Review Article - Interventional Cardiology (2011) Volume 3, Issue 6
Clinical advances in imaging: how useful is computed tomography for guiding and evaluating cardiac interventions
- Corresponding Author:
- Gabija Pundziute
Department of Cardiology, Thorax Center,
University Medical Center Groningen,
Hanzeplein 1, PO Box 30001, 9700 RB Groningen, The Netherlands
Tel: +31 503 612 355
Fax: +31 503 614 391
E-mail: g.pundziute@umcg.nl
Abstract
Keywords
aortic valve,cardiac intervention,computed tomography,coronary artery disease,electrophysiology,mitral valve
Following its introduction in the late 1990s and during the subsequent decade, multislice computed tomography (MSCT) has rapidly evolved as a modality allowing imaging of the heart. 3D imaging of the entire heart during a single breath hold is now possible. In particular, the potential of MSCT to visualize the coronary artery lumen and wall in a noninvasive manner has gained tremendous interest. Indeed, MSCT coronary angiography may be performed to assess coronary artery stenosis in symptomatic patients with suspected coronary artery disease (CAD) [1,2]. Moreover, MSCT coronary angiography may potentially be useful for guiding coronary interventions and in the evaluation of the results of treatment [[1,2]. In addition, 3D anatomical information obtained during the examination may be clinically useful in guiding interv-entions of the cardiac valves or treating rhythm disorders [1,2].
In this review we provide an overview of the current applications of MSCT, discussing the areas in which MSCT may replace invasive imaging and areas in which MSCT may be useful in guiding cardiac interventions.
Principles of imaging of the heart with MSCT & safety issues MSCT scanners consist of an x-ray
MSCT scanners consist of an x-ray source and detectors mounted on opposite sides of a gantry that continuously rotates around the patient. The scans are performed as the patient moves through the gantry. Computer systems can process these data to generate 3D volumetric information, which in turn can be viewed from multiple different projections on workstation monitors.
The most important parameters related to computed tomography (CT) image quality are the ability to depict differences between tissues, that is, spatial resolution as well as temporal resolution, and volume coverage during a single gantry rotation. The temporal resolution is essentially determined by the rotation speed of the gantry, the number of x-ray sources (single vs dual source MSCT) and the number of heart cycles used in a reconstruction. Indeed, the development of dual source MSCT, incorporating two x-ray tubes mounted at approximately 90°, allows images to be obtained during one fourth of a gantry rotation. Since the introduction of MSCT, the number of detector rows has grown from four to as many as 320, allowing the coverage of 16 cm in a single gantry rotation. A reduction in slice thickness, increase in gantry rotation speed and an increased number of detector rows were paralleled by a significantly improved image quality. With a spatial and temporal resolution of 0.4–0.6 mm and 83–175 ms, respectively, 64-slice MSCT scanners are currently considered a minimum prerequisite for noninvasive imaging of coronary arteries [3].
The beating heart can be scanned in two ways [4]. The first method is ECG-triggered prospective axial sequential scanning, whereby acquisition is triggered by the ECG signal in a predefined phase of the cardiac cycle. After individual axial image acquisition, the table moves (along the z-axis) to the next position for the next scan, which is repeated until the entire heart is scanned. The second method involves scanning with an application of an ECG-gated retrospective spiral mode with continuous data acquisition while the patient moves through the gantry, until the entire heart is covered.
Currently, invasive coronary angiography (ICA) is the gold standard technique for the detection of CAD. However, MSCT may serve as an alternative in certain patient populations, particularly due to its noninvasive approach (associated with a lower risk of procedural complications) and lower costs. Nevertheless, it is important to realize that the spatial and temporal resolution of invasive angiogram is superior compared with MSCT (0.2 mm and 20 ms, respectively). Accordingly, in order to acquire accurate results, it is important to understand which factors may influence the image quality of MSCT and how the drawbacks of MSCT may be overcome. This is particularly important for the visualization of the small, fast moving coronary arteries. A regular cardiac heart rhythm and a slow heart rate are prerequisites to achieve an appropriate image quality. A heart rate below 65 beats per minute (and even below 60 beats per minute with the new flash CT systems) is required, which is often achieved with the administration of b-blockers. Moreover, MSCT may be rejected in obese patients, as diagnostic image quality may be unacceptable due to high amounts of image noise. In addition, extensive calcifications may negatively influence the diagnostic accuracy of MSCT coronary angiography by increasing the chance of false-positive findings.
The disadvantages of MSCT include the necessity of iodinated intravenous contrast agent and radiation exposure [5]. Iodinated contrast agents may cause allergic reactions in a minority of patients (<1%) and are potentially associated with the development of renal dysfunction. Indeed, the administration of contrast exhibits a risk of significant contrast-induced nephropathy, although the risk is low even in patients with moderate-to-severe renal insufficiency [6]. Radiation exposure is of considerable concern with the growing use of imaging modalities involving ionizing radiation, since it may be associated with the development of malignancies. Consequently, enormous attention has been paid to dose-reduction measures. First, avoiding unnecessary MSCT studies is the most powerful strategy in reducing radiation exposure and MSCT should be used according to the appropriateness criteria [1]. Second,tube voltage determines the radiation exposure and adjusting the voltage depending on patient size is feasible. The most frequently applied tube voltage is 120 kV, however, adequate image quality may be achieved using 100 kV in non-obese adults [5,7,8]. Even further reduction of tube current to 80 kV may be feasible in patients with a body mass index ≤ 22.5 kg/ m2 [9]. Moreover, distinctive scan modes may have an important impact on radiation dose. Indeed, prospective ECG-triggered scanning may significantly reduce radiation exposure. Bischoff et al. observed a significant reduction of radiation dose, while maintaining image quality, when applying prospective as compared with helical scanning mode (3.6 vs 11.2 mSv; p < 0.001) [4]. Additionally, scan modes that cover the entire heart during a single heartbeat considerably reduce radiation dose. Novel 256- and 320-row CT systems allowing up to 16 cm z-axis coverage allow scanning of the entire heart during a single gantry rotation and, without loss of image quality, effective radiation doses of 1.7–2.1 mSv may be achieved [10,11]. High table speed through the gantry may be applied with the newest dual-source MSCT systems. These new-generation MSCT systems have an increased number of detectors and faster gantry rotation speed, allowing a temporal resolution of as low as 75 ms. Using spiral scanning mode with a high table speed, the entire heart may be scanned within one cardiac cycle, allowing radiation exposure in selected patients, even below 1 mSv [12]. Importantly, those values are lower than the values obtained with conventional ICA, which range between 2 and 15.8 mSv [13,14].
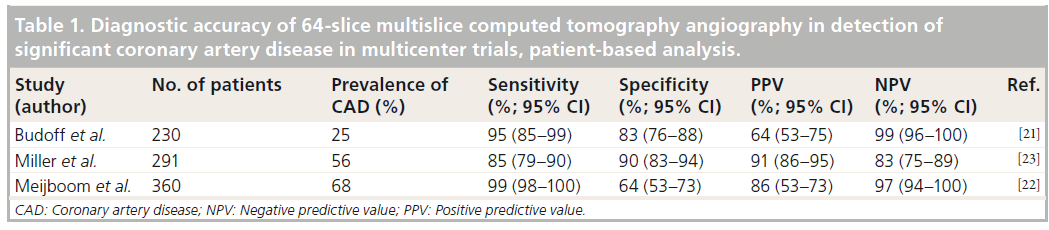
Areas in which MSCT can be superior to invasive imaging
▪ Evaluation of coronary artery stenosis
Currently, conventional ICA is considered the gold standard for the detection of coronary artery stenosis. Nevertheless, noninvasive MSCT has recently been proven a good alternative to ICA in certain patient populations. Several meta-analyses that evaluated the diagnostic performance of 64-slice MSCT and more recent scanners have been performed, demonstrating high per-patient sensitivity and specificity ranging from 97 to 100% and 82 to 91%, respectively [15–20]. Nevertheless, most studies were retrospective analyses in small samples of patients with high pretest likelihood of CAD, whereas the results were influenced by numerous biases. Accordingly, multicenter studies were performed to overcome the above limitations. In the recent multicenter trials aimed at identifying patients with coronary artery stenosis with 64-slice MSCT among individuals at low-tointermediate pretest likelihood for CAD and quantitative ICA serving as a standard of reference, MSCT was reported to have a sensitivity of 95–99% and a specificity of 64–83%, whereas the negative predictive value (NPV) was 97–99% (Table 1) [21,22]. In another recent multicenter, multivendor trial, the sensitivity and NPV of 64-slice MSCT angiography for detecting obstructive CAD were 85 and 83%, respectively (Table 1) [23]. Based on these data, it may be suggested that the diagnostic accuracy of MSCT is strongly influenced by the pretest likelihood of CAD, as also recently demonstrated by Meijboom et al. Indeed, in patients with varying degrees of pretest likelihood of CAD, the sensitivity, positive predictive value and NPV were 100, 75 and 100% for low, 84, 80 and 100% for intermediate and 98, 93 and 89% for high pretest likelihood of CAD, respectively [24]. Accordingly, MSCT angiography may be particularly suitable to exclude CAD in patients with low-to-intermediate pretest likelihood of CAD (Figure 1). As a result, with the application of MSCT coronary angiography in clinical practice, the number of diagnostic catheterizations associated with high costs and a certain risk of complications may be substantially decreased. MSCT coronary angiography may also be considered appropriate for the evaluation of patients who are unable to perform an exercise test or patients with equivocal test results. In a recent study including patients with inconclusive exercise tests, MSCT coronary angiography resulted in major cost savings, whereas the rate of ICA was reduced with no occurrence of adverse events [25]. Finally, the modality may also become an alternative to ICA in patients requiring coronary evaluation before noncoronary cardiac surgery or in patients with a new onset of heart failure of unknown etiology [1].
Table 1: Diagnostic accuracy of 64-slice multislice computed tomography angiography in detection of significant coronary artery disease in multicenter trials, patient-based analysis.
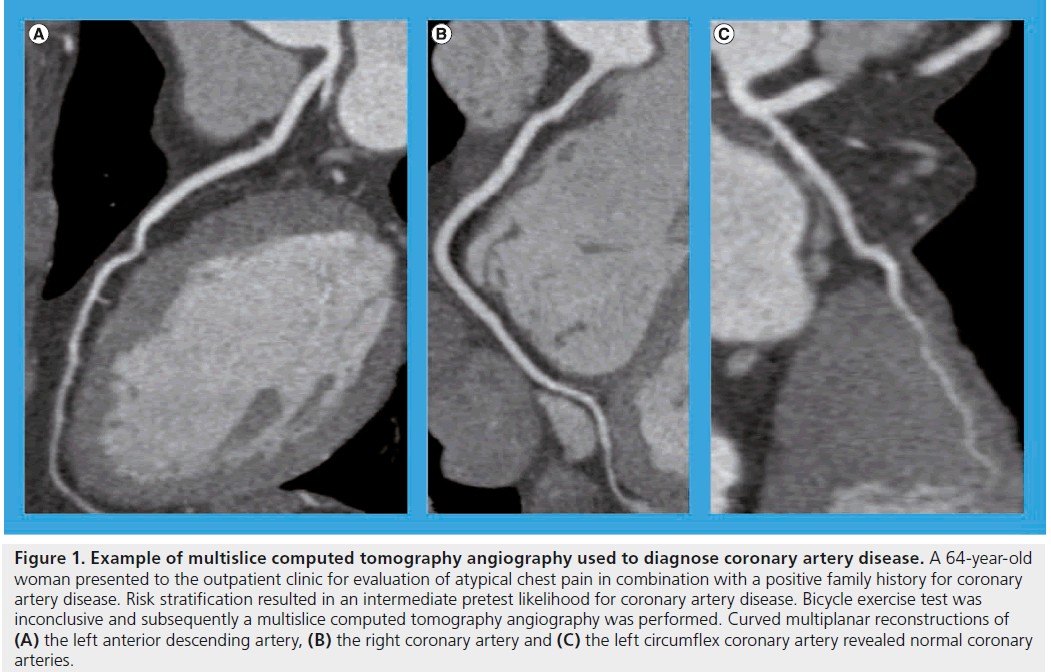
Figure 1: Example of multislice computed tomography angiography used to diagnose coronary artery disease. A 64-year-old woman presented to the outpatient clinic for evaluation of atypical chest pain in combination with a positive family history for coronary artery disease. Risk stratification resulted in an intermediate pretest likelihood for coronary artery disease. Bicycle exercise test was inconclusive and subsequently a multislice computed tomography angiography was performed. Curved multiplanar reconstructions of (A) the left anterior descending artery, (B) the right coronary artery and (C) the left circumflex coronary artery revealed normal coronary arteries.
The advantage of MSCT coronary angiography to rule out CAD is supported by prognostic studies performed in patients with suspected CAD. An important finding in these studies is that a normal MSCT coronary angiogram indicates an excellent prognosis. Indeed, in the meta-analyses including 7335 and 9592 patients with a median follow-up of 26.4 and 20 months, the annualized event rate (including death, myocardial infarction and revascularization) in patients with a normal MSCT coronary angiogram was as low as 1.1 [26] and 0.17%, respectively [27]. On the other hand, the presence of obstructive lesions is clearly associated with a worse prognosis. The presence of ≥1 significant coronary artery stenosis was associated with a tenfold higher risk for cardiovascular events [26]. Moreover, the risk of cardiovascular events may be increased with increasing severity of CAD.
▪ Clinical implications of evaluation of coronary artery stenosis in patients with stable CAD
The recent publication of the results of multicenter trials evaluating the outcomes in patients undergoing medical therapy compared with revascularization in nondiabetic and diabetic patients presenting with stable CAD, triggered a discussion on the role of medical therapy in the above patient population. In the COURAGE trial, 2287 patients with stable significant CAD and myocardial ischemia were included and randomized for an optimal medical therapy alone or in combination with percutaneous coronary intervention (PCI). Surprisingly, at 4.5 years, PCI did not reduce the risk of death, myocardial infarction or other cardiovascular event [28]. Similarly, in the BARI 2D trial, 2368 with Type 2 diabetes and stable CAD were stratified to undergo either prompt revascularization along with medical therapy or intensive medical therapy alone. At 5 years, no significant difference was observed in the rates of death and major cardiovascular events between patients undergoing revascularization and optimal medical therapy alone [29]. Even if the above studies have important limit-ations, the results of the studies may have implications also for the application of MSCT in clinical practice. In the COURAGE and the BARI 2D trials, the patient inclusion was based on the findings on ICA. Accordingly, the data may not be generalized to patients with lower risk coronary anatomy who do not undergo an initial ICA. As the reported diagnostic accuracy of MSCT coronary angiography to detect obstructive CAD is high, it is suggested that noninvasive MSCT may play a role in an initial evaluation of CAD in patients with stable angina in order to rule out three-vessel or left main disease. Indeed, based on the results of the COURAGE and BARI 2D trials, the need for intervention in patients with stable CAD may be decreased. Moreover, MSCT also allows detection of borderline obstructive or nonobstructive lesions, which may be considered for noninvasive management.
Nevertheless, several important issues need to be taken into consideration. First, due to the limited spatial resolution of MSCT compared with ICA, a number of false-positive findings may be observed on MSCT. Accordingly, an accurate assessment of severity of individual stenosis may be difficult in patients with extensive coronary artery calcifications, which is usually the case in patients with Type 2 diabetes. Second, high diagnostic accuracies of MSCT angiography have been reported in the studies performed in the centers of excellence. Nevertheless, one should realize that the diagnostic accuracy of MSCT coronary angiography may be substantially lower in the real world when it is performed in the centers with limited experience in performing and assessing MSCT coronary angiograms [30]. Future studies are clearly necessary to investigate this approach.
▪ Evaluation of patients with suspected acute coronary syndromes
The majority of studies evaluating the diagnostic accuracy of MSCT coronary angiography are performed in the patients presenting with chest pain, not in the emergency setting. Nevertheless, another possible application of MSCT is the diagnosis/exclusion of CAD in the clinical setting of suspected acute coronary syndromes (ACS). Indeed, up to 8% of patients with ACS are misdiagnosed and inappropriately discharged home [31]. A number of studies were performed with the aim of evaluating the effectiveness of MSCT in the early triage of patients with suspected ACS [32,33]. The ability of MSCT to exclude ACS after a normal MSCT scan or nonobstructive coronary lesions has been observed [33]. Moreover, Goldstein et al. compared two diagnostic strategies (standard of care vs MSCT coronary angiography), where the diagnostic efficiency, safety and cost-effectiveness of both strategies in patients presenting with low-risk ACS (no ECG changes and no elevation of cardiac enzymes) were evaluated [32]. The standard of care involved clinical observation, as well as serial testing with ECG and cardiac enzymes, followed by a myocardial perfusion scan with single photon emission CT (SPECT) for the evaluation of myocardial ischemia. The MSCT arm included initial MSCT, followed by ischemia testing with SPECT in the case of intermediate coronary lesions or nondiagnostic MSCT scans. The median diagnostic work-up duration in the standard of care arm was 15 h, compared with 3.4 h (p < 0.001) in the MSCT arm, resulting in significantly lower costs associated with MSCT arm (US$1872 vs 1586; p < 0.001). Nevertheless, this was at the expense of double or triple radiation exposure (MSCT, followed by SPECT and in some cases by ICA) in 24% of patients in the MSCT arm. Summarizing the above results, the experts consider MSCT coronary angiography as an appropriate modality for the diagnosis/exclusion of CAD in patients with low-risk ACS [1,34]. Nevertheless, as MSCT currently does not allow evaluation of hemodynamic significance of the lesions and because MSCT may be inconclusive, a proportion of patients with low probability of ACS may need additional testing. Moreover, performing MSCT coronary angiography in the setting of ACS may currently be logistically challenging in many centers. Future studies are necessary to explore different diagnostic protocols in the early triage of patients with ACS.
▪ Evaluation of coronary artery anomalies
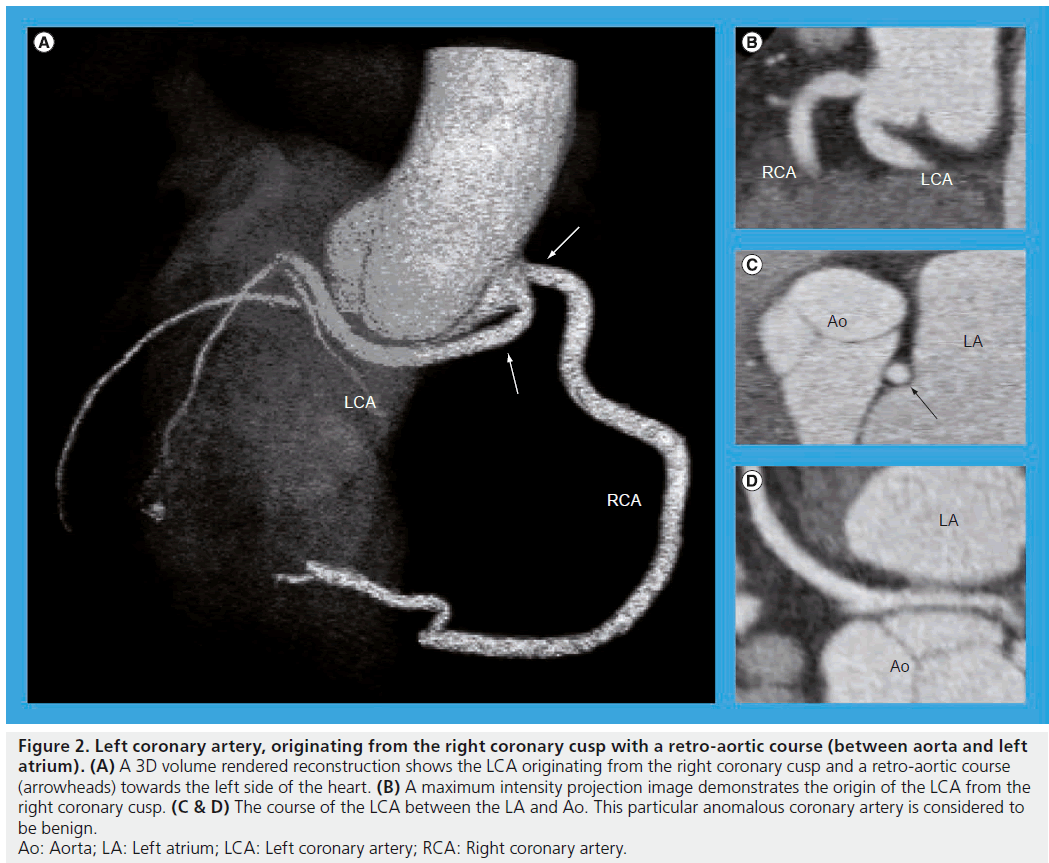
In a recent study, the prevalence of coronary anomalies with MSCT was 5.7% [35]. Coronary anomalies are reported to be correlated with sudden cardiac death, predominantly in young healthy individuals. Numerous studies have reported on the accuracy of MSCT to detect anomalous coronary arteries as compared with ICA. Indeed, in a study including 23 patients, the accuracy of MSCT to detect coronary artery anomalies was 100% [36]. In addition, in a series with 380 patients with coronary artery anomalies, 58 patients had MSCT performed after ICA. Among those patients, MSCT allowed a better assessment of the anatomic course and origin of the coronary arteries in eight cases (14%), whereas ICA failed to identify them in ten cases (17%) [37]. As ICA represents a 2D lumenogram of the coronary arteries, a reliable interpretation of the course of anomalous coronary arteries may be difficult. MSCT providing 3D information may be a more robust modality for the identification of malignant coronary anomalies requiring treatment (Figure 2).
Figure 2: Left coronary artery, originating from the right coronary cusp with a retro-aortic course (between aorta and left atrium). (A) A 3D volume rendered reconstruction shows the LCA originating from the right coronary cusp and a retro-aortic course (arrowheads) towards the left side of the heart. (B) A maximum intensity projection image demonstrates the origin of the LCA from the right coronary cusp. (C & D) The course of the LCA between the LA and Ao. This particular anomalous coronary artery is considered to be benign. Ao: Aorta; LA: Left atrium; LCA: Left coronary artery; RCA: Right coronary artery.
Although identification of anomalous coronary arteries with MSCT is possible, the clinical importance of the findings is less well established. Surgical correction of anomalous origin of coronary arteries is generally considered best for children and young adults. However, in a recent study, no positive impact on long-term survival could be demonstrated in older adults when surgical correction of the anomalous course of the aberrant coronary artery was performed compared with medical therapy [38]. The topic deserves further investigation.
Areas in which MSCT may assist in cardiac interventions
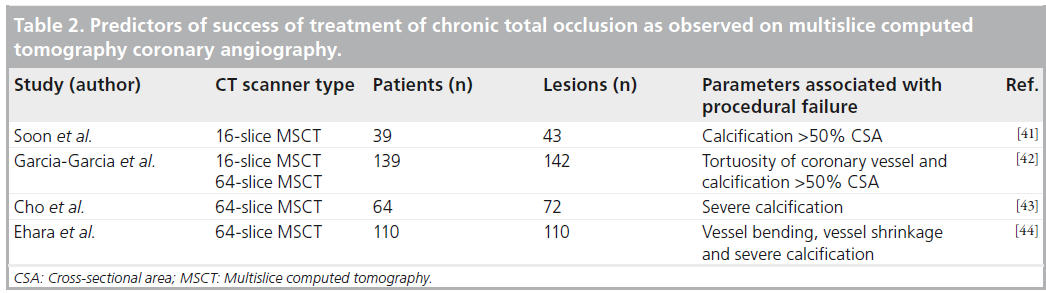
▪ Evaluation of chronic total occlusion
Chronic total occlusions (CTO) are common in patients referred for ICA [39]. Successful recanalization of a CTO is associated with substantial improvement in symptoms, physical limitation and quality of life [40]. Nevertheless, recanalization of a CTO remains challenging because of the inability to visualize the vessel lumen if the occluded segment is long and tortuous. Accordingly, the success rates in treating CTO still range between 55 and 85% [39].
Since MSCT allows visualization of not only the coronary artery lumen but also composition of the vessel wall, it may provide complementary information that could be helpful in planning the recanalization procedure and thereby increase the procedural success. CTOs appear as regions of the coronary artery that are not filled with intraluminal contrast. The distal part of the occluded vessel is filled with contrast through collaterals and is less intensively opacified. Calcifications can be clearly visible as bright structures along the occluded vessel (Figure 3). The factors associated with failure of recanalization, as identified with ICA, include occlusion length of ≥20 mm, severe calcification, blunt stump, tortuosity of occluded vessel, presence of bridging collaterals, as well as side branch at occlusion site. Several rather small studies have attempted to investigate the value of MSCT in predicting the outcomes of recanali-zation of CTO [41–44]. The findings on MSCT associated with procedural failure were various parameters of lesion calcification, tortuosity or bending of coronary vessel, as well as shrinkage of the target vessel (Table 2). Accordingly, it seems that MSCT currently does not provide information complementary to ICA that might be useful in planning recanalization. Moreover, due to superior spatial resolution, some parameters are even better visible on ICA (bridging collaterals or a side-branch at the entry point of occlusion). Keeping in mind still substantial radiation dose of current 64-slice MSCT systems (applied to patients who will subsequently undergo a high radiation exposure recanalization procedure), the need to administer nephrotoxic contrast and the lack of evidence that MSCT improves the outcomes, its application in planning of recanalization of CTO remains questionable. Additionally, MSCT may be useful in coregistration of images while performing recanalization procedures with magnetic navigation. Nevertheless, the value of MSCT in the above procedures needs to be demonstrated [45].
Figure 3: Computed tomography angiography in a 20-year-old male with known Kawasaki disease. Multislice computed tomography was performed for follow-up of known dilated coronary arteries. (A) Multiplanar reconstruction of the LAD shows a dilated proximal part of the coronary artery with a totally occluded segment beyond a calcified plaque. (B) Distally the coronary artery is small in diameter. Invasive coronary angiography shows an aneurysmatically dilated LAD with a chronic total occlusion at the site where the first diagonal branch splits from the LAD. (C) Extensive collaterals (arrowheads) from the right coronary artery allow filling the distal LAD with contrast. D1: First diagonal branch; LAD: Left anterior descending coronary artery; RCA: Right coronary artery.
▪ Evaluation of the aortic valve in transcatheter valve replacement
Transcatheter aortic valve implantation (TAVI) is a novel method to treat symptomatic severe aortic stenosis in patients with high surgical risk [46]. Although the results of the procedure are encouraging, several issues remain a concern [47]. A mild aortic regurgitation is observed in 50% of patients, whereas 13–18% of patients develop a moderate regurgitation [46,48]. Moreover, vascular complications remain a safety concern. Accordingly, before the aortic valve implantation, extensive planning of the procedure is mandatory, in order to increase the chance of success. Currently two devices are used, namely the self-expandable CoreValve® revalving system (Medtronic, MN, USA) and the balloon expandable Edwards Sapien valve (Edwards Lifesciences, CA, USA). Proper device sizing and expansion, as well as vascular access, are of particular importance in the application of both devices.
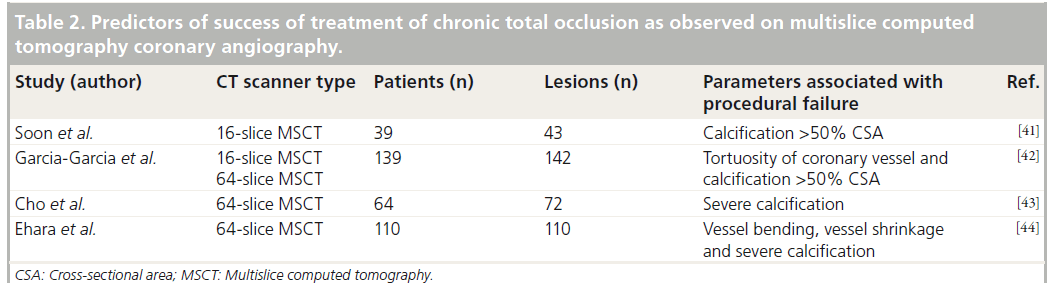
The assessment of the aortic valve, aorta and the peripheral vessels is usually performed using 2D transthoracic and transoesophageal echocardiography and angiography. Accurate measurement of the aortic annulus is one of the key steps in selecting the correct device. Indeed, an undersized device may lead to suboptimal expansion and paravalvular regurgitation, whereas an oversized device may increase the risk of tissue rupture. However, the measurements with 2D echocardiography are limited since they are based on single annular plane, assuming the aortic annulus is a circle. Nevertheless, using 3D imaging modalities the annulus appeared to be a complex oval-shaped structure (Figure 4) [49]. Indeed, Messika-Zeitoun et al. evaluated 45 patients referred for TAVI; despite good agreement of the sagittal diameter of the aortic annulus between transthoracic and transesophageal echocardiography (the mean difference between the measurements was 0.6 ± 0.8 mm; p = NS), the difference between MSCT and transthoracic (1.22 ± 1.3 mm; p = 0.03) or transesophageal echocardiography was larger (1.52 ± 1.1 mm; p < 0.001) [50]. Moreover, Schultz et al. suggested that device selection could be more accurate if a mean diameter of an oval-shaped aortic annulus (a mean of sagittal and coronal diameters, as observed on 3D examination) was used instead of making a decision based on a single diameter measurement (Figure 4) [51]. In addition, the measurement demonstrated a good reproducibility [52].
Figure 4: Assessment of the aortic valve annulus by multislice computed tomography. Oblique transverse view allows assessment of the degree of calcification of (A) the aortic valve (which in this case is functionally bicuspid) and (B) the oval-shaped annulus size. The size of the aortic valve annulus is measured in the (C) coronal and (D) sagittal views, providing an estimation of the eccentricity of the aortic valve annulus. Ao: Aorta; LA: Left atrium; LV: Left ventricle.
Moreover, MSCT allows assessment of the peripheral arteries and thoracic aorta and may help identify patients with unfavorable anatomy, such as small lumen diameter, tortuosity and extensive atherosclerosis. Indeed, Kurra et al. observed that 35% of patients referred for TAVI had unfavorable atherosclerotic iliofemoral disease, the majority of these patients having a luminal narrowing of <8 mm in the iliofemoral arteries [56].
Finally, after TAVI, MSCT may be a valuable and complementary tool to evaluate the procedural results (deployment and location of the prosthesis), in order to understand the underlying mechanisms of postprocedural aortic regurgitation.
Currently, a novel modality to obtain 3D information in the cardiac interventional laboratory is emerging. The C-arm in the interventional laboratory can be used as a CT scanner. Accordingly, the same information, which is obtained on preprocedural MSCT, can be obtained intra-operatively. This includes the characterization of the aortic valve calcification, the assessment of the relationship between the coronary ostia and the aortic leaflets and the information necessary for the valve sizing and planned localization of the implant. Moreover, the application of the C-arm CT may enable the coregistration of the reconstructed image with the C-arm and fluoroscopy, such that the image rotates with it during positioning, providing the operator an additional information to improve placement of the aortic valve [57].
Additional studies are necessary to establish a reference method for preprocedural evaluation of the patients referred for TAVI and to demonstrate whether the risks associated with MSCT (radiation exposure and administration of nephrotoxic contrast) are justified by a better outcome (lower postprocedural aortic regurgitation rate and fewer vascular complications).
▪ Evaluation of the mitral valve in percutaneous procedures
Despite recent advances in treatment strategies, surgery is denied in 49% of high-risk patients with severe mitral regurgitation [58]. Consequently, transcatheter and minimally invasive surgical techniques have been developed as an alternative approach. Currently, clinical data are available on the use of a MitraClip® device (Abbott Vascular, CA, USA) for leaflet repair, as well as on Carillon® (Cardiac Dimensions Inc., WA, USA) and Monarc™ (Edwards Lifesciences, CA, USA) devices for coronary sinus annuloplasty. Accordingly, the broadening options for mitral valve repair have increased the demands on the reliability of morphologic assessment of the mitral valve. Echocardiography is currently the most widely used imaging modality in therapy decision making. However, 3D imaging modalities such as MSCT may be useful in the assessment of anatomy and geometry of the mitral valve. Indeed, MSCT could clearly have an additional value in the assessment of the coronary sinus and its relationship with the coronary arteries and the mitral valve annulus before percutaneous mitral valve annuloplasty. Tops et al. demonstrated the course of the circumflex coronary artery between the coronary sinus and the mitral valve annulus in as many as 68% of patients (which might result in acute ischemia during the procedure) [59]. Moreover, coronary sinus coursed superiorly to the mitral annulus in the majority of patients. The feas-ibility of a comprehensive mitral valve evaluation with MSCT was nicely demonstrated in a study by Delgado et al. [60]. Additionally, Feuchtner et al. demonstrated in 112 patients a sensitivity of 96% and a specificity of 93% in diagnosing mitral valve prolapse with MSCT as compared with 2D echocardiography [61]. Nevertheless, echocardiography remains the most important tool for the evaluation of patients referred for mitral valve intervention, whereas MSCT may be a reserve option in case the echocardiography fails to provide insight into the mechanisms of mitral valve disease.
▪ Evaluation before catheter ablation of atrial fibrillation
In patients with atrial fibrillation (AF) who remain symptomatic under optimal medical therapy, catheter ablation is an effective treatment method [62]. MSCT may play a role in patient selection during the catheter ablation procedure, as well as in the evaluation of the procedural complications.
MSCT may be used in the assessment of the left atrial size as well as the presence of thrombus. Indeed, MSCT is a reliable method to measure the left atrial volume [63]. Nevertheless, due to radiation exposure, the need for contrast agent with MSCT and good results available with echocardiography, it is not routinely performed for the assessment of the left atrial size.
Moreover, thrombus in the left atrium or left atrium appendage has to be excluded before ablation. In a study by Dorenkamp et al. the diagnostic accuracy of thrombus detection by MSCT was assessed as compared with transesophageal echocardiography in 329 patients scheduled for pulmonary vein isolation. MSCT showed low sensitivity (29%) and a high specificity (98%) among patients with a 2.1% prevalence of thrombus of on echocardiography [64]. Accordingly,MSCT is an inappropriate modality for left atrial thrombus detection.
3D MSCT is a reliable modality in imaging of the pulmonary veins before the catheter ablation of AF as it allows superior accuracy of pulmonary vein assessment as compared with echocardiography [65]. In the majority of cases, four pulmonary veins are present. Nevertheless, anatomical variants have been described in pulmonary vein anatomy. A recent study showed a higher prevalence of a single pulmonary vein ostium on the left side in patients with AF compared with a control group (33.7 vs 19.9%; p = 0.004) [66]. In addition, ostial diameters of the veins were larger in case of AF.
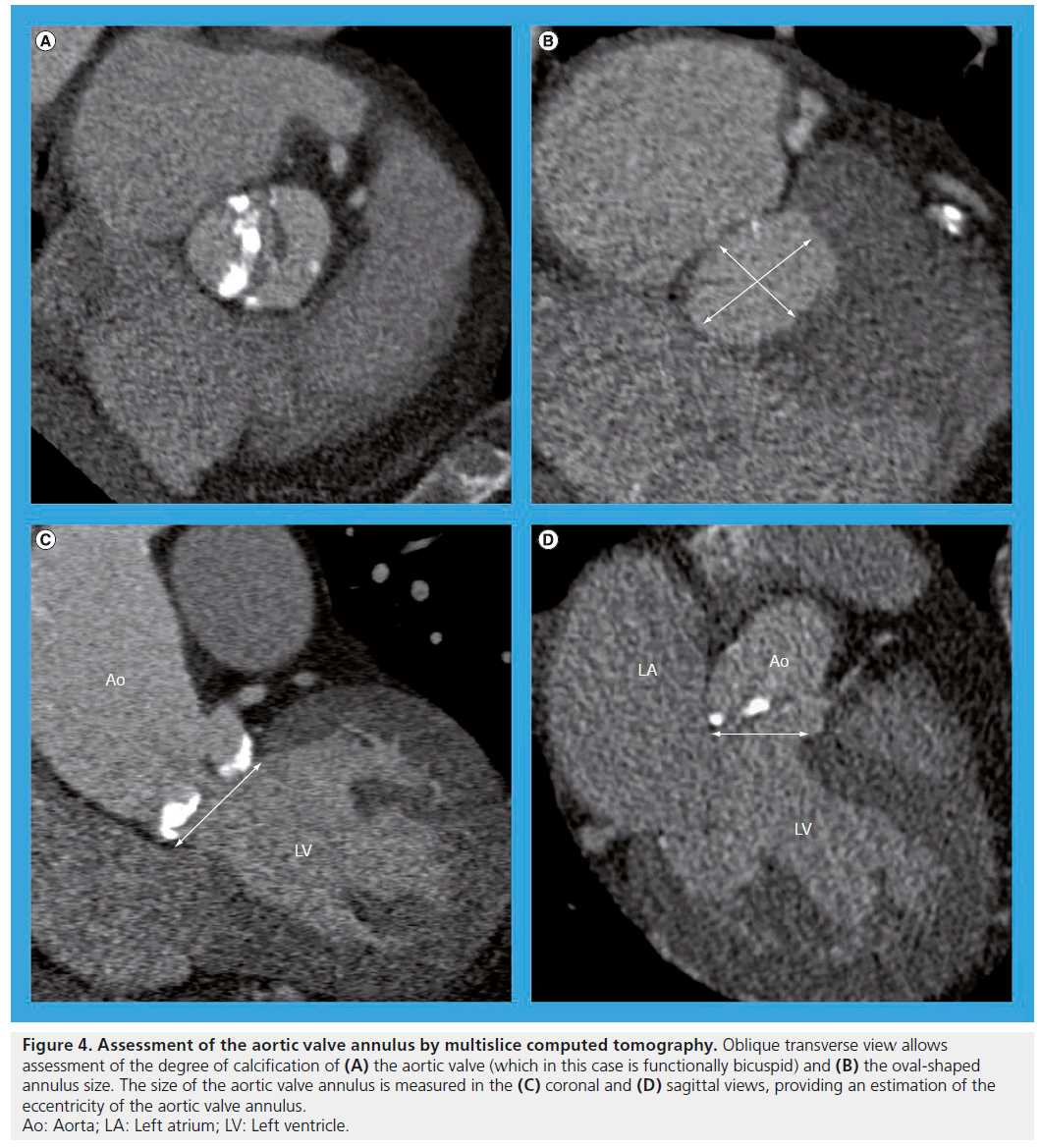
During the ablation procedure, fluoroscopy and electroanatomic mapping systems are used to identify sites for ablation. Integration of these modalities with MSCT has been introduced over recent years and data demonstrating an improvement of the procedural outcomes have become available. In a randomized study including 290 patients (145 patients with image integration and 145 with conventional mapping), the arrhythmia-free survival rate was significantly higher in image integration group compared with conventional mapping group (88 vs 69%; p = 0.017) (Figure 5) [67].
Figure 5: Posterior view of (A) multislice computed tomography reconstruction and (B) electro-anatomical mapping image of the left atrium. (C) Fusion of both images is helpful in anatomical orientation during ablation procedures. The white dotted points represent locations where electrical ablation was performed.
Finally, MSCT may help detect pulmonary vein stenosis after ablation. Indeed, pulmonary vein stenosis may occur in up to 10% of patients undergoing catheter ablation of AF [62].
▪ Evaluation in ventricular tachycardia ablation
Patients who suffer from appropriate implantable cardioverter defibrillator shocks for ventricular tachycardia, despite treatment with anti-arrhythmic medications, may be candidates for radiofrequency ablation of ventricular tachycardia. Endocardial voltage mapping is normally applied for this purpose and has a limited ability to detect intramyocardial or epicardial scar. Moreover, suboptimal catheter contact can result in false low-voltage measurements. Accordingly, integration of MSCT images into the mapping systems may facilitate the ablation of ventricular tachycardia. A recent study demonstrated the feasibility of integration of MSCT derived data into mapping system in guiding catheter ablation [68]. It has also been demonstrated that the fusion of MSCT images with real-time electroanatomic mapping data is accurate, and may enhance the safety of epicardial catheter ablation procedures of ventricular tachycardia by establishing the relationship between the catheter tip and coronary arteries [69]. However, keeping in mind the emerging role of magnetic resonance imaging, nuclear imaging and intracardiac echocardiography for this purpose, more studies are necessary before MSCT may have a role in ablation of ventricular arrhythmias.
Other applications of MSCT related to interventional cardiology
▪ Evaluation of coronary artery stents
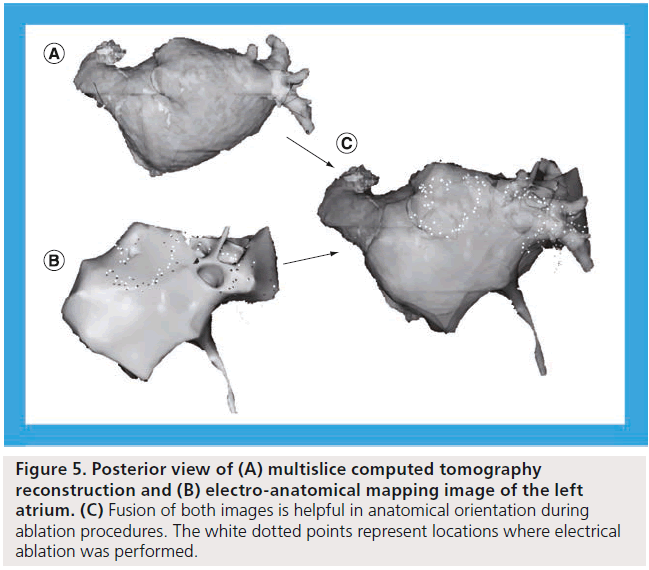
Detection of CAD in patients with a history of PCI may be clinically relevant. Over recent years, the diagnostic accuracy of MSCT for the detection of in-stent restenosis remained a topic of investigation. Even with improved image quality with 64-slice MSCT and dual-source MSCT as compared with previous scanner generations, visualization of the lumen of coronary artery stents remains a challenge. Carrabba et al. performed a meta-analysis of nine studies with 64-slice MSCT including 598 patients and 978 stents [70]. In total, an average of 91% of stents were interpretable (the uninterpretable stents ranging from 0 to 42%). In the stents with good image quality, the sensitivity to detect in-stent restenosis as compared with ICA was 86%, whereas a NPV was 97%. Nevertheless, the positive predictive value was limited to 70%, indicating that the degree of in-stent restenosis is frequently overestimated with MSCT. The stent diameter has been reported to be a major predictor of stent evaluability (Figure 6) [71]. Accordingly, based on the available data, the experts currently consider MSCT appropriate for follow-up of asymptomatic patients after left main coronary artery stenting if a stent diameter is ≥3 mm [1].
Figure 6: Coronary computed tomography angiography evaluation of coronary stents. Two patients, who were both evaluated for atypical complaints and already had a history of percutaneous coronary intervention. (A) A left anterior descending coronary artery with two stents of a diameter of 2.75 mm. It is difficult to assess the presence of any stenosis within the stents; however, there is adequate run-off of contrast medium distal to the stents. (B) Three stents are visible in the left anterior descending coronary artery, two proximal stents of a diameter of 3 mm and a distal stent of a diameter of 2.75 mm. Some growth of neo-intima (arrows) can be assessed in both stents of 3 mm diameter.
▪ Evaluation of coronary artery plaques
It is recognized that the morphology of coronary plaques plays an important role in the development of ACS [72]. The possibility of characterizing coronary plaques with MSCT and investigating whether there is a correlation between certain findings and the development of coronary events is clinically relevant.
Coronary atherosclerosis may be assessed with MSCT in two ways. Scans performed without contrast injection enable quantification of calcified component of coronary plaques (Figure 7). Calculation of Agatston score is the most widely accepted method for this purpose. Calcium scoring was performed in large patient populations and in different clinical settings ranging from asymptomatic patients to patients presenting with ACS. In general, coronary calcifications occur almost solely as a result of atherosclerosis, and the amount of calcification correlates approximately to the extent of atherosclerotic burden [73]. Currently, data from large studies and with long follow-up are available on the value of calcium scoring in risk stratification of asymptomatic individuals. Indeed, a 6.8‑year follow-up of 25,253 asymptomatic individuals by Budoff et al. demonstrated an increased risk for all-cause mortality with the increased calcium scores [74]. Nevertheless, the correlation between individual lesion severity on ICA and the amount of coronary calcium is weak [73]. Moreover, the absence of coronary calcium does not rule out CAD in symptomatic patients, particularly when young and presenting with acute symptoms [75].
Figure 7: Visualization of coronary atherosclerotic plaques by computed tomography. (A) Visualization of coronary calcifications by means of contrast-free scan for coronary calcium scoring, demonstrating extensive calcification in the left anterior descending coronary artery and the first diagonal branch. (B–D) Curved multiplanar reconstructions of different types of coronary plaques, indicated by arrow and with cross-sectional images. (B) A calcified plaque in the circumflex coronary artery, (C) a mixed plaque in the left anterior descending coronary artery and, (D) a noncalcified coronary artery plaque in the left anterior descending coronary artery of a different patient.
Another way to visualize coronary plaques involves the use of MSCT coronary angiography, which (due to intravenous injection of iodinated contrast) enables characterization of calcified and noncalcified plaque. Coronary plaques may be classified into noncalcified, calcified and mixed (containing both calcified and noncalcified components) (Figure 7). A recent meta-analysis including 22 studies demonstrated excellent diagnostic accuracy of MSCT for the detection of coronary plaques, with a sensitivity of 90% (95% CI: 83–94%) and a specificity of 92% (95% CI: 90–93%) as compared with intravascular ultrasound [76]. However, if the plaques observed on MSCT angiograms are to be used for risk stratification and possibly going to be treated, detection of a plaque is not sufficient and a more detailed characterization of plaques is necessary.
The data are currently available on the prognostic value of coronary plaques observed on MSCT. In a study including 1059 symptomatic patients who underwent diagnostic MSCT coronary angiography, the presence of coronary plaques with positive remodeling and plaques with low attenuation were associated with the development of ACS at 27 months of followup [77]. Based on these preliminary observations,it may be concluded that coronary plaque imaging with MSCT may provide prognostic information. Nevertheless, further research is necessary to examine more detailed characterization of coronary plaque with MSCT before the information obtained on MSCT may be used for risk stratification or treatment purposes.
Future perspective
Non-invasive evaluation of CAD with MSCT will remain a topic of investigation in the near future, especially with new scanning techniques allowing further reduction of radiation dose. With a low radiation exposure with the scans, the characteristics of potentially vulnerable coronary plaques will be explored, since the information on coronary atherosclerosis obtained may potentially become a tool in patient risk stratification. Moreover, studies exploring prognostic value of MSCT with a long-term follow-up in various study populations are to be expected.
MSCT may provide additional information on anatomic relations of cardiac and adjacent structures in cardiac interventions, such as ablation in cardiac arrhythmias or procedures of cardiac valves. Prospective studies exploring the clinical advantages of use of MSCT need to further clarify the position of MSCT in the above procedures.
Importantly, appropriate use of MSCT which involves x-ray exposure will remain mandatory. Thereby optimal image quality with low radiation exposure will remain essential in cardiovascular applications of MSCT.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Principals of imaging the heart with multislice computed tomography & safety issues
▪▪ Appropriate use of multislice computed tomography (MSCT) in cardiovascular applications is the most powerful strategy in reducing radiation exposure.
▪▪ A regular heart rhythm and a slow heart rate are prerequisites for high image quality in MSCT scans, and in the application of low-dose imaging protocols.
Areas in which MSCT can be superior to invasive imaging
▪▪ MSCT coronary angiography may accurately exclude coronary artery disease in patients with low and intermediate pretest likelihood for coronary artery disease, and thereby may reduce the number of diagnostic catheterizations and associated costs.
Areas in which MSCT may assist in cardiac interventions
▪▪ MSCT may provide useful anatomical information for planning percutaneous procedures of the cardiac valves; it is currently unknown whether use of MSCT in planning of aortic valve procedures leads to better outcomes.
▪▪ Echocardiography remains the most important tool for planning the percutaneous mitral valve procedures.
▪▪ Fusion of multiple imaging modalities, including MSCT, improves outcomes of catheter ablation of atrial fibrillation.
Other applications of MSCT related to interventional cardiology
▪▪ The application of MSCT coronary angiography after revascularization procedures remains limited.
▪▪ Currently, noninvasive characterization of coronary artery plaques may not be used in prediction of acute coronary syndromes or for treatment of those plaques.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Taylor AJ, Cerqueira M, Hodgson JM et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/ NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J. Am. Coll. Cardiol. 56(22), 1864–1894 (2010).
- Schroeder S, Achenbach S, Bengel F et al. Cardiac computed tomography: indications, applications, limitations and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur.Heart J. 29(4), 531–556 (2008).
- Abbara S, Arbab-Zadeh A, Callister TQ et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J. Cardiovasc. Comput. Tomogr. 3(3), 190–204(2009).
- Bischoff B, Hein F, Meyer T et al. Comparison of sequential and helical scanning for radiation dose and image quality: results of the Prospective Multicenter Study on Radiation Dose Estimates of Cardiac CT Angiography (PROTECTION) I Study. Am. J. Roentgenol. 194(6), 1495–1499 (2010).
- Hausleiter J, Meyer T, Hermann F et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 301(5), 500–507 (2009).
- Thomsen HS, Morcos SK. Risk of contrast-medium-induced nephropathy in high-risk patients undergoing MDCT: a pooled analysis of two randomized trials. Eur. Radiol. 19(4), 891–897 (2009).
- Blankstein R, Bolen MA, Pale R et al. Use of 100 kV versus 120 kV in cardiac dual source computed tomography: effect on radiation dose and image quality. Int.Cardiovasc. Imaging 27(4), 579–586(2011).
- Hausleiter J, Martinoff S, Hadamitzky M et al. Image quality and radiation exposurewith a low tube voltage protocol for coronary CT angiography results of the PROTECTION II Trial. JACC Cardiovasc. Imaging 3(11), 1113–1123 (2010).
- Wang D, Hu XH, Zhang SZ et al. Image quality and dose performance of 80 kV low-dose scan protocol in high-pitch spiral coronary CT angiography: feasibility study. Int. J. Cardiovasc. Imaging doi:10.1007/s10554-011-9822-5 (2011) (Epub ahead of print).
- Kitagawa K, Lardo AC, Lima JA et al. Prospective ECG-gated 320 row detector computed tomography: implications for CT angiography and perfusion imaging. Int.Cardiovasc. Imaging 25(Suppl. 2),201–208 (2009).
- Zhang C, Zhang Z, Yan Z et al. 320-row CT coronary angiography: effect of 100-kV tube voltages on image quality, contrast volume, and radiation dose. Int. J. Cardiovasc. Imaging 27(7), 1059–1068 (2011).
- Achenbach S, Marwan M, Ropers D et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur.Heart J. 31(3), 340–346 (2010).
- Kaul P, Medvedev S, Hohmann SF et al. Ionizing radiation exposure to patients admitted with acute myocardial infarction in the United States. Circulation 122(21), 2160–2169 (2010).
- Mettler FA Jr, Huda W, Yoshizumi TT et al. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 248(1), 254–263 (2008).
- Abdulla J, Abildstrom SZ, Gotzsche O et al. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur. Heart J. 28(24), 3042–3050 (2007).
- Guo SL, Guo YM, Zhai YN et al. Diagnostic accuracy of first generation dual-source computed tomography in the assessment of coronary artery disease: a meta-analysis from 24 studies. Int. J. Cardiovasc. Imaging 27(6), 755–771 (2011).
- Mowatt G, Cook JA, Hillis GS et al. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart 94(11), 1386–1393 (2008).
- Sun Z, Lin C, Davidson R et al. Diagnostic value of 64-slice CT angiography in coronary artery disease: a systematic review. Eur.J. Radiol. 67(1), 78–84 (2008).
- Paech DC, Weston AR: A systematic review of the clinical effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of suspected coronary artery disease. BMC Cardiovasc. Disord. 11, 32 (2011).
- von Ballmoos MW, Haring B, Juillerat P et al. Meta-analysis: diagnostic performance of low-radiation-dose coronary computed tomography angiography. Ann. Intern. Med. 154(6), 413–420 (2011).
- Budoff MJ, Dowe D, Jollis JG et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 52(21), 1724–1732 (2008).
- Meijboom WB, Meijs MF, Schuijf JD et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J. Am. Coll. Cardiol. 52(25), 2135–2144(2008).
- Miller JM, Rochitte CE, Dewey M et al. Diagnostic performance of coronary angiography by 64-row CT. N. Engl. J. Med. 359(22), 2324–2336 (2008).
- Meijboom WB, van Mieghem CA, Mollet NR et al. 64-Slice Computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J. Am. Coll. Cardiol. 50(15), 1469–1475 (2007).
- Menon M, Lesser JR, Hara H et al. Multidetector CT coronary angiography for patient triage to invasive coronary angiography: performance and cost in ambulatory patients with equivocal or suspected inaccurate noninvasive stress tests. Catheter. Cardiovasc. Interv. 73(4), 497–502(2009).
- Bamberg F, Sommer WH, Hoffmann V et al. Meta-analysis and systematic review of the long-term predictive value of assessment of coronary atherosclerosis by contrast-enhanced coronary computed tomography angiography. J. Am. Coll. Cardiol. 57(24), 2426–2436(2011).
- Hulten EA, Carbonaro S, Petrillo SP et al. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 57(10), 1237–1247 (2011).
- Boden WE, O’Rourke RA, Teo KK et al. Optimal medical therapy with or without PCI for stable coronary disease. N. Engl. J. Med. 356(15), 1503–1516 (2007).
- Frye RL, August P, Brooks MM et al.A randomized trial of therapies for Type 2 diabetes and coronary artery disease. N. Engl. J. Med. 360(24), 2503–2515 (2009).
- Ovrehus KA, Munkholm H, Bottcher M et al. Coronary computed tomographicangiography in patients suspected of coronary artery disease: impact of observer experience on diagnostic performance and interobserver reproducibility. J. Cardiovasc. Comput. Tomogr. 4(3), 186–194 (2010).
- Mehta RH, Eagle KA. Missed diagnoses of acute coronary syndromes in the emergency room: continuing challenges. N. Engl.Med. 342(16), 1207–1210 (2000).
- Goldstein JA, Gallagher MJ, O’Neill WW et al. A randomized, controlled trial ofmulti-slice coronary computed tomography for evaluation of acute chest pain. J. Am. Coll. Cardiol. 49(8), 863–871 (2007).
- Hoffmann U, Bamberg F, Chae CU et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. Am. Coll. Cardiol. 53(18), 1642–1650(2009).
- Hamm CW, Bassand JP, Agewall S et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. doi:10.1093/eurheartj/ehr236 (2011) (Epub ahead of print).
- Bazzocchi G, Romagnoli A, Sperandio M et al. Evaluation with 64-slice CT of theprevalence of coronary artery variants and congenital anomalies: a retrospective study of 3236 patients. Radiol. Med. 116(5), 675–689 (2011).
- Kacmaz F, Ozbulbul NI, Alyan O et al. Imaging of coronary artery anomalies: the role of multidetector computed tomography. Coron. Artery Dis. 19(3), 203–209 (2008).
- Andreini D, Mushtaq S, Pontone G et al. Additional clinical role of 64-slice multidetector computed tomography in the evaluation of coronary artery variants and anomalies. Int. J. Cardiol. 145(2), 388–390 (2010).
- Krasuski RA, Magyar D, Hart S et al. Long-term outcome and impact of surgery on adults with coronary arteries originating from the opposite coronary cusp. Circulation 123(2), 154–162 (2011).
- Magro M, Schultz C, Simsek C et al. Computed tomography as a tool for percutaneous coronary intervention of chronic total occlusions. EuroIntervention (Suppl. 6)G, G123–G131 (2010).
- Grantham JA, Jones PG, Cannon L et al. Quantifying the early health status benefits of successful chronic total occlusion recanalization: results from the lowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ. Cardiovasc. Qual. Outcomes 3(3), 284–290(2010).
- Soon KH, Cox N, Wong A et al. CT coronary angiography predicts the outcome of percutaneous coronary intervention of chronic total occlusion. J. Interv. Cardiol. 20(5), 359–366 (2007).
- Garcia-Garcia HM, van Mieghem CA, Gonzalo N et al. Computed tomography in total coronary occlusions (CTTO registry): radiation exposure and predictors of successful percutaneous intervention. EuroIntervention 4(5), 607–616 (2009).
- Cho JR, Kim YJ, Ahn CM et al. Quantification of regional calcium burden in chronic total occlusion by 64-slice multi-detector computed tomography and procedural outcomes of percutaneous coronary intervention. Int.J. Cardiol. 145(1), 9–14 (2010).
- Ehara M, Terashima M, Kawai M et al. Impact of multislice computed tomography to estimate difficulty in wire crossing in percutaneous coronary intervention for chronic total occlusion. J. Invasive Cardiol. 21(11), 575–582 (2009).
- Ramcharitar S, van der Giessen WJ, van der Ent M et al. The feasibility and safety of applying the Magnetic Navigation System to manage chronically occluded vessels: a single centre experience. EuroIntervention 6(6), 711–716 (2011).
- Webb JG, Pasupati S, Humphries K et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 116(7), 755–763 (2007).
- Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363(17), 1597–1607 (2010).
- Grube E, Buellesfeld L, Mueller R et al. Progress and current status of percutaneous aortic valve replacement: results of three device generations of the CoreValve Revalving® system. Circ. Cardiovasc. Interv. 1(3), 167–175 (2008).
- Tops LF, Wood DA, Delgado V et al. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc. Imaging 1(3), 321–330 (2008).
- Messika-Zeitoun D, Serfaty JM, Brochet E et al. Multimodal assessment of the aorticannulus diameter: implications for transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 55(3), 186–194 (2010).
- Schultz CJ, Moelker AD, Tzikas A et al. Cardiac CT: necessary for precise sizing for transcatheter aortic implantation. EuroIntervention (Suppl. 6)G, G6–G13(2010).
- Tzikas A, Schultz CJ, Piazza N et al. Assessment of the aortic annulus by multislice computed tomography, contrast aortography, and trans-thoracic echocardiography in patients referred for transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv.
- John D, Buellesfeld L, Yuecel S et al. Correlation of device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc. Interv. 3(2), 233–243 (2010).
- Kurra V, Kapadia SR, Tuzcu EM et al. Preprocedural imaging of aortic root orientation and dimensions: comparison between X-ray angiographic planar imaging and 3-dimensional multidetector row computed tomography. JACC Cardiovasc. Interv. 3(1), 105–113 (2010).
- Gurvitch R, Wood DA, Leipsic J et al. Multislice computed tomography for prediction of optimal angiographic deployment projections during transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 3(11), 1157–1165 (2010).
- Kurra V, Schoenhagen P, Roselli EE et al. Prevalence of significant peripheral artery disease in patients evaluated for percutaneous aortic valve insertion: preprocedural assessment with multidetector computed tomography. J. Thorac. Cardiovasc. Surg.137(5), 1258–1264 (2009).
- Schwartz JG, Neubauer AM, Fagan TE et al. Potential role of three-dimensional rotational angiography and C-arm CT for valvular repair and implantation. Int. J. Cardiovasc. Imaging doi:10.1007/s10554-011-9839-9(2011) (Epub ahead of print).
- Mirabel M, Iung B, Baron G et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur. Heart J. 28(11), 1358–1365 (2007).
- Tops LF, Van de Veire NR, Schuijf JD et al. Noninvasive evaluation of coronary sinus anatomy and its relation to the mitral valve annulus: implications for percutaneous mitral annuloplasty. Circulation 115(11), 1426–1432 (2007).
- Delgado V, Tops LF, Schuijf JD et al. Assessment of mitral valve anatomy and geometry with multislice computed tomography. JACC Cardiovasc. Imaging 2(5), 556–565 (2009).
- Feuchtner GM, Alkadhi H, Karlo C et al. Cardiac CT angiography for the diagnosis of mitral valve prolapse: comparison with echocardiography1. Radiology 254(2), 374–383 (2010).
- Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: the task force for the Management of atrial fibrillation of the European Society of Cardiology (ESC). Europace 12(10), 1360–1420 (2010).
- Hof I, Arbab-Zadeh A, Dong J et al. Validation of a simplified method to determine left atrial volume by computed tomography in patients with atrial fibrillation. Am. J. Cardiol. 102(11), 1567–1570 (2008).
- Dorenkamp M, Sohns C, Vollmann D et al. Detection of left atrial thrombus during routine diagnostic work-up prior to pulmonary vein isolation for atrial fibrillation: Role of transesophageal echocardiography and multidetector computed tomography. Int. J. Cardiol. doi:10.1016/j.ijcard.2011.06.124(2011) (Epub ahead of print).
- To AC, Gabriel RS, Park M et al. Role of Transesophageal echocardiography compared with computed tomography in evaluation of pulmonary vein ablation for atrial fibrillation (ROTEA Study). J. Am. Soc. Echocardiogr.24(9), 1046–1055 (2011).
- Bittner A, Monnig G, Vagt AJ et al. Pulmonary vein variants predispose to atrial fibrillation: a case–control study using multislice contrast-enhanced computed tomography. Europace 13(10), 1394–1400 (2011).
- Della Bella P, Fassini G, Cireddu M et al. Image integration-guided catheter ablation of atrial fibrillation: a prospective randomized study. J. Cardiovasc. Electrophysiol. 20(3), 258–265 (2009).
- Tian J, Jeudy J, Smith MF et al. Three-dimensional contrast-enhanced multidetector CT for anatomic, dynamic, and perfusion characterization of abnormal myocardium to guide ventricular tachycardia ablations. Circ. Arrhythm. Electrophysiol. 3(5), 496–504(2010).
- Zeppenfeld K, Tops LF, Bax JJ et al.Images in cardiovascular medicine. Epicardial radiofrequency catheter ablation of ventricular tachycardia in the vicinity of coronary arteries is facilitated by fusion of 3-dimensional electroanatomical mapping with multislice computed tomography. Circulation 114(3), e51–e52 (2006).
- Carrabba N, Schuijf JD, de Graaf FR et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography for the detection of in-stent restenosis: a meta-analysis. J. Nucl. Cardiol. 17(3), 470–478 (2010).
- Pugliese F, Weustink AC, Van Mieghem C et al. Dual source coronary computedtomography angiography for detecting in-stent restenosis. Heart 94(7), 848–854 (2008).
- Kolodgie FD, Virmani R, Burke AP et al. Pathologic assessment of the vulnerable human coronary plaque. Heart 90(12), 1385–1391 (2004).
- Greenland P, Bonow RO, Brundage BH et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation clinical expert consensus task force (ACCF/AHA writing committee to update the 2000 expert consensus document on electron beam computed tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J. Am. Coll. Cardiol. 49(3), 378–402 (2007).
- Budoff MJ, Shaw LJ, Liu ST et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J. Am. Coll. Cardiol. 49(18), 1860–1870 (2007).
- Marwan M, Ropers D, Pflederer T et al. Clinical characteristics of patients with obstructive coronary lesions in the absence of coronary calcification: an evaluation by coronary CT angiography. Heart 95(13), 1056–1060 (2009).
- Voros S, Rinehart S, Qian Z et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc. Imaging 4(5), 537–548(2011).
- Motoyama S, Sarai M, Harigaya H et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J. Am. Coll. Cardiol. 54(1), 49–57 (2009).
▪▪ Appropriate use criteria for cardiac computed tomography supported by multiple professional societies.
▪▪ Guidelines on performing coronary computed tomographic angiography.
▪ Study representing the possibility of coronary artery imaging with multislice computed tomography (MSCT) with very low radiation doses.
▪ Multicenter study on the diagnostic accuracy of 64-slice MSCT angiography.
▪ Multicenter study on the diagnostic accuracy of 64-slice MSCT angiography.
▪ Summarizes prognostic value of state-of-the-art MSCT.
▪▪ Study on noninvasive evaluation of the aortic valve annulus with MSCT prior to transcatheter aortic valve replacement.
▪ Prognostic study evaluating MSCT characteristics associated with development of acute coronary syndromes.