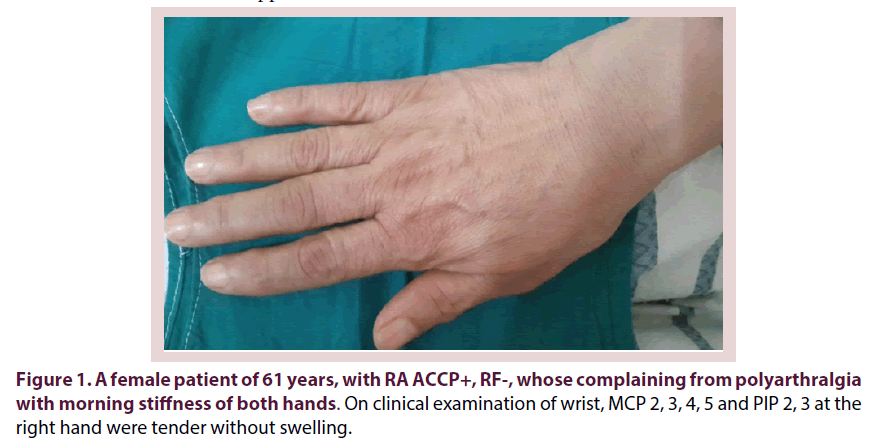
Research Article - International Journal of Clinical Rheumatology (2017) Volume 12, Issue 3
Clinical and ultrasound concordance in the detection of synovitis in rheumatoid arthritis: a transversal study about 50 patients
- *Corresponding Author:
- Safaa Belghali
IBN EL JAZZAR Medical School
Farhat Hached Hospital
4002 Sousse, Tunisia
E-mail: Safaa.belghali@yahoo.fr
Abstract
Objectives: Assessing the clinical ultrasound concordance in the detection of hands and wrists synovitis and determining the factors associated with such a concordance. Patients and methods: Single centre cross-sectional study related to 50 patients with Rheumatoid Arthritis (RA), included consecutively over a period of 21 months. The concordance between the clinical synovitis and the ultrasound one was assessed by calculating Cohen (k) coefficient. A correlation study between the concordance percentage at the patient scale with the clinical and biological parameters was conducted. Results: The concordance between the clinical examination and ultrasound in the detection of synovitis was too weak. The kappa coefficient varied from 0, 03 to 0, and 16. Likewise, the concordance between joint pain and ultrasound synovitis was overall at a low level (kappa between -0, 005 to 0, and 31) as well as the one between clinical signs (pain and/ or swelling) as well as ultrasound ones (synovial hypertrophy, effusion, Doppler signal) together, kappa coefficient was between 0, 03 and 0, 28. We objectified statistically significant positive correlations between the average concordance percentage and the low disease activity (DAS28˂3, 2). Conclusion: Concordance between clinical examination and ultrasound in the synovitis detection was overall at a low level. These observations indicate the best ultrasound sensitivity. Disease activity was the major factor influencing such a concordance in the present study.
Keywords
rheumatoid arthritis, synovitis, clinical examination, hand ultrasound, concordance, associated factors
Introduction
Rheumatoid Arthritis (RA) displays rapid joint damage responsible for a major functional impairment. In order to avoid such a structural damage, the objectives of RA care evolved considerably aiming at the gain of a rapid as well as lasting clinical remission and the prevention of osteocartilaginous degradations [1]. Therefore, an early diagnosis together with a rigorous monitoring based on objective criteria is essential.
The clinical examination, of hands joints during RA, can prove to be tricky and often suffers from lack of sensitivity as well as objectivity. Some studies suggest that our examination is only able to detect less than half of the synovitis cases and often fail in the tenosynovitis detection [2-4]. Besides, patients within seeming clinic recovery can keep sub-clinical synovitis. Yet, the number of synovitis underestimation can be an obstacle to the treatment optimization. The control of the inflammation is then insufficient with a considerable risk of structural progression [5] and of recurrence in the short term [6-8]. A valid, accessible, reproducible and sensitive to changes examination is an undeniable need to help the practitioner in his therapeutic decisions.
The osteoarticuar ultrasound occupies for a long time a growing place in rheumatology [9-11]. Indeed, it allows the detection of synovitis and sub-clinical tenosynovitis, then displays, thanks to the power Doppler (DP), the synovial vascularization which is correlated to the histological synovial inflammation [12,13] and synonymous with the disease inflammatory activity.
We have established as an objective, during this study, to assess the concordance between the clinical examination and ultrasound in the detection of synovitis within a group of patients affected with RA and to look for the factors influencing such a concordance.
Patients and methods
Patients
This monocentric cross-sectional study, the first in Tunisian Centre, focused on patients affected with RA, meeting the criteria of 1987 Rheumatology American College [14].
The patients were included consecutively whatever the level of the illness activity. The non-inclusion criteria were manifested in the presence of severe or irreducible joint distortions compromising the completion of hands and wrists ultrasound, an osteoarthritis coexistence as well as hand surgery antecedent.
Methods
A rheumatologist was responsible, the same day for implementing the ultrasound and without having access to it, to collect the following data: age, sex, the duration of the illness evolution, the Patient’s Global Assessment (PGA) by using a 0-100 Visual Analog Scale (VAS), the Number of Painful Joints (NPJ) and the Number of Swollen Joints (NSJ) of hands and wrists.
For each joint, pain and swelling were rated using a 0-1 scale. The disease activity was assessed by the Disease Activity Score 28 (DA S28) [15] and the functional consequences were estimated by the Health Assessment Questionnaire (HAQ) [16].
The erythrocyte sedimentation rate (ESR) first thing, the C-reactive protein (CRP), the presence or lack of the rheumatoid factor (RF) and of anti-cyclic citrullinated peptides (anti CCP) and the treatment were identified.
The whole hands and wrists ultrasounds were undertaken by a single radiologist with a Philips IU22 ultrasound scanner equipped with multifrequency linear catheter (7.5 MHz).
The exploration was first carried out through dorsal stream then the palmar one. A total of 22 joints were explored for each patient: the wrists were studied through a cross-sectional and longitudinal section of the dorsal surface, wrist in a neutral position and probe centred on the third ray then through palmer scanning of the flexor tendons axial and longitudinal plane. As for the ultrasound data, were applied the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) group definitions [17,18].
Statistics
The data were analyzed using the Statistical Package for the Social Sciences (SPSS 18.0, Chicago, IL) software.
The concordance study between clinical and ultrasound data was assessed by calculating the percentage of concordance and the kappa coefficient for each of the 22 articulations (joints), whether it is the right or left side. κ interpretation was the following:
• κ < 0 means a discordance.
• 0 < κ ≤ 0.20 means a very low concordance.
• 0.20 < κ ≤ 0.40 means a low concordance.
• 0.40 < κ ≤ 0.60 means a moderate concordance.
• 0.60 < κ ≤ 0.80 means a good concordance.
• 0.811 < κ ≤ 1.00 means a very good concordance.
The links between two quantitative variables were studied by the Pearson correlation coefficient «r» which varies from -1 (perfect negative correlation: the higher a variable is, the lower the other and vice-versa to +1 (perfect positive correlation: the higher a variable is, the higher the other) by way of zero: No correlation. The meaning corresponds to «p», the meaning threshold being fixed at 0.05.
Results
Clinical and ultrasound data
One thousand one hundred joints were studied for 50 patients included in the study. Our population general characteristics are summarized in Table 1. Within the 1100 joints, the NPJ was of 224 (average NPJ about 4.4 ± 4.1). The NSJ was about 309 (average NSJ about 6.1 ± 4.5). An ultrasound synovial hypertrophy was detected in 738 articulations with an ultrasound synovitis number average number of 14.7 ± 6.1. The overall distribution of the painful and swollen articulations detail as well as the ultrasound data according to the articulation are illustrated in Table 2.
| Sex | Women n (%) | 40 (80) |
|---|---|---|
| Men n (%) |
10 (20) | |
| Average age (years) | 51.3 ± 15 | |
| Average disease duration (years) | 5.5 ± 7.3 | |
| Average DAS28 DAS28 >3.2 n (%) |
4.4 ± 1.5 40 (80) |
|
| Average HAQ | 1.4 ± 0.8 | |
| Positive RF n (%) | 37 (74) | |
| Positive Anti CCP n (%) | 35 (70) | |
| Average ESR (accelerated SR%) | 24.4 ± 17.9 (52) | |
| CRP (High CRP %) | 19.9 ± 30.5 (43.3) | |
| Disease-modifying drug n (%) | cDMARDs | 24 (48) |
| bDMARDs | 11 (22) | |
| Corticosteroid therapy * n (%) | 40 (80) | |
n: Number; %: Percentage; DAS28: Disease Activity Score; HAQ: Health Assessment Questionnaire; Anti CCP: Anti-Cyclic Citrullinated Peptide; RF: Rheumatoid Factor; ESR: Erythrocyte Sedimentation Rate; CRP: C Reactive Protein; cDMARDs: Conventional Disease Modifying Antirheumatic Drug; bDMARDs: Biologic Disease Modifying Antirheumatic Drug; *8 patients received le methylprednisolone in the form of boli the week before the ultrasound realization
Table 1. The population study general characteristics.
| NPJ n(%) |
NSJ n(%) |
Ult S n(%) |
DP (+) n(%) |
|
|---|---|---|---|---|
| Total | 224 (20.3) | 309 (28) | 738 (67) | 39 (3.5) |
| Wrists | 54 (54) | 52 (52) | 61 (61) | 14 (14) |
| MCP | 110 (22) | 180 (36) | 325 (65) | 25 (5) |
| IPP | 50 (10) | 71 (14.2) | 352 (70.4) | - |
MCP: Metacarpophangeal Joint; IPP: Proximal Interphalangeal Joint; NPJ: Number of Painful Joints; NSJ: Number of Swollen Joints; Ult S: Ultrasound Synovitis; DP (+): Positive Power Doppler signal
Table 2. Global clinical and ultrasound data according to articulations.
The clinical ultrasound concordance
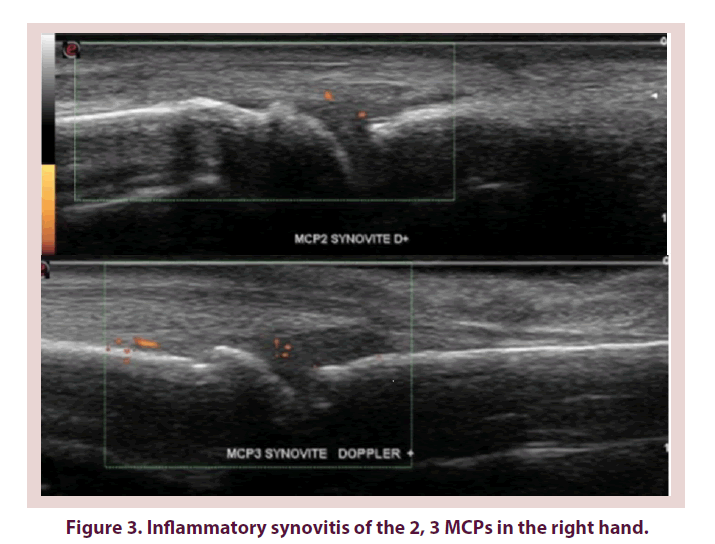
Concerning the concordance between the clinical (joint swelling) and ultrasound synovitis detection (synovial hypertrophy), kappa coefficient equaled 0, 13 at the level of wrists, it varied from 0, 06 to 1, 6 at the level of MCP joint then from 0, 03 to 0,12 at the level of IPPs (Figure 1).
The percentage of concordance was of 57% at the level of wrists, it varied from 56 to 70% at the level of MCP and from 32 to 55% at the level of IPPs. Table 3 details concordance between clinical and ultrasound synovitis.
| Articulation | C S | Ult S | Kappa | IC à 95% | Concordance in% |
95% IC | |
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| Wrists | Yes No |
35 26 |
17 22 |
0.13 | 0-0.32 | 57 | 46.7-66.7 |
| MCP1 | Yes No |
21 36 |
8 35 |
0.16 | 0-0.32 | 56 | 45.7-65.8 |
| MCP2 | Yes No |
67 28 |
2 3 |
0.08 | 0-0.23 | 70 | 59.8-78.5 |
| MCP3 | Yes No |
50 36 |
5 9 |
0.11 | 0-0.26 | 59 | 48.7-68.6 |
| MCP4 | Yes No |
12 43 |
3 42 |
0.14 | 0.01-0.26 | 54 | 43.7-63.9 |
| MCP5 | Yes No |
5 27 |
7 61 |
0.06 | 0-0.23 | 66 | 55.7-74.9 |
| IPP1 | Yes No |
9 43 |
2 46 |
0.12 | 0.01-0.24 | 55 | 44.7-64.8 |
| IPP2 | Yes No |
25 63 |
1 11 |
0.06 | 0-0.12 | 36 | 26.8-46.2 |
| IPP3 | Yes No |
19 62 |
3 16 |
0.03 | 0-0.17 | 35 | 25.9-45.2 |
| IPP4 | Yes No |
9 47 |
2 42 |
0.10 | 0-0.28 | 51 | 40.8-6.10 |
| IPP5 | Yes No |
7 68 |
0 25 |
0.05 | 0-0.17 | 32 | 23.2-42.1 |
MCP: Metacarpianphalangeal Joint; IPP: Proximal Interphalangeal Joint; CI: Confidence Interval; CS: Clinical Synovitis; Ult S: Ultrasound Synovitis
Table 3. Concordance between clinical and ultrasound synovitis.
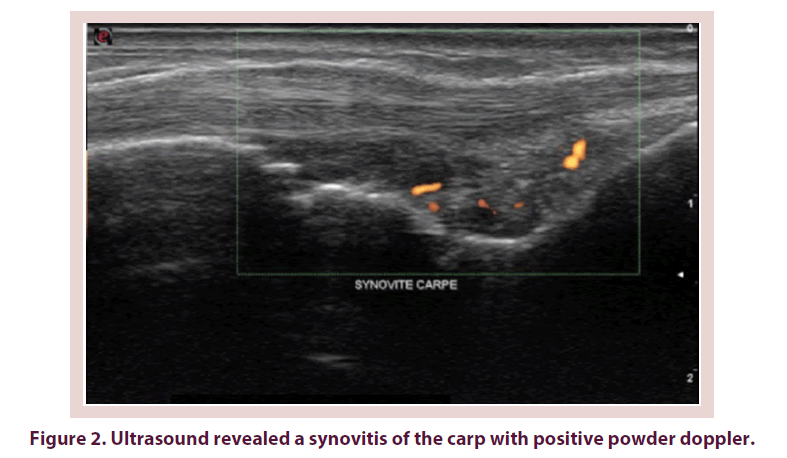
For the concordance between the joint pain and the ultrasound one, kappa coefficient and the concordance percentage were respectively 0, 12 and 57% at the level of wrists (Figure 2). Kappa coefficient varied from 0, 0A to 0.01 at the level of MCP and from -0.55 and 0.04 at the level of IPPs. The concordance percentage varied between 30 and 74% at the level of MCP and between 20 and 50% at the level of IPP. These concordance details are summed up in Table 4.
| Joint | JP | Ult S | Kappa | 95% CI | Concordance in % | 95% CI | |
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| Wrists | Yes No |
36 25 |
18 21 |
0.12 | 0-0.31 | 57 | 46.7-66.7 |
| MCP1 | Yes No |
11 46 |
8 35 |
0.06 | 0-0.14 | 46 | 36-56.2 |
| MCP2 | Yes No |
28 70 |
0 2 |
0.01 | 0-0.03 | 30 | 21.4-40.1 |
| MCP3 | Yes No |
29 57 |
3 11 |
0.04 | 0-0.12 | 40 | 30.4-50.3 |
| MCP4 | Yes Non |
13 42 |
2 43 |
0.17 | 0.05-0.3 | 56 | 45.7-65.8 |
| MCP5 | Yes No |
11 21 |
5 63 |
0.31 | 0.11-0.5 | 74 | 64.1-82 |
| IPP1 | Yes No |
5 47 |
3 45 |
0.03 | 0-0.13 | 50 | 39.9-60.1 |
| IPP2 | Yes No |
9 79 |
1 11 |
0.005 | 0-0.04 | 20 | 12.9-29.4 |
| IPP3 | Yes No |
11 70 |
5 14 |
-0.55 | - | 25 | 17.1-34.8 |
| IPP4 | Yes No |
8 48 |
5 39 |
0.02 | 0-0.14 | 47 | 37-57.2 |
| IPP5 | Yes No |
9 50 |
4 37 |
0.04 | 0-0.15 | 46 | 36-56.2 |
MCP: Metacarpianphalangeal joint; IPP: Proximal Interphalangeal Joint; CI: Confidence Interval; JP: Joint Pain; Ult S: Ultrasound Synovitis
Table 4. Concordance between joint pain and ultrasound synovitis.
Finally, concerning global concordance between the combined clinical and ultrasound signs, kappa coefficient equaled 0.13 and the concordance percentage equaled 62% at the level of wrists. At the level of MCP, kappa coefficient varied from 0.02 to 0.28 and the concordance percentage varied from 56 to 68%. At the level of IPP, kappa coefficient and concordance percentage varied respectively between 0.02 and 0.13 and from 31 to 64%. These global concordance data are illustrated in Table 5.
| Joint | CE | UB+DP | Kappa | 95% CI | Concordance in % | 95% CI | |
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| Wrists | Yes No |
44 18 |
22 16 |
0.13 | 0-0.33 | 62 | 49.7-69.5% |
| MCP1 | Yes No |
25 38 |
6 31 |
0.20 | 0.05-0.34 | 56 | 45.7-65.8 |
| MCP2 | Yes No |
67 31 |
1 1 |
0.02 | 0-0.11 | 68 | 57.8 –76.7 |
| MCP3 | Yes No |
53 36 |
4 7 |
0.10 | 0-0.24 | 60 | 49.7-695 |
| MCP4 | Yes No |
18 40 |
4 38 |
0.19 | 0.05-0.33 | 56 | 45.7-65.8 |
| MCP5 | Yes No |
17 29 |
5 42 |
0 .28 | 0.12-0.45 | 64 | 55.7-7.49 |
| IPP1 | Yes No |
10 43 |
2 45 |
0.13 | 0.02-0.25 | 54 | 44.7-64.8 |
| IPP2 | Yes No |
21 69 |
0 10 |
0.02 | 0.01-0.09 | 31 | 22.3-41.1 |
| IPP3 | Yes No |
20 65 |
3 13 |
0.03 | 0-0.08 | 33 | 23.8-42.8 |
| IPP4 | Yes No |
14 45 |
4 37 |
0.12 | 0-0.24 | 51 | 40.8-61 |
| IPP5 | Yes No |
9 68 |
0 23 |
0.05 | 0.01-0.1 | 32 | 23.2-42.1 |
MCP: Metacarpianphalangeal joint; IPP: Proximal Interphalangeal Joint; CI: Confidence Interval; CE: Clinical Examination (Joint Pain and/or Swelling); UB+DP: B-Mode Ultrasound and Power Doppler Signal (synovial hypertrophy and /or effusion et/ou doppler sign)
Table 5. Global concordance between joint pain and ultrasound synovitis.
Factors associated with the clinical-ultra-sound concordance
At the patient’s scale, the average concordance percentage between clinical and ultrasound in synovitis detection equaled 52.5% ± 19.2 (13, 6-81, 8%) (Figure 3). Correlations between this concordance percentage and the clinical parameters (age, evolution duration, DAS28, HAQ, EGP), as well as the biological parameters are illustrated in Table 6. Statistically significant correlations were objectified with the DAS28 and the EGP.
| Biological and clinical parameters | Concordance % | r | p |
|---|---|---|---|
| Age (year) <40 ≥40 et<60 ≥60 |
55.6 48.7 51.5 |
0.03 0.06 0.06 |
0.90 0.79 0.82 |
| Disease duration (year) < 2 ≥ 2 et<10 ≥10 |
52.1 47.2 60.3 |
0.23 0.51 -0.54 |
0.34 0.83 0.10 |
| DAS28 <3,2 ≥3,2 et<5,2 ≥5,2 |
59.3 77 53.8 |
0.68 -0.19 -0.094 |
0.03 0.39 0.71 |
| HAQ <1 ≥1 et<2 ≥2 |
55 47.4 56.3 |
-0.087 0.2 -0.43 |
0.72 0.39 0.18 |
| PGA <5 ≥5 |
53 50.1 |
0. 33 -0.04 |
0.09 0.84 |
| ESR (mm/h) <20 ≥20 |
54.2 49.8 |
-0.20 0.17 |
0.36 0.38 |
| CRP <6 ≥6 |
53.5 48.9 |
0.32 0.31 |
0.14 0.12 |
DAS28: Disease Activity Score; HAQ: Health Assessment Questionnaire; ESR: Erythrocyte Sedimentation Rate; CRP: Creactive protein; PGA: Patient’s global assessment.
Table 6. Correlations between the concordance percentage in the detection of synovitis and the clinical and biological data.
Discussion
Concordance between the clinical and the ultrasound synovitis
Concordance between the clinical and the ultrasound synovitis was globally very low. These results are lower than those of Le Boedec et al. [19] and Garrigues et al. [20] and comparable to those of Ceponis et al. [21] who studied 612 joints (wrists and MCP) in 51 patients with former PR.
Concordance between clinical and ultrasound synovitis was globally too low. These results were lower than those of Le Boedec et al. [19] and comparable to those of Ceponis et al. [21] who studied 612 joints (wrists and MCP) in 51 patients with former PR.
The concordance between joint swelling and synovial hypertrophy was very low with a kappa coefficient going from 0.06 to 0.17. Such a low concordance can be largely explained by the superiority of the ultrasound examination in the detection of synovitis and its complementary potential interest to the clinical examination for an objective assessment of the disease progression. Moreover, the confusion between synovitis and tenosynovitis (false synovitis) during the clinical examination also contributes to such a low concordance [2,21].
Concordance between joint pain and ultrasound synovitis
The concordance between joint pain and ultrasound synovitis is very low at the level of wrists, MCP and IPPs. These results are similar to the study of Ceponis al. [21] where concordance equaled 0.11 at the level of wrists and varied between 0.01 and 0.17 at the level of MCPs. This concordance was lower than that of the clinical synovitis. This can be explained by several factors. We quote the high frequency of degenerative phenomena in particular at the level of IPPs (it may be recalled that the average age in our study was 53) and joint destructions secondary to old-established PR responsible for pains having no ultrasound translation especially without synovial hypertrophy [19].
Furthermore, the fibromyalgia associated with PR can be a plausible cause for joint pain. That’s why some authors advise not to refer to DAS28 for the assessment of the disease activity in case of its association with fibromyalgia [22,23]. But rather to the Doppler ultrasound data [24,25] that reflect the PR real activity. Finally, some cold synovitis may ache.
Global concordance between clinical and ultrasound signs
The study of concordance between the reunited clinical parameters (pain and/or swelling) and the ultrasound ones (Doppler effusion, and/ hypertrophy and/or hyperemia) wasn’t able to improve the concordance coefficient kappa except for MCP5. This coefficient remained globally low or even very low. These results are lower than those of Szkudlarek et al. [3] and al who found a concordance percentage equaling between 70 and 78% at the level of MCPs and of between 77% and 87% at the level of IPP by making this global comparison.
How to explain the difference between the clinical and ultrasound in the detection of synovitis?
Factors relative to assessment means
The main factor that could explain the gap between clinical and ultrasound is the non-objective character of our clinical examination attested by the important inter-practitioners variability as well as its non-discriminating character between articular and peri articular lesions [26-28]. It is commonly accepted that the clinical examination depends essentially on the examiner experience, so that a standardized formation in the clinical examination practice may reduce the variation in the detection of painful joints.
However, its impact on the swollen joints remains uncertain [29]. In Ogasawara et al. [30] study, 108 patients (1944 joints) were examined by the same practitioner. Afterwards, he achieved by him an osteoarticuar ultrasound in order to compare the clinical and ultrasound noticing. The concordance between the two was assessed at both the beginning and the end of the study. The final results were in favour of the improving of the concordance coefficient and the detection sensitivity of synovitis (40%) to the detriment of specificity decline by 18%. This auto-feedback rapidly improved the practitioner clinical competence. This study suggests that the ultrasound done by the rheumatologist himself improves his clinical examination which was not the case in the present work because of the ultrasound inaccessibility in rheumatologic department.
Ultrasound makes possible the sub-clinical synovitis detection. Its sensitivity is confirmed by taking the MRI as a reference method [3]. Although it is operator dependent, reproducibility between operators is good perhaps even excellent for an adequate apprenticeship of the ultrasound examination [31-34]. In this study, the ultrasound synovitis was assessed in a binary fashion. This binary response offers as benefit a good reproducibility in general, yet it is little sensitive to change [35,36]. The realization of an MRI as part of this study was not possible given the difficulty of its realization concurrently an ultrasound and especially its substantial cost.
Factors relative to patients and disease
Factors related to patients (age) and to disease (duration of the illness, biological and clinical activity signs), able to influence the concordance between the clinical and ultrasound in the detection of synovitis, have been studied. The influence of age is explained by the degenerative phenomena particularly at the level of IPP, able to compromise the synovial hypertrophy assessment which tends to be overestimated [31,37].
The influence of the disease progression duration is explained by the periarticular fibrosis and the structural damages without active inflammation during the old PR that can be confounded with a clinical synovitis [19]. A better concordance during recent PR than the established one was noticed in different studied [3,19]. In this way, the longer, the disease duration is, the worst the concordance is. This study found a non-significant negative correlation between an evolution duration superior to 10 years and the clinical-ultrasound concordance.
By contrast, this concordance was correlated in a positive way, with a significant difference, to the low disease activity attested by a lower than 3, 2 DAS 28. These results join those of Le Boedec et al. [19]. Finally, the HAQ, the EGP, the SR (Sedimentation Rate) and the CRP weren’t significantly correlated to the clinical ultrasound concordance.
Methodology critics and the study limits
The number of patients was sufficient to allow the statistical analysis. Indeed, 50 patients were included and 1100 articulations were studied. Concordances between clinical and ultrasound data were practiced using Cohen Kappa coefficient which numbers the intensity or the capacity of the effective agreement between two variables by getting away from the random component.
However, the ultrasound results weren’t compared to those of the MRI that remains the Gold standard in the detection of synovitis, of inflammation signs and of bone erosion.
Thus, the ultrasound results were not valid by a performing examination. This is due to the impossibility of access to the MRI within a short time in respect to the clinical examination as well as the ultrasound. In the absence of comparison with a gold standard, it is then impossible to provide a data comparison of the sensitivity and specificity of ultrasound in the detection of synovitis.
Conclusion
At the end of this pilot study, the first one in the Tunisian centre, we can conclude that there is a low concordance, between the clinical and the ultrasound examination in the detection of synovitis. This highlights the superiority of ultrasound in the assessment of PR activity. Some factors seem to influence this concordance in particular the disease activity. The osteoarticuar ultrasound integration in PR management, in current practice, is nowadays an undeniable need in order to improve the clinical examination unlikely to be enough to ensure the early diagnosis and the PR follow-up.
Declaration of interests
The authors declare not to have any interest conflicts in relation to this article.
References
- Smolen JS, Landewé R, Breedveld FC et al. EULAR recommendations for the management of rheumatoid arthritis with syntheticand biological disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 69(6), 964–975 (2013).
- Wakefield R, Green M, Marzo-Ortega H et al. Should oligoarthritis be reclassified? Ultrasound reveals a high prevalence of subclinical disease. Ann. Rheum. Dis. 63(4), 382–385 (2004).
- Szkudlarek M, Klarlund M, Narvestad E et al. Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res. Ther. 8(2), R52 (2006).
- Funck-Brentano T, Gandjbakhch F, Etchepare F et al. Prediction of radiographic damage in early arthritis by sonographic erosions and power Doppler signal: a longitudinal observational study. Arthritis Care Res. 65(6), 896–902 (2013).
- Brown AK, Conaghan PG, Quinn MA et al. An explanation for the apparent dissociationbetween clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 58(10):2958–2967 (2008).
- Nguyen H, Ruyssen-Witrand A, Gandjbakhch F et al. Prevalence of ultrasound-detected residual synovitis and risk of relapse and structural progression in rheumatoid arthritis patients in clinical remission: a systematic review and meta-analysis. Rheumatol. 53(11), 2110–2118 (2014).
- Mouterde G, Morel J. Place de l’échographie dans la polyarthrite rhumatoïde en rémission. Revue du Rhumatisme Monographies. 82(4), 225–232 (2015).
- Boutry N, Cotten A. Apport de l’échographie dans les rhumatismes inflammatoires (polyarthrite rhumatoïde, pseudopolyarthrite rhizomélique et spondylarthropathies). Revue de Medecine Interne. 31(1), 29–40 (2010).
- D’Agostino MA, Breban M. Pourquoi les rhumatologues doivent-ils s’intéresser à l’échographie dans les rhumatismes inflammatoires? Revue du Rhumatisme. 69(5), 473–477 (2002).
- Kang T, Lanni S, Nam J et al. The evolution of ultrasound in rheumatology. Ther. Adv. Musculoskeletal Dis. 4(6), 399–411 (2012).
- Lee S-J, Lee S-W. Rehabilitative Ultrasound Imaging Findings of the Change in the Pennation Angle of the Extensor Carpi Radialis Brevis in Patients with Tennis Elbow. Indian J. Sci. Technol. 8(7), 1–8 (2015).
- Walther M, Harms H, Krenn V et al. Correlation of power Doppler sonography with vascularity of the synovial tissue of the knee joint in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 44(2), 331–338 (2001).
- Motomura H, Seki E, Kimura T. Correlation of power doppler ultrasonographic findings with site-matched histopathology of the synovial tissue. Ann. Rheum. Dis. 73(3), 1590–1595 (2013).
- Arnett F, Edworthy S, Bloch D et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31(1), 315–324 (1988).
- Prevoo M, Van Riel P, Van 't Hof M et al. Validity and reliability of joint indices: A longitudinal study in patients with recent onset rheumatoid arthritis. Rheumatol. 32(7), 589–594 (1993).
- Guillemin F, Briancon S, Pourel J. Mesure de la capacité fonctionnelle dans la polyarthrite rhumatoïde : adaptation française du health assessment questionnaire (HAQ). Revue du Rhumatisme. 58(6), 459–465 (1991).
- Wakefield RJ, Balint PV, Szkudlarek M et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J. Rheumatol. 32(12), 2485–2487 (2005).
- Alcalde M, D'Agostino MA, Bruyn GA et al. A systematic literature review of US definitions, scoring systems and validity according to the OMERACT filter for tendon lesion in RA and other inflammatory joint diseases. Rheumatol. 51(7), 1246–1260 (2012).
- Le Boedec M, Jousse-Joulin S, Ferlet JF et al. Factors influencing concordance between clinical and ultrasound findings in rheumatoid arthritis. J Rheumatol. 40(3), 244–252 (2013).
- Garrigues F, Jousse-Joulin S, Bouttier R et al. Concordance between clinical and ultrasound findings in rheumatoid arthritis. Joint Bone Spine. 80(6), 597–603 (2013).
- Ceponis A, Onishi M, Bluestein HG et al. Utility of the ultrasound examination of the hand and wrist joints in the management of established rheumatoid arthritis. Arthritis Care Res. 66(2), 236–244 (2014).
- Wolfe F, Michaud K. Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemographic disadvantage characterize (RA) patients with fibromyalgia. J. Rheumatol. 31(4), 695–700 (2004).
- Leeb BF, Andel I, Sautner J et al. The DAS28 in rheumatoid arthritis and fibromyalgia patients. Rheumatol. 43(12), 1504-1507 (2004).
- Da Silva Chakr RM, Brenol JC, Behar M et al. Is ultrasound a better target than clinical disease activity scores in rheumatoid arthritis with fibromyalgia? A case-control study. PloS ONE. 10(3), 1–8 (2015).
- Ghib LJ, Tamas MM, Damian LO et al. The role of ultrasonography in assessing disease activity in patients with rheumatoid arthritis and associated fibromyalgia. Med. Ultrasonog. J. 17(3), 339–344 (2015).
- Salaffi F, Filippucci E, Carotti M et al. Inter-observer agreement of standard joint counts in early rheumatoid arthritis: a comparison with grey scale ultrasonography- a preliminary study. Rheumatol. 47(1), 54–58 (2008).
- Murayama G, Ogasawara M, Nemoto T et al. Clinical miscount of involved joints denotes the need for ultrasound complementation in usual practice for patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 31(4), 506–514 (2013).
- Cheung PP, Gossec L, Mak A et al. Reliability of joint count assessment in rheumatoid arthritis: A systematic literature review. Semin. Arthritis and Rheum. 43(6), 721–729 (2014).
- Scott IC, Scott DL. Joint counts in inflammatory arthritis. Clin. Exp. Rheumatol. 32( 85), 7–12 (2014).
- Ogasawara M, Murayama G, Yamada Y et al. Autofeedback from ultrasound images provides rapid improvement in palpation skills for identifying joint swelling in rheumatoid arthritis. J Rheumatol. 39(6), 1207–1214 (2012).
- Scheel AK, Schmidt WA, Hermann KG et al. Interobserver reliability of rheumatologists performing musculoskeletal ultrasonography: results from a EULAR "Train the trainers" course. Ann. Rheum. Dis. 64(7), 1043–1049 (2005).
- Kissin EY, Nishio J, Yang M et al. Self-directed learning of basic musculoskeletal ultrasound among rheumatologists in the United States. Arthritis Care Res. 62(2), 155–160 (2010).
- Marhadour T, Jousse-Joulin S, Chalès G et al. Reproducibility of joint swelling assessments in long-lasting rheumatoid arthritis: influence on Disease Activity Score-28 values (SEARepro study part I). J. Rheumatol. 37(5), 932–937 (2010).
- Cheung PP, Dougados M, Gossec L. Reliability of ultrasonography to detect synovitis in rheumatoid arthritis: a systematic literature review of 35 studies. Arthritis Care Res. 62(3), 323–334 (2010).
- Szkudlarek M, Court-Payen M, Jacobsen S et al. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 48(4), 955–962 (2003).
- Jousse-Joulin S, d'Agostino MA, Marhadour T et al. Reproducibility of joint swelling assessment by sonography in patients with long-lasting rheumatoid arthritis (SEA-Repro study part II). J. Rheumatol. 37(5), 938–945 (2010).
- Szkudlarek M, Wakefield RJ, Backhaus M et al. The discriminatory capacity of ultrasound in rheumatoid arthritis: active vs inactive, early vs. advanced and more. J. Rheumatol. 51(7), 6–9 (2012).