer infusion catheters and PMT techniques, endovascular revascularization is a viable option for more patients. Following revascularization, the patient remains at considerable risk owing to complications of reperfusion syndrome. Continued evaluation and treatment for metabolic abnormalities, renal failure, cardiac arrhythmias and compartment syndrome is crucial to prevent the devastating consequences.
Future perspective
We can expect continued advances in technology and techniques of CDT and PMT. This will result in improved reperfusion times. As a result, endovascular revascularization will continue to play an increasingly significant role in the management of ALI. Although arterial bypass will remain an important therapy for patients with diffuse underlying PAD, with improved durability of infra-inguinal endovascular revascularization, fewer patients will require these operations. Over time, improvement in morbidity and mortality associated with ALI will be realized.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Incidence and etiology
▪ Acute limb ischemia (ALI) is either due to embolic or in situ thrombotic occlusion.
Presentation
▪ ALI is confirmed by the presence of pain, pallor, polar, pulselessness, parasthesia and paralysis.
▪ A Doppler assessment should be used to confirm diagnosis of ALI.
▪ The clinical categorization of ALI determines the urgency and type of revascularization, as well as the prognosis of the affected limb.
Treatment
▪ All patients with ALI should receive parenteral anticoagulation therapy with the standard being intravenous unfractionated heparin.
▪ A ‘vascular’ specialist familiar with the treatment options available should be involved in the evaluation of these patients.
▪ After initial surgical or endovascular treatment of thrombus, follow-up angiography is necessary to identify residual significant disease requiring revascularization.
Post-revascularization treatment
▪ Compartment syndrome must be recognized and treated promptly with fasciotomies.
Randomized trials
▪ Although the utilization of open procedures has decreased and endovascular procedures increased from 1996 to 2005, the likelihood of amputation has significantly decreased.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Peeters P, Verbist J, Keirse K, Deloose K, Bosiers M: Endovascular management of acute limb ischemia. J. Cardiovasc. Surg. (Torino) 51(3), 329–336 (2010).
- Dormandy J, Heeck L, Vig S: Acute limb ischemia. Semin. Vasc. Surg. 12(2), 148–153 (1999).
- Tawes RL Jr, Harris EJ, Brown WH et al.: Arterial thromboembolism. A 20-year perspective. Arch. Surg. 120(5), 595–599 (1985).
- Panetta T, Thompson JE, Talkington CM, Garrett WV, Smith BL: Arterial embolectomy: a 34-year experience with 400 cases. Surg. Clin. North Am. 66(2), 339–353 (1986).
- Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann. Surg. S220(3), 251–266; discussion 66–68 (1994).
- Walker TG: Acute limb ischemia. Tech. Vasc. Interv. Radiol. 12(2), 117–129 (2009).
- Anton GE, Hertzer NR, Beven EG, O’Hara PJ, Krajewski LP: Surgical management of popliteal aneurysms. Trends in presentation, treatment, and results from 1952 to 1984. J. Vasc. Surg. 3(1), 125–134 (1986).
- Varga ZA, Locke-Edmunds JC, Baird RN: A multicenter study of popliteal aneurysms. Joint Vascular Research Group. J. Vasc. Surg.
20(2), 171–177 (1994). - Thompson MM, Bell PR: ABC of arterial and venous disease. Arterial aneurysms. BMJ 320(7243), 1193–1196 (2000).
- Whitehouse WM Jr, Wakefield TW, Graham LM et al.: Limb-threatening potential of arteriosclerotic popliteal artery aneurysms. Surgery 93(5), 694–699 (1983).
- Abbott WM, Maloney RD, McCabe CC, Lee CE, Wirthlin LS: Arterial embolism: a 44 year perspective. Am. J. Surg. 143(4), 460–464 (1982).
- Elliott JP Jr, Hageman JH, Szilagyi E, Ramakrishnan V, Bravo JJ, Smith RF: Arterial embolization: problems of source, multiplicity, recurrence, and delayed treatment. Surgery 88(6), 833–845 (1980).
- Henke PK: Contemporary management of acute limb ischemia: factors associated with amputation and in-hospital mortality. Semin. Vasc. Surg. 22(1), 34–40 (2009).
- Rutherford RB: Clinical staging of acute limb ischemia as the basis for choice of revascularization method: when and how to intervene. Semin. Vasc. Surg. 22(1), 5–9 (2009).
- O’Connell JB, Quinones-Baldrich WJ: Proper evaluation and management of acute embolic versus thrombotic limb ischemia. Semin. Vasc. Surg. 22(1), 10–16 (2009).
- Plecha FR, Pories WJ: Intraoperative angiography in the immediate assessment of arterial reconstruction. Arch. Surg. 105(6), 902–907 (1972).
- Wissgott C, Richter A, Kamusella P, Steinkamp HJ: Treatment of critical limb ischemia using ultrasound-enhanced thrombolysis (PARES Trial): final results. J. Endovasc. Ther. 14(4), 438–443 (2007).
- Ouriel K, Veith FJ, Sasahara AA: A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N. Engl. J. Med. 338(16), 1105–1111 (1998).
- Sarac TP, Hilleman D, Arko FR, Zarins CK, Ouriel K: Clinical and economic evaluation of the trellis thrombectomy device for arterial occlusions: preliminary analysis. J. Vasc. Surg. 39(3), 556–559 (2004).
- Beyersdorf F, Schlensak C: Controlled reperfusion after acute and persistent limb ischemia. Semin. Vasc. Surg. 22(1), 52–57 (2009).
- Trummer G, Brehm K, Siepe M, Heilmann C, Schlensak C, Beyersdorf F: The management of acute limb ischemia. Minerva Chir. 65(3), 319–328 (2010).
- Green RM, DeWeese JA, Rob CG: Arterial embolectomy before and after the Fogarty catheter. Surgery 77(1), 24–33 (1975).
- Ouriel K, Shortell CK, DeWeese JA et al.: A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J. Vasc. Surg. 19(6), 1021–1030 (1994).
- Ouriel K, Veith FJ, Sasahara AA: Thrombolysis or peripheral arterial surgery: Phase I results. TOPAS Investigators. J. Vasc. Surg. 23(1), 64–73; discussion 4–5 (1996).
- Rowe VL, Lee W, Weaver FA, Etzioni D: Patterns of treatment for peripheral arterial disease in the United States: 1996–2005. J. Vasc. Surg. 49(4), 910–917 (2009).
▪ Concise review of relevant randomized trials is presented.
▪▪ The etiology and evaluation of acute limb ischemia is clearly described. A management algorithm for acute limb ischemia is also presented.
▪▪ The clinical staging of acute limb ischemia and the basis for selecting a revascularization strategy is outlined.
▪ Good discussion of reperfusion syndrome is presented.