Perspective - Interventional Cardiology (2014) Volume 6, Issue 5
Chronic total occlusion angioplasty: no more excuses
- Corresponding Author:
- J Aaron
Grantham
University of Missouri Kansas City, Saint Luke’s Mid America Heart Institute, NSI 5th floor, 4401 Wornall Rd, Kansas City, MO 64111, USA
Tel: +1 816 932 5475
E-mail: jgrantham@saint-lukes.org
Abstract
Chronic total occlusion percutaneous intervention (CTO-PCI) technique has evolved coincident with improvements in success rate and safety of the procedure. Despite this, many patients are not offered this treatment even after medical therapy fails to alleviate their symptoms. The frequently stated reasons for this include the absence of randomized trial data, excessive costs and the time burden of the procedures. In this article our aim is to dispel the myths surrounding the benefits, costs and time burden of CTO-PCI and suggest that these excuses for not attempting CTO-PCI should be avoided so that patients receive the treatment they need, not simply the treatment their provider or institution prefers.
Chronic total occlusion percutaneous intervention (CTO-PCI) technique has evolved coincident with improvements in success rate and safety of the procedure. Despite this, many patients are not offered this treatment even after medical therapy fails to alleviate their symptoms. The frequently stated reasons for this include the absence of randomized trial data, excessive costs and the time burden of the procedures. In this article our aim is to dispel the myths surrounding the benefits, costs and time burden of CTO-PCI and suggest that these excuses for not attempting CTO-PCI should be avoided so that patients receive the treatment they need, not simply the treatment their provider or institution prefers.
‘Why didn’t my doctor recommend this 3 years ago?’ asked Mr J the day after successful chronic total occlusion (CTO) angioplasty. Mr J had suffered with angina after failure of his saphenous vein graft to the right coronary artery (RCA) left him with a ‘well collateralized’ CTO 3 years earlier. His local cardiologist placed him on three anti-anginal drugs and told him ‘nothing more can be done’. After two visits to the emergency department, two stress tests confirming RCA ischemia, a repeat cardiac catheterization reconfirming the only culprit to be the CTO of the RCA, even a stent placement to the grafted left anterior descending artery (LAD) providing him with no benefit, Mr J’s family convinced him to seek a second opinion. Questions similar to this are frequently posed by grateful patients after successful CTOpercutaneous coronary intervention (PCI). The answer is complicated: evidence suggests that patients with CTOs are being treated or not treated based on physician preference and institutional biases, not their needs; many excuses are given for not treating appropriate patients with PCI; the real problem is that physicians have not been taught how to treat them with PCI and achieve high success rates and low complication rates when PCI is deemed appropriate. The problem is worthy of consideration since CTOs are discovered in 18% [1] of patients referred for coronary angiography but account for less than 5% of all angioplasties performed [2] in real world PCI registries.
Physician preference & institutional bias influences CTO-PCI decision-making
There is evidence that the presence of a CTO drives the decision-making over how to treat a patient with coronary artery disease. In the Bypass Angioplasty Revascularization Investigation (BARI) registry, when non-CTO disease was discovered PCI was performed approximately 35% of the time, while when a CTO was discovered, 10% of patients were treated with PCI and a larger proportion were treated with medical therapy [3].
Using the National Cardiovascular Disease Registry’s CathPCI Registry we found that operator PCI volume was independently associated with CTO-PCI attempt rate such that low volume operators (<50 PCIs/year) were half as likely as intermediate (50–200 PCIs/year) or high volume (>200 PCIs/year) operators to attempt a CTO once discovered [4]. In a Canadian registry, one center performed PCI for 1% of patients with a CTO [1], and another center performed PCI in 16% of patients with a CTO. While treatment variability may represent under or overutilization, it is reasonable to suggest that the hospital treating only 1% of cases with PCI may be underutilizing PCI given the multitude of barriers to CTO-PCI performance, including operator preference. Operator- and institutional- based variability is not justifiable since there is no difference between the symptom-relieving benefits of PCI when a vessel is 100% compared with those that are sub-totally occluded [5].
Taken together, these observations support our contention that many patients with CTOs such as Mr J, are treated according to operator- and institutional treatment biases, not their clinical needs. The real concern in his case was that he was not referred by his cardiologist to a center with CTO-PCI capabilities; he was simply told that ‘nothing more can be done’.
Excuses for not offering CTO-PCI
There are two commonly used reasons for not offering appropriately selected patients CTO-PCI or referring them to a CTO center. Operators cite insufficient evidence of benefits and express concerns related to economic barriers of CTO-PCI.
CTO-PCI evidence of benefit
It is true that there is a paucity of evidence from randomized trials proving the benefits of CTO-PCI as compared with medical therapy. The OAT trial, which indicated that PCI of an occluded culprit artery 3–28 days after myocardial infarction did not reduce the occurrence of death, reinfarction or heart failure during 4 years of follow-up [6], is often misapplied. OAT did not study CTOs, rather recent occlusions and OAT-excluded patients with evidence of large ischemic territories or symptoms suggestive of ischemia and/or viability, the primary therapeutic targets for CTO-PCI.
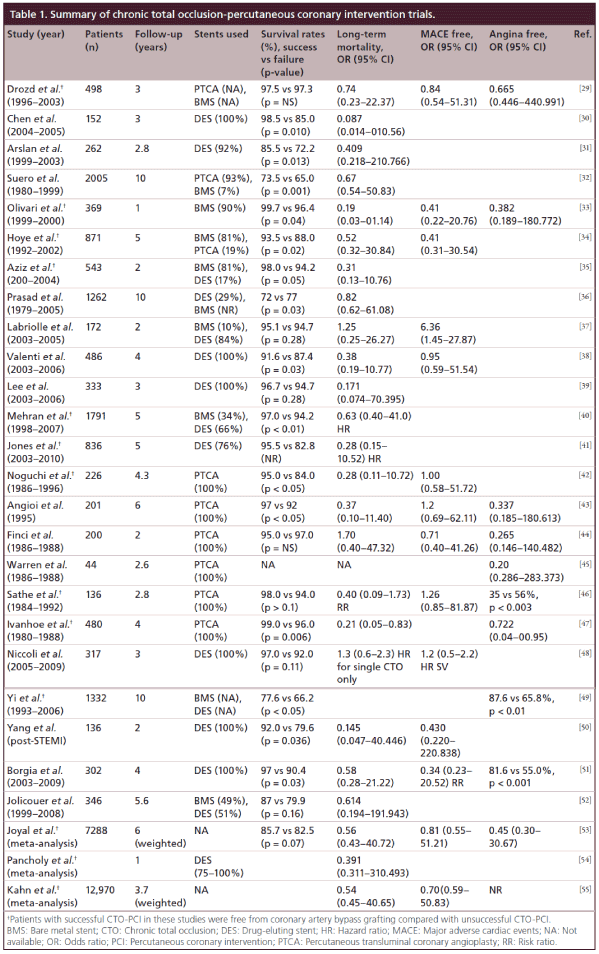
Because of this lack of randomized trial data some cardiologists still choose to ignore the evidence that does exist. There has been an emphasis on whether successful CTO-PCI improves mortality. The studies describing an association between successful CTO-PCI and survival compared with unsuccessful CTO-PCI are summarized in Table 1. They all suffer from the same limitations. The failure groups may represent a different cohort, with higher lesion complexity and disease burden resulting in poorer outcomes, among other confounders. The controversy is furthered by disparate findings between studies. Fefer et al. suggest that the presence of a CTO is not independently associated with an adverse long-term outcome, and failure to revascularize a non-LAD CTO is not associated with a higher mortality [7].
Differences in patient or angiographic characteristics in the non-revascularized group may account for overall increased mortality. While Godino et al. found that non-revascularized patients (those without an attempt and failed procedures) experienced a higher rate of cardiac mortality and sudden cardiac death compared with those with successful revascularization. Within the non-revascularized cohort, the presence of low-left ventricular ejection fraction (LVEF), chronic renal failure, or diabetes were associated with a fourfold increased risk of cardiac death as compared with those without the same risk factors [8]. Recently a meta-analysis of all of these studies suggests that CTO-PCI success as compared with failure is associated with mortality (Table 1; Khan et al. [55]). Thus each asymptomatic patient should be considered individually based on their risk profile. The indications, risks and limitations of the available evidence supporting CTO-PCI for survival advantage should be discussed in a balanced way. Simply saying ‘there is no data’ is just as disingenuous as saying there is proof of a survival advantage of CTO-PCI. Patients deserve the full story and the opportunity to engage in fully informed shared decision-making.
The studies confirming the symptom relieving potential of CTO-PCI are compelling and summarized in Table 1 as well. Again there is a paucity of randomized controlled trial data but the recent finding by Safley et al. [5] that CTO patients derive similar self reported quality of life improvements as compared with non-CTO patients after PCI leads one to wonder why there should be a double standard between CTO and non-CTO disease revacularization rates. We often challenge operators to ask themselves, ‘if it were 90% would you stent it?’ This decision should be based entirely on the patient’s symptoms, risk, and response to medical therapy, not the angiographic severity of the stenosis in the culprit artery.
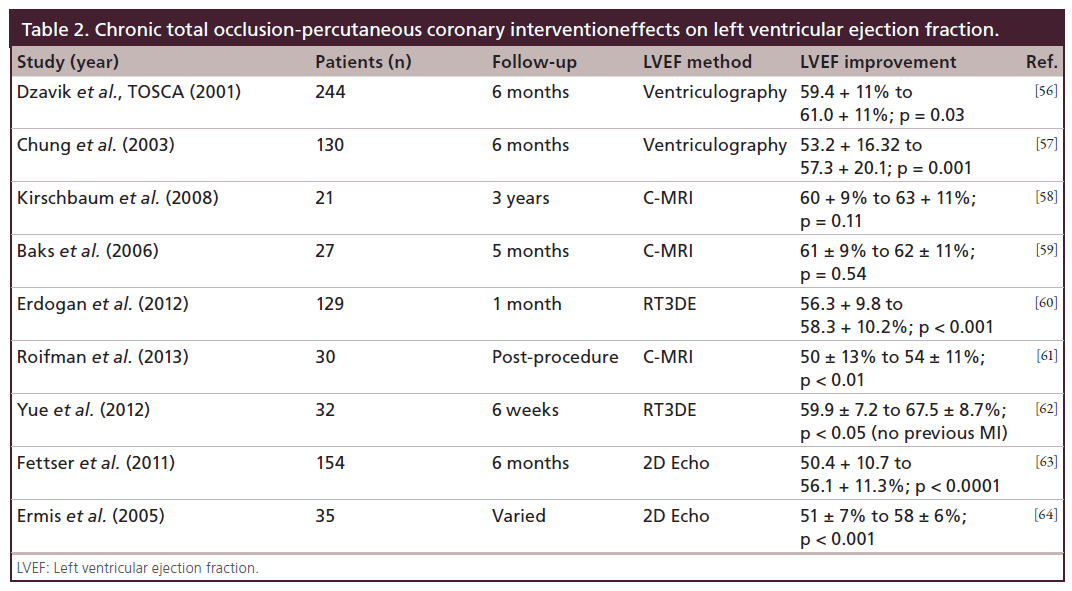
Other reported advantages of CTO-PCI include improved left ventricular function (Table 2), and avoidance of other procedures such as coronary artery bypass grafting (CABG), automatic implantable cardioverter defibrillators (AICDs) and cardiac transplant [9,10]. These studies are also summarized in Table 1. These multiple registries and studies have been considered by the American College of Cardiology (ACC), American Heart Association (AHA), and The Society for Cardiology Angiography and Interventions (SCAI) in their indications and appropriate use criteria for revascularization [11]. We strongly encourage rigorous application of these guidelines in the selection of patients for CTO-PCI, even though there is a paradoxical downgrading in the appropriateness of CTO-PCI as compared with non-CTO-PCI [12].
Economic disincentives
There are several economic barriers to performing and referring patients for CTO-PCI. There is only one study that attempted to determine the cost–effectiveness of CTO-PCI from a societal perspective. Many institutions are concerned with the increased supply costs relative to non-CTO-PCI, lost productivity due to prolonged procedures and the potential for lost coronary artery bypass graft volume. Some operators worry about the potential loss of patients or professional reputation after referring patients to a CTO-PCI center.
An estimated 600,000 to 1.8 million Americans suffer from refractory angina [11] at an estimated lifetime expense of US$1 million each. Upwards of 70% of these patients are deemed non-revascularizable due to a CTO. Chronic angina is commonly experienced by patients with CTOs [9,13,14] and successful CTO-PCI is associated with improvements in angina status and quality of life (Table 1). These treatment costs of patients suffering from chronic stable angina exceeds that of other conditions such as non-cancer pain [15]. In a comparative cost-effective analysis using a Markov model the cost of CTO-PCI was higher as compared with optimal medical therapy (OMT). However, CTO-PCI was associated with much greater qualityadjusted life-years (2.38 vs 1.99), yielding a better cost–effectiveness ratio [16]. Again, the case of Mr J highlights the added costs to society of undertreated CTOs, where repeated hospital admissions, imaging and catheter studies were performed before his problem was ultimately successfully addressed.
Institutional concern over higher supply costs is warranted. The tool kit required to implement a CTO program costs approximately US$70,000. Added to that is the expense of higher stent use per case (typically 2.4 stents per case compared with 1.7 per case in non-CTO-PCI) [4]. Given the parsimonious increase in reimbursement for complex PCI compared with non-CTO (i.e., routine) PCI in the USA, hospitals are right to question the fairness of the current reimbursement system in the USA. Outside the USA similar restrictions on procedural reimbursements exist that effectively preclude the use of newer, more expensive technologies. Clearly better data on the cost and cost–effectiveness of CTO as compared with medical therapy is needed so that a complete understanding of the value of CTO-PCI can be obtained. It may be penny-wise and pound-foolish to continue in the current state of CTO-PCI denial for patients suffering with refractory angina, from a payor perspective.
From a hospital perspective, Karmpaliotis and colleagues determined that the contribution margin of CTO-PCI was only US$400 less for CTO than non- CTO-PCI [17]. This obviously requires careful management of payor mix and could be drastically impacted by healthcare reimbursement changes (e.g., the two midnight rule by Medicare) or price gouging by CTO device vendors. Nevertheless, we as practitioners and patient advocates must in every case do what is best for patients and continue to argue for better reimbursement for these procedures to the extent that the data supports them, and work with vendors to negotiate fair prices for CTO specialized equipment. A more detailed cost analysis of CTO-PCI is underway in the US multi-center OPEN-CTO study.
Lost cath lab and operator productivity due to prolonged procedures is being addressed by improved procedural efficiency as has been demonstrated using the hybrid approach to CTO-PCI [18], which is discussed below. This issue is less acute recently as interventional volumes have declined [19] and hospitals interested in filling this time look toward new business opportunities so that their fixed costs are not wasted.
Lost CABG volume is not a necessary effect of initiating a CTO-PCI program. CABG remains an attractive alternative to PCI for patients with intermediate and high disease burden [20]. Additionally, once recognized for expertise in the management of complex coronary artery disease, CTO centers often experience an increase in bypass surgery volume.
Operators committed to the right therapy for the right patient every time should also consider that bypass surgery for CTOs may not always be the best solution for the patient. Durability of a saphenous vein graft to an RCA or LCX CTO is limited. One report suggested 1-year patency rates as low as 23% [21]. Finally, many patients with CTO have already undergone CABG. Repeat coronary artery bypass graft surgery for an isolated RCA or LCX saphenous vein graft is not warranted when native vessel CTO-PCI is an option. In the US 30% of CTO-PCIs are performed on patients with prior CABG [22].
The loss of a patient to a referral center remains a prevalent fear that limits any center of excellence model in the delivery of cardiovascular care. However, in the age of informed medical consumerism and direct-toconsumer marketing, many CTO centers will seek to inform the public of the PCI option for CTOs. As patients begin to understand that they are not receiving the same information from their doctors, the negative impact on a medical practice could be just as significant.
Lack of physician training & technical expertise
Advancements in guidewire technology, refinements in support and balloon catheters, novel re-entry devices and the retrograde technique have all contributed to higher procedural success, adequate safety and improved procedural efficiency [23]. The complex technical decision-making of CTO-PCI has been simplified by the development and implementation of the hybrid approach [24,25]. This approach is teachable and has now been adopted at over 100 centers in the USA and abroad. While not every interventionalist can or should become a CTO operator, enough will adopt this approach so that every patient will soon have access to a CTO-PCI operator. Operators and institutions interested in developing a CTO-PCI program now have at their disposal a wealth of resources to help them in this effort. National and international meetings committed to teaching CTO-PCI techniques, web-based training modules such as www.CTOfundamentals.org, and systematic industry-sponsored proctoring are readily available.
Conclusion
The case of Mr J highlights many of the challenges in delivering appropriate percutaneous coronary interventional services to patients with complex coronary artery disease. Patients with CTOs are routinely given excuses by their physicians for not considering PCI. Many patients who are excellent candidates for PCI are offered medical therapy or bypass surgery instead. While a better understanding of the benefits of CTO-PCI might help, the ultimate solution lies in teaching interventional cardiologists about the solutions to the barriers to CTO-PCI. The hybrid approach is designed to address most of these concerns. With the hybrid approach success rates over 90% [26], major cardiac adverse events (MACE) less than 4% [27] and highly efficient procedures (80% can be completed in less than 90 min) [28] can be achieved by enough operators to meet the goal of providing access to PCI for every appropriately selected patient with a CTO.
Future perspective
The field of CTO-PCI has evolved. With the development of new techniques and implementation of the Hybrid Approach success rates of 90% with low complication rates and lower procedure times are possible. Barriers to CTO-PCI adoption still exist in the minds of many operators and institutions. The Hybrid Approach [24,25] is teachable and has now been adopted at over 100 centers in the USA and abroad. While not every interventionalist can or should become a CTO operator, enough will adopt this approach so that every patient will soon have access to a CTO-PCI when it is appropriate. Operators and Institutions interested in developing a CTO-PCI program now have at their disposal a wealth of resources to help them in this effort. National and international meetings committed to teaching CTO-PCI techniques, web based training modules such as www.CTOfundamentals.org, and systematic industry-sponsored proctoring are readily available. To further inform physicians and patients in this decision trials designed to better define the benefits, appropriateness and cost of CTO-PCI such as the OPEN CTO (www.clinicaltrials.gov) are underway.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
• A case example of inappropriately deferred percutaneous coronary intervention (PCI) is presented to highlight the problem of underutilization of PCI for chronic total occlusion (CTO).
• The evidence suggesting that CTOs are being treated or not treated based on physician preference and institutional biases, not patient needs, is reviewed.
• The reasons for not treating appropriate patients with PCI are discussed along with the evidence refuting these excuses.
• Solutions to these barriers to CTO-PCI adoption are described.
References
Papers of special note have been highlighted as: • of interest
- Fefer P, Knudtson ML, Cheema AN et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J. Am. Coll. Cardiol. 59(11), 991–997 (2012).
- Abbott JD, Kip KE, Vlachos HA et al. Recent trends in the percutaneous treatment of chronic total coronary occlusions. Am. J. Cardiol. 97(12), 1691–1696, (2006).
- Group BDS, Frye RL, August P et al. A randomized trial of therapies for Type 2 diabetes and coronary artery disease. N. Engl. J. Med. 360(24), 2503–2515 (2009).
- Grantham JA, Marso SP, Spertus J, House J, Holmes DR Jr, Rutherford BD. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc. Interv. 2(6), 479–486 (2009)
- Safley DM, Grantham JA, Hatch J, Jones PG, Spertus JA. Quality of life benefits of percutaneous coronary intervention for chronic occlusions. Catheter. Cardiovasc. Interv. 84(4), 629–634 (2013)
- Hochman JS, Lamas GA, Buller CE et al. Coronary intervention for persistent occlusion after myocardial infarction. N. Engl. J.Med. 355(23), 2395–2407 (2006).
- Fefer P, Gannot S, Kochkina K E et al. Impact of coronary chronic total occlusions on long-term mortality in patients undergoing coronary artery bypass grafting. Interact. Cardiovasc. Thorac. Surg. 18(6), 713–716 (2014).
- Godino C, Bassanelli G, Economou FI et al. Predictors of cardiac death in patients with coronary chronic total occlusion not revascularized by PCI. Int. J. Cardiol. 168(2), 1402–1409 (2013)
- Olivari Z, Rubartelli P, Piscione F et al. Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions: data from a multicenter, prospective, observational study (TOASTGISE). J. Am. Coll. Cardiol. 41(10), 1672–1678 (2003).
- Chung CM, Nakamura S, Tanaka K et al. Effect of recanalization of chronic total occlusions on global and regional left ventricular function in patients with or without previous myocardial infarction. Catheter. Cardiovasc. Interv. 60(3), 368–374 (2003).
- Harold JG, Bass TA, Bashore TM et al. ACCF/AHA/ SCAI 2013 Update of the Clinical Competence Statement on Coronary Artery Interventional Procedures: a Report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training (Writing Committee to Revise the 2007 Clinical Competence Statement on Cardiac Interventional Procedures). Catheter. Cardiovasc. Interv. 82(2), E69–E111 (2013).
- Marso SP, Teirstein PS, Kereiakes DJ, Moses J, Lasala J, Grantham JA. Percutaneous coronary intervention use in the United States. Defining measures of appropriateness. JACC Cardiovasc. Interv. 5(2), 229–235 (2012).
- Werner GS, Gitt AK, Zeymer U et al. Chronic total coronary occlusions in patients with stable angina pectoris: impact on therapy and outcome in present day clinical practice. Clin. Res. Cardiol. 98(7), 435–441 (2009).
- Rubartelli P, Niccoli L, Verna E et al. Stent implantation versus balloon angioplasty in chronic coronary occlusions: results from the GISSOC trial. J. Am. Coll. Cardiol. 32(1), 90–96 (1998).
- McGillion M, Croxford R Watt-Watson J, LeFort S, Stevens B, Coyte P. Cost of chronic stable angina. Can. J. Cardiol. 24(10), 759–764 (2008).
- Gada H, Whitlow PL, Marwick TH. Establishing the cost–effectiveness of percutaneous coronary intervention for chronic total occlusion in stable angina: a decision-analytic model. Heart 98(24), 1790–1797 (2012).
- Karmpaliotis D, Lembo N, Kalynych A et al. Development of a high-volume, multiple-operator program for percutaneous chronic total coronary occlusion revascularization: procedural, clinical, and cost-utilization outcomes. Catheter. Cardiovasc. Interv. 82(1), 1–8 (2013).
- Pershad A, Eddin M, Girotra S, Cutogno R, Daniels D, Lombardi W. Validation and incremental value of the hybrid algorithm for CTO PCI. Catheter. Cardiovasc. Interv. (2014) (In Press).
- Riley RF, Don CW, Powell W, Maynard C, Dean LS. Trends in coronary revascularization in the United States from 2001 to 2009: recent declines in percutaneous coronary intervention volumes. Circ. Cardiovasc. Qual. Outcomes 4(2), 193–197 (2011).
- Mohr FW, Morice M-C, Kappetein AP et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 381(9867), 629–638 (2013).
- Widimsky P, Straka Z, Stros P et al. One-year coronary bypass graft patency: a randomized comparison between off-pump and on-pump surgery angiographic results of the PRAGUE-4 trial. Circulation 110(22), 3418–3423 (2004).
- Michael TT, Karmpaliotis D, Brilakis ES et al. Impact of prior coronary artery bypass graft surgery on chronic total occlusion revascularisation: insights from a multicentre US registry. Heart 99(20), 1515–1518 (2013).
- Whitlow PL, Burke MN, Lombardi WL et al. Use of a novel crossing and re-entry system in coronary chronic total occlusions that have failed standard crossing techniques: results of the FAST-CTOs (Facilitated Antegrade Steering Technique in Chronic Total Occlusions) trial. JACC Cardiovasc. Interv. 5(4), 393–401 (2012).
- Brilakis ES, Grantham JA, Rinfret S et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc. Interv. 5(4), 367–379 (2012).
- Syrseloudis D, Secco GG, Barrero EA et al. Increase in J-CTO lesion complexity score explains the disparity between recanalisation success and evolution of chronic total occlusion strategies: insights from a single-centre 10- year experience. Heart 99(7), 474–479 (2013).
- Karmpaliotis D, Michael TT, Brilakis ES et al. Retrograde coronary chronic total occlusion revascularization. Procedural and in-hospital outcomes from a multicenter registry in the United States. JACC Cardiovasc. Interv. 5(12), 1273–1279 (2012).
- Jones DA, Weerackody R, Rathod K et al. Successful recanalization of chronic total occlusions is associated with improved long-term survival. JACC Cardiovasc. Interv. 5(4), 380–388 (2012).
- Michael TT, Mogabgab O, Fuh E et al. Application of the “hybrid approach” to chronic total occlusion interventions: a detailed procedural analysis. J. Interv. Cardiol. 27, 36–43 (2014).
- Drozd J, Wojcik J, Opalinska E, Zapolski T, Widomska- Czekajska T. Percutaneous angioplasty of chronically occluded coronary arteries: long-term clinical follow-up. Kardiol. Pol. 64(7), 667–674 (2006).
- Chen JP. Benefits of percutaneous coronary revascularization in patients with adequately collateralized chronic total occlusions. South. Med. J. 101(9), 881–882 (2008).
- Arslan U, Balcioglu AS, Timurkaynak T, Cengel A. The clinical outcomes of percutaneous coronary intervention in chronic total coronary occlusion. Int. Heart J. 47(6), 811–819 (2006).
- Suero JA, Marso SP, Jones PG et al. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: a 20-year experience. J. Am. Coll. Cardiol. 38(2), 409–414 (2001).
- Olivari Z, Rubartelli P, Piscione F et al. TOAST-GISE Investigators. Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions: data from a multicenter, prospective, observational study (TOAST-GISE). J. Am. Coll. Cardiol. 41(10), 1672–1678 (2003).
- Hoye A, van Domburg RT, Sonnenschein K, Serruys PW. Percutaneous coronary intervention for chronic total occlusions: the Thoraxcenter experience 1992–2002. Eur. Heart J. 2005 26(24), 2630–2636.
- Aziz S, Stables RH, Grayson AD, Perry RA, Ramsdale DR. Percutaneous coronary intervention for chronic total occlusions: improved survival for patients with successful revascularization compared with a failed procedure. Catheter. Cardiovasc. Interv. 70(1), 15–20 (2007).
- Prasad A, Rihal CS, Lennon RJ et al. Trends in outcomes after percutaneous coronary intervention for chronic total occlusions: a 25-year experience from the Mayo Clinic. J. Am. Coll. Cardiol. 49, 1611–1618 (2007).
- de Labriolle A, Bonello L, Roy P et al. Comparison of safety, efficacy, and outcome of successful versus unsuccessful percutaneous coronary intervention in ‘true’ chronic total occlusions. Am. J. Cardiol. 102, 1175–1181 (2008).
- Valenti R, Migliorini A, Signorini U et al. Impact of complete revascularization with percutaneous coronary intervention on survival in patients with at least one chronic total occlusion. Eur. Heart J. 29, 2336–2342 (2008).
- Lee SW, Lee JY, Park DW et al. Long-term clinical outcomes of successful versus unsuccessful revascularization with drug-eluting stents for true chronic total occlusion. Catheter. Cardiovasc. Interv. 78, 346–353 (2011).
- Mehran R, Claessen BE, Godino C et al. Multinational Chronic Total Occlusion Registry. Long-term outcome of percutaneous coronary intervention for chronic total occlusions. JACC Cardiovasc. Interv. 4, 952–961 (2011).
- Jones DA, Weerackody R, Rathod K et al. Successful recanalization of chronic total occlusions is associated with improved long-term survival. JACC Cardiovasc. Interv. 5, 380–388 (2012).
- Noguchi T, Miyazaki M, Morii I. Percutaneous transluminal coronary angioplasty of chronic total occlusions. determinants of primary success and long-term clinical outcome. Catheter. Cardiovasc. Interv. 49, 258–264 (2000).
- Angioi M, Danchin N, Juilliere Y et al. Is percutaneous transluminal coronary angioplasty in chronic total coronary occlusion justified? Long term results in a series of 201 patients. Arch. Mal. Coeur Vaiss. 88(10), 1383–1389 (1995).
- Finci L, Meier B, Favre J, Righrtti A, Rutishauser W. Long-term results of successful and failed angioplasty for chronic total coronary arterial occlusion. Am. J. Cardiol. 66, 660–662 (1990).
- Warren RJ, Black AJ, Valentine PA, Manolas EG, Hunt D. Coronary angioplasty for chronic total occlusion reduces the need for subsequent coronary bypass surgery. Am. Heart J. 120, 270–274 (1990).
- Sathe S, Alt C, Black A, Manolas E, Warren R, Valentine P. Initial and long-term results of percutaneous transluminal balloon angioplasty for chronic total occlusions: an analysis of 184 procedures. Aust. NZ J. Med. 24(3), 277–281 (1994).
- Ivanhoe R, Weintraub W, Douglas J et al. Percutaneous transluminal coronary angioplasty of chronic total occlusions. Primary success, restenosis, and long-term clinical follow-up. Circulation 85, 106–115 (1992).
- Niccoli G, De Felice F, Belloni F et al. Late (3 years) follow-up of successful versus unsuccessful revascularization in chronic total coronary occlusions treated by drug eluting stent. Am. J. Cardiol. 110(7), 948–953 (2012).
- Yi XH, Han YL, Li Y et al. Long-term clinical outcomes of patients undergoing successful or failed percutaneous coronary intervention for chronic total occlusions of coronary arteries. Zhonghua Xin Xue Guan Bing Za Zhi 37(9), 773–776 (2009).
- Yang ZK, Zhang RY, Hu J, Zhang Q, Ding FH, Shen WF. Impact of successful staged revascularization of a chronic total occlusion in the non-infarct-related artery on longterm outcome in patients with acute ST-segment elevation myocardial infarction. Int. J. Cardiol. 165(1), 76–79 (2013).
- Borgia F, Viceconte N, Ali O et al. Improved cardiac survival, freedom from MACE and angina-related quality of life after successful percutaneous recanalization of coronary artery chronic total occlusions. Int. J. Cardiol. 161(1), 31–38 (2012).
- Jolicoeur EM, Sketch MJ, Wojdyla DM et al. Percutaneous coronary interventions and cardiovascular outcomes for patients with chronic total occlusions. Catheter. Cardiovasc. Interv. 79(4), 603–612 (2012).
- Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and metaanalysis. Am. Heart J. 160(1), 179–187 (2010).
- Pancholy SB, Boruah P, Ahmed I, Kwan T, Patel TM, Saito S. Meta-analysis of effect on mortality of percutaneous recanalization of coronary chronic total occlusions using a stent-based strategy. Am. J. Cardiol. 111(4), 521–525 (2013).
- Khan MF, Wendel CS, Thai HM, Movahed MR. Effects of percutaneous revascularization of chronic total occlusions on clinical outcomes: a meta-analysis comparing successful versus failed percutaneous intervention for chronic total occlusion. Catheter. Cardiovasc. Interv. 82, 95–107 (2013).
- Dzavik V, Carere RG, Mancini GB et al. Predictors of improvement in left ventricular function after percutaneous revascularization of occluded coronary arteries: a report from the Total Occlusion Study of Canada (TOSCA). Total Occlusion Study of Canada Investigators. Am. Heart. J. 142, 301–308 (2001).
- Cheng AS, Selvanayagam JB, Jerosch-Herold M et al. Percutaneous treatment of chronic total coronary occlusions improves regional hyperemic myocardial blood flow and contractility: insights from quantitative cardiovascular magnetic resonance imaging. JACC Cardiovasc. Interv. 1, 44–53 (2008).
- Kirschbaum SW, Rossi A, Boersma E et al. Combining magnetic resonance viability variables better predicts improvement of myocardial function prior to percutaneous coronary intervention. Int. J. Cardiol. 159, 192–197 (2012).
- Baks T, van Geuns RJ, Duncker DJ et al. Prediction of left ventricular function after drug-eluting stent implantation for chronic total coronary occlusions. J. Am. Coll. Cardiol. 47, 721–725 (2006).
- Erdogan E, Akkaya M, Bacaksiz A et al. Early assessment of percutaneous coronary interventions for chronic total occlusions analyzed by novel echocardiographic techniques. Clinics (Sao Paulo) 68(10), 1333–1337 (2013).
- Roifman I, Paul GA, Zia MI et al. The effect of percutaneous coronary intervention of chronically totally occluded coronary arteries on left ventricular global and regional systolic function. Can. J. Cardiol. 29(11), 1436–1442 (2013).
- Yue WW, Huangfu FT, Yin J, Wang T, Wang GF, Jia RY. Assessment of recanalization of chronic total occlusions on left ventricular function in patients with or without previous myocardial infarction by real-time threedimensional echocardiography. Cell .Biochem. Biophys. 62(1), 83–86 (2012).
- Fettser DV, Batyraliev TA, Niiazova-Karben ZA, Sercelik A, Abdramanov KA, Belenkov IuN. Clinical and angiographical factors influencing improvement of left ventricular function after implantation of bare-metal stents in patients with chronic total occlusions of coronary arteries. Kardiologia 51(2), 52–58 (2011).
- Ermis C, Boz A, Tholakanahalli V et al. Assessment of percutaneous coronary intervention on regional and global left ventricular function in patients with chronic total occlusions. Can. J. Cardiol. 21, 275–280 (2005).
• Highlights contemporary issues with chronic total occlusion (CTO)-percutaneous coronary intervention (PCI) in the USA.
• Illustrates how successful CTO-PCI improves quality of life.
• Clinical characteristics associated with higher mortality in non-revascularized CTO.
• Cost–effectiveness of CTO-PCI.
• How the use of the hybrid algorithm improves CTO-PCI success in the real world.
• Illustrates the incidence of failure or non-arterial coronary artery bypass grafts.
• The use of the hybrid algorithm in CTO-PCI.
• Up-to-date meta-analysis on survival of successful CTO-PCI versus failed CTO-PCI.