Research Article - Interventional Cardiology (2015) Volume 7, Issue 6
Clinical outcome of 2nd generation drug-eluting stents versus bare-metal stents in percutaneous intervention on vein grafts
- Corresponding Author:
- Jawad Mazhar
Department of Cardiology, The Canberra Hospital
Garran ACT 2605, Australia
Tel: +61 451 829 029 +61 262 442 222 (work)
E-mail: jawadmc2@gmail.com
Submitted: 20 August 2015; Accepted: 1 October 2015; Published online: 12 November 2015
Abstract
Background: Superiority of drug-eluting stents (DES) over bare-metal stents (BMS) for treatment of saphenous vein graft (SVG) lesions is controversial. Methods: This is an observational study comparing the incidence of target vessel revascularization, allcause death, myocardial infarction (MI), stroke and stent thrombosis in patients who underwent SVG percutaneous coronary intervention, using DES versus BMS. Results: Out of 174 cases of SVG percutaneous coronary intervention; 87 received BMS, 66 received DES and 21 received no stents. The majority (94%) of the DES were second generation. There was no difference in target vessel revascularization at 12 months. On multivariate analysis the only predictor of major adverse cardiovascular event was stent length >20 mm. Conclusion: There was no difference in the incidence of death, MI, stroke or stent thrombosis.
Keywords
bare metal stents, coronary angioplasty, drug-eluting stents, percutaneous coronary intervention, revascularization, saphenous vein graft
The safety and efficacy of drug-eluting stents (DES) compared with bare-metal stents (BMS) in reducing target lesion restenosis and repeat revascularization in percutaneous coronary intervention (PCI) to native coronary arteries is well established [1–3]. Interventions to saphenous vein graft (SVG) lesions remain technically challenging, and are associated with higher rates of periprocedural myocardial infarction (MI), in-hospital mortality, restenosis and chronic occlusion compared with PCI to native coronary arteries, largely because of the friable atheroma and thrombotic debris that develop when the SVG degenerates [4,5].
The results of studies investigating the safety and efficacy of DES in SVG intervention have been inconsistent. There have been a few small randomized trials, several registries and mostly observational studies comparing DES with BMS in patients undergoing SVG PCI. These studies have shown conflicting results in short- and long-term follow-up in terms of mortality, MI, stent thrombosis, revascularization and major adverse cardiovascular events (MACE) [6–16]. It is important to note that the drug-eluting stents utilized in most of these studies were mainly first-generation DES. Our aim was to assess the long-term outcome of SVG interventions in our center with BMS and DES using mainly second-generation DES. We also sought to determine other potential predictors of outcomes after SVG interventions.
Materials & methods
Study population
We conducted a retrospective analysis of all SVG PCI performed at The Canberra Hospital from 2006 to 2012. Using a search of our PCI database we identified 174 consecutive patients. The creation and maintenance of the PCI registry was approved by the ACT Health Human Research Ethics Committee and all patients provided written consent for inclusion in the registry and follow-up. Demographic and procedural characteristics and the indication for the procedure were obtained from the database. The Canberra Hospital is the region’s major public hospital, providing specialist and acute care to more than 700,000 people. We perform approximately 2000 diagnostic angiograms, 800 PCI and 150 primary PCI procedures per year.
Procedure
The majority of PCI procedures were performed through the femoral artery using 6 Fr sheaths. All patients were treated with dual antiplatelet therapy including aspirin and either clopidogrel or prasugrel prior to the procedure. Aspirin was continued indefinitely and clopidogrel or prasugrel was recommended for 12 months. Intravenous heparin was administered to achieve an activated clotting time of 300 seconds. The use of glycoprotein IIb/IIIa inhibitors, distal protection devices and the type of stent was at the discretion of the interventional cardiologist. All patients underwent pre- and post-intervention ECG. Plasma creatine kinase and troponin-I levels were measured the following day to detect new ischemic events.
Study end points
Primary end point for the study was the incidence of target vessel revascularization (TVR). Secondary end points included all cause death, myocardial infarction, stent thrombosis and stroke.
Definitions
Procedural success was defined as <20% residual stenosis and TIMI 3 flow in the target vessel. MI was defined according to the Third Universal Definition of MI [17]. TVR was defined as a repeat procedure anywhere in the target vessel, including repeat PCI or coronary artery bypass graft surgery. Stent thrombosis was defined as angiographically documented stent thrombosis. Clinical in-stent restenosis was defined as in-stent restenosis of >50% found on an angiogram performed for clinical indications.
Follow-up
In-hospital clinical events were recorded by a research nurse prior to discharge. Long-term follow-up was conducted by letter, phone calls and review of hospital records at 12 months. In case of adverse events, further details were obtained from the patient’s medical records, physician or from other hospitals.
Statistical analysis
The baseline clinical characteristics of the two groups were compared using the student’s t-test for continuous variables and the chi-square test for categorical variables. Cox proportional hazard multivariate analysis was performed to determine univariate and multivariate predictors of TVR during follow-up. Variables in the model included age >70 years, gender, diabetes mellitus, age of graft >12 years, presentation with acute coronary syndrome, use of >1 stent, PCI for in-stent restenosis, use of glycoprotein IIb/ IIIa inhibitor, stent diameter ≥3.5 mm, stent length >20 mm and use of DES. A p-value <0.05 was considered significant. All statistical analysis was performed using SPSS Statistics for Windows, Version 22.0.
Results
PCI was performed on 174 patients with SVG disease between 2006 and 2012. BMS were implanted in 87 patients, DES in 66 patients and 21 patients received no stents. In this study we compared the results for DES and BMS in patients treated for SVG disease. The two groups were similar in their baseline characteristics (see Table 1). The proportion of diabetics was numerically higher in the DES group, although not statistically significant. The age of the vein graft was 13.3 ± 6.0 years in the BMS group and 14.3 ± 6.5 years in the DES group (p = 0.34). The majority (94%) of DES were 2nd generation.
| Characteristic | Bare-metal stent | Drug-eluting stent | p-value | |
|---|---|---|---|---|
| Number | 87 | 66 | – | |
| Age | 71 ± 9.1 | 69± 9.8 | 0.18 | |
| Female sex | 16 (18%) | 10 (15%) | 0.60 | |
| Diabetes | 29 (33%) | 29 (44%) | 0.18 | |
| Hypertension | 57 (66%) | 37 (56%) | 0.23 | |
| Smoking | 6 (7%) | 8 (12%) | 0.27 | |
| Reformed smoker | 38 (44%) | 33 | (50%) | 0.44 |
| Hyperlipidemia | 52 (60%) | 42 (64%) | 0.63 | |
| Family history of IHD | 26 (30%) | 29 (44%) | 0.073 | |
| BMI | 28.0 ± 4.6 | 28.5 ± 4.0 | 0.68 | |
| Vein graft age (years) | 13.3 ± 6.0 | 14.3 ± 6.5 | 0.34 | |
BMI: Body mass index; IHD: Ischemic heart disease.
Table 1: Baseline characteristics for patients with vein graft disease treated with bare-metal and drug-eluting stents.
Procedural data for the two groups are shown in Table 2. There were no significant differences between the groups with regards to indication for the procedure and lesion complexity. Procedural success was achieved in over 96% of cases in both groups. There were more restenotic lesions at baseline in the DES group compared with the BMS group (23 vs 5%, p = 0.0007), see Table 2. Glycoprotein IIb/IIIa inhibitors were used in 40% of BMS compared with 24% of DES cases (p = 0.036). This may have been partly because more patients with acute myocardial infarction received BMS (Table 2). Mean number of stents implanted per patient in the BMS group was 1.4 ± 0.80 compared with 1.6 ± 0.90 for DES (p = 0.16). Mean stent diameter was 3.4 ± 0.66 for BMS and 3.31 ± 0.50 for DES (p = 0.45). Mean total stent length was higher in the DES group (28 ± 21.2 mm vs 21 ± 11.4 mm, p = 0.011).
| BMS (n = 87) | DES (n = 66) | p-value | |
|---|---|---|---|
| Presentation | |||
| STEMI | 12 (14%) | 5(8%) | 0.25 |
| NSTEMI | 25(29%) | 17(27%) | 0.78 |
| Unstable angina | 21(24%) | 22(35%) | 0.16 |
| Stable angina | 28(33%) | 19(30%) | 0.76 |
| Lesion characteristics | |||
| Lesion type B2/C | 70 (84%) | 57 (86%) | 0.73 |
| Restenosis lesion | 4 (5%) | 15 (23%) | 0.0008 |
| Procedural variables | |||
| GP IIb/IIIa Inhibitor | 35 (40%) | 16 (24%) | 0.036 |
| Protection device | 3(3.5%) | 4 (6%) | 0.45 |
| Mean number of stents per patient | 1.4 + 0.80 | 1.6 + 0.90 | 0.12 |
| Mean stent diameter (mm) | 3.4 ± 0.66 | 3.31 ± 0.50 | 0.45 |
| Mean total stent length (mm) | 21 + 11.4 | 28 + 21.2 | 0.011 |
| Maximum balloon pressure | 14.4 ± 0.39 | 16.5 ± 3.5 | 0.0003 |
| Final TIMI flow <3 | 8 (9%) | 2 (3%) | 0.11 |
Table 2: Procedural variables for vein graft interventions treated with bare-metal stents or drugeluting stents.
Mean duration of follow-up was 401 ± 450 days for the DES and 383 ± 298 days for the BMS group (p = 0.77). Incidence of adverse events during follow-up is shown in Table 3. There was no significant difference in the primary outcome of target vessel revascularization (18 vs 17%, p = 0.78). Even after excluding patients with underlying in-stent restenosis from both groups, there was no difference in the incidence of TVR (18 vs 14%, p = 0.51). There was no significant difference in the incidence of death (3.45 vs 0%, p = 0.13), MI (3.45 vs 7.6%, p = 0.26) or stent thrombosis (4.6 vs 1.5%, p = 0.27) between BMS and DES groups during follow-up. Stroke did not occur in either group. Repeat angiography was performed for clinical indications in 36% of patients in the BMS and 29% of patients in the DES group (p = 0.37). There was no significant difference in the rate of clinical in-stent restenosis (16 vs 15%, p = 0.87).
| BMS (n = 87) | DES (n = 66) | p-value | |
|---|---|---|---|
| Primary end point | |||
| TVR | 16 (18%) | 11 (17%) | 0.78 |
| Secondary end points | |||
| Death | 3 (3.45%) | 0 (0%) | 0.13 |
| MI | 3 (3.45%) | 5 (7.6%) | 0.26 |
| Stent thrombosis | 4 (4.6%) | 1 (1.5%) | 0.27 |
| Target vein PCI | 11 (13%) | 6 (9%) | 0.49 |
| CABG | 3 (3.5%) | 5 (7.6%) | 0.26 |
| Clinical in-stent restenosis | 14 (16%) | 10 (15%) | 0.87 |
| Stroke | 0 | 0 | – |
Table 3: Incidence of adverse events after vein graft interventions with bare-metal stents or drugeluting stents during 12 months follow-up.
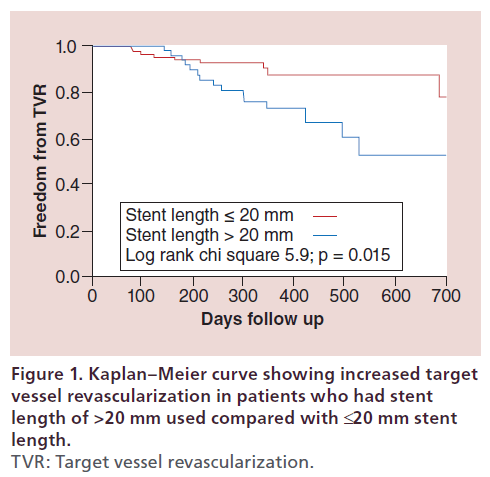
On multivariate analysis, the only significant predictor of TVR was total stent length >20 mm (hazard ratio 2.97, 95% CI: 1.23–7.57, p = 0.01) (Table 4). Incidence of TVR for SVG interventions with a stent length >20 mm was 27% compared with 11% for stent length ≤20 mm (p = 0.012). Kaplan–Meier curve for freedom from TVR in patients with total stent length ≤20 mm and >20 mm is shown in Figure 1 (log-rank chi-square 5.9, p = 0.015). Use of DES was not a significant predictor of TVR during follow-up.
| Variables | Hazard ratio | CI | p-value |
|---|---|---|---|
| Age >70 years | 0.79 | 0.28–2.08 | 0.64 |
| Male | 2.53 | 0.62–17.3 | 0.21 |
| Diabetes mellitus | 0.86 | 0.34–2.05 | 0.74 |
| Presentation with ACS | 0.90 | 0.36–2.45 | 0.83 |
| Age of graft >12 years | 0.63 | 0.26–1.5 | 0.30 |
| Use of glycoprotein IIb/ | 1.38 | 0.69–2.76 | 0.36 |
| IIIa inhibitor | |||
| Underlying in-stent | 1.3 | 0.39–3.74 | 0.64 |
| restenosis | |||
| Stent diameter ≥3.5 mm | 1.34 | 0.55–3.6 | 0.52 |
| Stent length >20 mm | 2.97 | 1.23–7.57 | 0.01 |
| Use of DES | 0.75 | 0.29–1.82 | 0.53 |
Table 4: Multivariate analysis to determine variables associated with target vessel revascularization.
Discussion
In this observational study comparing PCI outcomes with DES and BMS in patients with SVG disease, the two groups were similar in their baseline characteristics, clinical presentation, age of the vein graft and lesion complexity. As expected in an observational study, there were some significant differences between the two groups. There was a higher prevalence of restenotic lesions at baseline in patients treated with DES (23 vs 5%, p = 0.0007). It is possible that some operators deployed DES in these patients in the hope of reducing the risk of restenosis, although there is no convincing evidence of benefit for DES in this setting. However, even after excluding patients with instent restenosis at baseline, there was no difference in the incidence of TVR. Mean total stent length used was longer in the DES group (28 vs 21 mm, p = 0.011). Operators treating longer lesions may have preferred DES in the hope of reducing the risk of restenosis. Use of glycoprotein IIb/IIIa inhibitors was higher in the BMS group (40 vs 24%, p = 0.036), but there was no association between glycoprotein IIb/IIIa inhibitor use and outcomes on univariate or multivariate analysis. Similarly, in a pooled analysis of five randomized controlled trials (RCTs), the use of glycoprotein IIb/IIIa inhibitors in SVG PCI did not improve outcomes [5].
In our study, after a mean follow-up of approximately 400 days in both groups, there was no difference in the incidence of TVR, all-cause death, MI, stent thrombosis or stroke. Several registries comparing DES with BMS in SVG disease have shown conflicting results [6–11]. Registries with short follow-up of 6 to 12 months have shown a reduction in MACE with DES [6,11]. However, some registries with longer follow-up of 3 and 4 years have shown no reduction in TVR or MACE with DES [8,10]. A large registry of 1418 patients with 4-year follow-up showed a reduction in TVR with DES, especially in diabetics and in patients with a long segment of stent (≥30 mm), but there was no reduction in death or myocardial infarction [9]. It is possible that the use of DES delays but does not prevent the process of disease progression in degenerated vein grafts. In our study the only significant predictor of TVR was total stent length >20 mm. Longer total stent length likely represents more diffuse and severe atherosclerotic disease affecting the vein graft, with a higher risk of long-term occlusion.
There have been three RCTs comparing the use of DES with BMS in patients with SVG disease [12–16]. The largest trial was ISAR-CABG with 610 patients, which showed a significant reduction in target lesion revascularization with use of DES, but no difference in myocardial infarction, all-cause death or stent thrombosis [12]. The main limitation of ISAR-CABG was a short follow-up of 1 year [12]. Another small RCT of 80 patients (SOS trial) with 3-year follow-up showed a reduction in target lesion revascularization and MI with the use of DES, but no difference in mortality [13,14]. On the contrary, in a small RCT of 75 patients (delayed RRISC trial) the reduction in TVR seen at 6 months was lost at longer follow-up of 2.5 years and there was increased mortality with the use of DES [15,16]. A recent comprehensive meta-analysis of all randomized controlled trials comparing clinical outcomes of PCI using DES or BMS in patients with SVG disease showed no difference in all-cause death or myocardial infarction, but a reduction in repeat revascularization with DES [18].
Most of the above-mentioned registries and RCTs used 1st-generation DES. Two recent registries comparing 1st- and 2nd-generation DES showed no significant difference in death, TVR and overall MACE [19,20]. Another registry comparing 1st- and 2nd-generation DES in 331 patients with SVG disease showed a reduction in MACE mainly driven by a reduction in TVR with 2nd-generation DES [21]. In our study there was no reduction in TVR despite pre-dominant use of 2nd-generation DES. However, this effect may have been reduced due to higher prevalence of underlying in-stent restenosis in patients treated with DES and the longer length of stent used in the DES group.
In our study there was no difference in the incidence of stent thrombosis in the two groups, although our study population is not large enough to detect differences in this rare event. Meta-analyses of observational studies and randomized trials have not shown a difference in the incidence of stent thrombosis with DES and BMS in patients with SVG disease [22,23].
Recent European Society of Cardiology guidelines on myocardial revascularization recommend DES for PCI of vein grafts [24]. Compared with PCI of native vessels, SVG PCI is associated with a twofold increase in mortality at 30 days and 6 months and higher rates of repeat revascularization [5]. In our study, incidence of TVR for SVG interventions with a stent length >20 mm was 27% compared with 11% for stent length ≤20 mm (p = 0.012). Repeat coronary artery bypass grafting is associated with a threefold increase in peri-operative mortality, as the procedure is technically challenging and the patients are usually older with multiple co-morbidities [25]. Hence, whenever possible, it is reasonable to consider PCI of native vessels as an alternative to SVG PCI [24]. In patients with diffuse and severe SVG disease whose native vessels are not suitable for PCI, repeat CABG should be considered [24].
Study limitations
The small sample size of this observational study may be the reason for the lack of difference seen in the two groups. However, the study included consecutive real life patients over a 6-year period, including those with high-risk features such as acute ST-elevation MI and restenotic lesions, which may not have been included in randomized studies. Also, the TVR rate of 18 versus 17% in BMS versus DES groups during a mean follow-up of around 400 days gives a realistic indication of outcomes in these two groups. There were more complex lesions in DES group (more in-stent restenosis and longer stent length) that may have made the results neutral. It is likely that operators favored the use of DES in the treatment of restenotic and longer lesions. Embolic protection devices were used infrequently in our cohort, but the usage was not different between the two groups. Another limitation is that SVGs can occlude silently without any symptoms. This can lead to an underestimation of the true graft failure rate. It is possible that this may have occurred in a small number of cases. Despite the neutral results of our study regarding reduction of TVR with DES, we cannot rule out a benefit from DES in vein grafts due to the small size of our cohort and potential treatment bias.
Conclusion
In this observational study comparing predominantly 2nd-generation DES with BMS in patients with SVG disease; there was no difference in MACE or TVR at 12 months.
Acknowledgements
We acknowledge the contribution of all the Cardiac Catheterization Laboratory nurses and technicians in caring for our patients and for their assistance in data collection.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Executive summary
• This study compares the incidence of target vessel revascularization, all-cause death, myocardial infarction, stent thrombosis and stroke in patients who underwent saphenous vein graft (SVG) percutaneous coronary intervention (PCI), using drug-eluting stents (DES) versus bare-metal stents (BMS).
• A retrospective analysis of the PCI database at Canberra Hospital for all cases of SVG PCI from 2006 to 2012 was conducted.
• Mean follow-up was 393 ± 390 days.
• Out of 174 cases of SVG PCI; 87 received BMS, 66 received DES and 21 received no stents. The majority (94%) of the DES were second generation.
• Mean number of stents per patient was 1.4 ± 0.80 versus 1.6 ± 0.90 (p = 0.16), mean stent diameter 3.4 ± 0.66 versus 3.31 ± 0.50 mm (p = 0.45) and mean stent length 21 ± 11.4 versus 28 ± 21.2 mm (p = 0.01) for BMS and DES, respectively.
• There was no difference in target vessel revascularization (18 vs 17%; p = 0.78) at 12 months.
• On multivariate analysis the only predictor of major adverse cardiovascular events was stent length >20 mm.
• There was no difference in the incidence of death (3.45 vs 0%; p = 0.13), myocardial infarction (3.45 vs 7.6%; p = 0.26) or stent thrombosis (4.6 vs 1.5%; p = 0.27).
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest.
- Stettler C, Wandel S, Allemann S et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370(9591), 937–948 (2007).
- Weisz G, Leon MB, Holmes DR Jr Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) trial. J. Am. Coll. Cardiol. 53(17), 1488–1497 (2009).
- Ellis SG, Stone GW, Cox DA et al. Long-term safety and efficacy with paclitaxel-eluting stents: 5 year final results of the TAXUS IV clinical trial (TAXUS IV-SR: Treatment of De Novo Coronary Disease Using a Single Paclitaxel-Eluting Stent). JACC Cardiovasc. Interv. 2(12), 1248–1259 (2009)
- Stone GW, Reifart NJ, Moussa I et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part II. Circulation 112(16), 2530– 2537 (2005).
- Roffi M, Mukherjee D, Chew DP et al. Lack of benefit from intravenous platelet glycoprotein IIb/IIIa receptor inhibition as adjunctive treatment for percutaneous interventions of aortocoronary bypass grafts: a pooled analysis of five randomized clinical trials. Circulation 106(24), 3063–3067 (2002).
- Lee MS, Shah AP, Aragon J et al. Drug-eluting stenting is superior to bare metal stenting in saphenous vein grafts. Catheter. Cardiovasc. Interv. 66(4), 507–511 (2005).
- Brodie BR, Wilson H, Stuckey T et al. Outcomes with drug-eluting versus bare-metal stents in saphenous vein graft intervention results from the STENT (strategic transcatheter evaluation of new therapies) group. JACC Cardiovasc. Interv. 2(11), 1105–1112 (2009).
- Goswami NJ, Gaffigan M, Berrio G et al. Long-term outcomes of drug-eluting stents versus bare-metal stents in saphenous vein graft disease: results from the Prairie “Real World” Stent Registry. Catheter. Cardiovasc. Interv. 75(1), 93–100 (2010).
- Ko DT, Guo H, Wijeysundera HC et al. Long-term safety and effectiveness of drug-eluting stents for the treatment of saphenous vein grafts disease: a population-based study. JACC Cardiovasc. Interv. 4(9), 965–973 (2011).
- Nair S, Fath-Ordoubadi F, Clarke B et al. Late outcomes of drug eluting and bare metal stents in saphenous vein graft percutaneous coronary intervention. EuroIntervention 6(8), 985–991 (2011).
- Tolerico PH, Cohen DJ, Kleiman NS et al. In-hospital and 1 year outcomes with drug-eluting versus bare metal stents in saphenous vein graft intervention: a report from the EVENT registry. Catheter. Cardiovasc. Interv. 80(7), 1127–1136 (2012).
- Mehilli J, Pache J, Abdel-Wahab M et al. Drug-eluting versus bare-metal stents in saphenous vein graft lesions (ISAR-CABG): a randomised controlled superiority trial. Lancet 378(9796), 1071–1078 (2011).
- Brilakis ES, Lichtenwalter C, de Lemos JA et al. A randomized controlled trial of a paclitaxel-eluting stent versus a similar bare-metal stent in saphenous vein graft lesions the SOS (Stenting of Saphenous Vein Grafts) trial. J. Am. Coll. Cardiol. 53(11), 919–928 (2009).
- Brilakis ES, Lichtenwalter C, Abdel-karim AR et al. Continued benefit from paclitaxel-eluting compared with bare-metal stent implantation in saphenous vein graft lesions during long-term follow-up of the SOS (Stenting of Saphenous Vein Grafts) trial. JACC Cardiovasc. Interv. 4(2), 176–182 (2011).
- Vermeersch P, Agostoni P, Verheye S et al. Randomized double-blind comparison of sirolimus-eluting stent versus bare-metal stent implantation in diseased saphenous vein grafts: six-month angiographic, intravascular ultrasound, and clinical follow-up of the RRISC Trial. J. Am. Coll. Cardiol. 48(12), 2423–2431 (2006).
- Vermeersch P, Agostoni P, Verheye S et al. Increased late mortality after sirolimus-eluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC Trial. J. Am. Coll. Cardiol. 50(3), 261–267 (2007).
- Thygesen K, Alpert JS, Jaffe AS et al. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 60(16), 1581–1598 (2012).
- Alam M, Bandeali SJ, Virani SS et al. Clinical outcomes of percutaneous interventions in saphenous vein grafts using drug-eluting stents compared with bare-metal stents: a comprehensive meta-analysisof all randomized clinical trials. Clin. Cardiol. 35(5), 291–296 (2012).
- Costopoulos C, Latib A, Naganuma T et al. Comparison of first- and second-generation drug-eluting stents in saphenous vein grafts used as aorto-coronary conduits. Am. J. Cardiol. 112(3), 318–322 (2013).
- Pokala NR, Menon RV, Patel SM et al. Long-term outcomes with first- vs. second-generation drug-eluting stents in saphenous vein graft lesions. Catheter. Cardiovasc. Interv. doi:10.1002/ccd.25982 (2015) (Epub ahead of print).
- Kitabata H, Loh JP, Pendyala LK et al. Two-year follow-up of outcomes of second-generation everolimus-eluting stents versus first-generation drug-eluting stents for stenosis of saphenous vein grafts used as aortocoronary conduits. Am. J. Cardiol. 112(1), 61–67 (2013).
- Hakeem A, Helmy T, Munsif S et al. Safety and efficacy of drug eluting stents compared with bare metal stents for saphenous vein graft interventions: a comprehensive meta-analysis of randomized trials and observational studies comprising 7,994 patients. Catheter. Cardiovasc. Interv. 77(3), 343–355 (2011).
- Wiisanen ME, Abdel-Latif A, Mukherjee D, Ziada KM. Drug-eluting stents versus bare-metal stents in saphenous vein graft interventions: a systematic review and meta-analysis. JACC Cardiovasc. Interv. 3(12), 1262–1273 (2010).
- Windecker S, Kolh P, Alfonso F et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 35(37), 2541–2619 (2014).
- Yap CH, Sposato L, Akowuah E et al. Contemporary results show repeat coronary artery bypass grafting remains a risk factor for operative mortality. Ann. Thorac. Surg. 87(5), 1386–1391 (2009).
• This pooled analysis of five randomized controlled trials showed that use of glycoprotein IIb/IIIa inhibitors does not improve outcomes in percutaneous coronary intervention (PCI) of vein grafts.
•• Largest randomized controlled trial showing reduction in target vessel revascularization (TVR) with drug-eluting stents (DES) but no difference in mortality.
• This large registry with long follow-up of 4 years showed a reduction in TVR with DES but no reduction in mortality.
•• Meta-analysis of all randomized controlled trials comparing DES with bare-metal stents in vein graft PCI showed a reduction in TVR but no difference in mortality.
• Registry comparing 1st-generation DES with 2ndgeneration DES in saphenous vein graft (SVG) PCI showed no difference in outcomes.
• Recent registry comparing 1st-generation DES with 2nd-generation DES in SVG PCI showed no difference in outcomes.
• Registry comparing 1st-generation DES with 2ndgeneration DES in SVG PCI showed reduction in TVR.