Research Article - Interventional Cardiology (2021) Volume 13, Issue 6
Clinical outcomes of everolimus bioresorbable scaffolds versus metallic everolimus-eluting stents for coronary arterial disease: A meta-analysis of randomized clinical trials
- Corresponding Author:
- Rohan Madhu Prasad
Department of Internal Medicine,
Michigan State University,
East Lansing,
Michigan,
USA,
E-mail: rohanmaprasad@msu.edu
Received date: September 02, 2021 Accepted date: September 16, 2021 Published date: September 30, 2021
Abstract
Background: Bioresorbable Scaffolds (BRS) were previously shown to have no significant benefit in clinical outcomes versus metallic Drug-Eluting Stents (DES) at one-year follow-up duration. However, the presence of long-term side effects is currently unknown. This meta-analysis was conducted to compare the mid-term clinical outcomes of everolimus BRS versus metallic Everolimus-Eluting Stents (EES) in patients with coronary arterial disease.
Methods: A comprehensive review of all relevant manuscripts and abstracts studies from inception to March 2021 were obtained. A meta-analysis was performed using a random effect model to calculate Odds Ratios (OR) with 95% Confidence Intervals (CI).
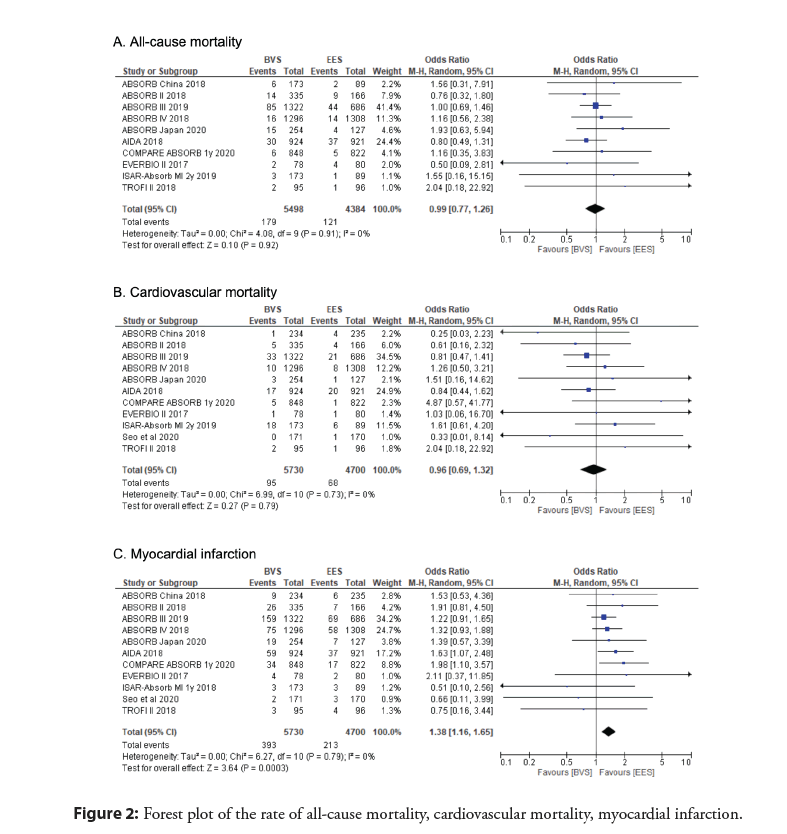
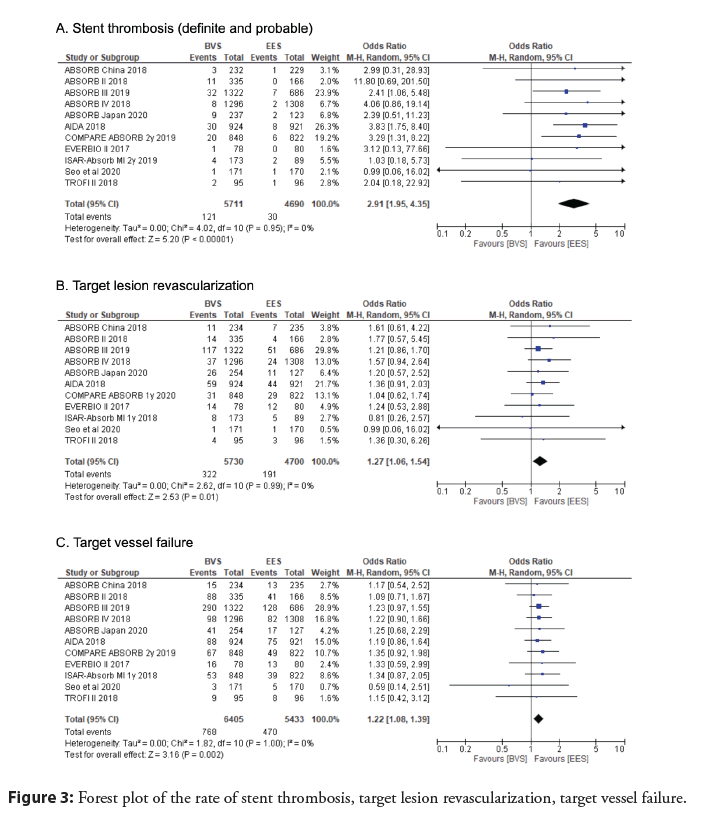
Results: Eleven Randomized Controlled Trials (RCTs) were included with a total of 10,430 patients and a median-weighted follow-up period of 2.66 years. A pooled analysis of the data showed no significant difference in all-cause mortality (OR 0.99; 95% CI 0.77 to 1.26; p=0.92) or cardiovascular mortality (OR 0.96; 95% CI 0.69 to 1.32; p=0.79). However, myocardial infarction (OR 1.38, 95% CI 1.16 to 1.65, p=0.0003), stent thrombosis (OR 2.91, 95% CI 1.95 to 4.35, p<0.00001), TLR (OR 1.27, 95% CI 1.06 to 1.54, p=0.01), and TVF (OR 1.22, 95% CI 1.08 to 1.39, p=0.002) were all determined to be significantly higher in the BRS group.
Conclusion: Mid-term follow-up data reveals that everolimus BRS has no significant difference in terms of all-cause mortality when compared to EES. However, everolimus BRS does have an increased rate of cardiovascular events and thrombosis in the mid- term setting.
Keywords
Everolimus • Bioresorbable scaffolds • Drug-eluting stents • Coronary arterial disease • Meta-analysis
Introduction
Metallic Drug-Eluting Stents (DES) have been used to provide scaffolding of the coronary vessels, which prevents acute and subacute vessel closure as well as constrictive remodeling. The response of cell proliferation is blunted by the elution of medications [1]. However, DES has long-term side effects of decreasing normal vasomotion, adaptive arterial remodeling precluding bypass surgery, and causing a foreign body- induced inflammatory reaction [2]. Therefore, fully Bioresorbable vascular Scaffolds (BRS) were developed to overcome these limitations as they were designed to dissolve over time. The most well-recognized form is Absorb (Absorb BRS; Abbott Vascular), which entails an everolimus scaffold [2]. There are different metallic DES equivalents, such as the Cobalt-Chromium Everolimus-Eluting Stent (EES) (CoCr-EES; Abbott Vascular or Xience Prime; Abbott) or the platinum EES (Promus Element EES, Boston Scientific).
Randomized Clinical Trials (RCTs) that compared clinical outcomes of everolimus BRS reported increased cardiovascular events and thrombotic events at one-year follow-up when compared to metallic DES [3-5]. Subsequently in 2018, the European Society of Cardiology and European Association of Percutaneous Cardiovascular Interventions Task Force reported that patients who receive conventional metallic DES have favorable prognosis clinical outcomes. Despite the evidence of late adverse events with the metallic DES, the current recommendations indicate to use them in daily practice. However, ongoing studies with mid-term clinical safety outcomes may alter these guidelines [3].
Recently, multiple RCTs published their results after 2-5 years of follow-up, hence we conducted this meta-analysis to compare the clinical outcomes between these two treatment modalities with a longer median-weighted follow-up period.
Materials and Methods
We conducted a comprehensive review of previous publications of all relevant studies from inception to March 2021. We searched the electronic databases of PUBMED, EMBASE and COCHRANE for clinical studies and scientific session abstracts. Additionally, oral and presentations and/or expert slide presentations were obtained from TCT (www.tctmd.com), EuroPCR (www.europcr. com), ACC (www.acc.org), AHA (www.aha.org) and ESC (www. escardio.org).
The inclusion criteria consisted of: (1) a randomized controlled trial evaluating the clinical outcomes between everolimus BRS versus metallic EES for the treatment of coronary artery disease. (2) The study reported more than one clinical outcome.
Exclusion criteria were (1) follow-up data in less than 90% of patients, (2) ongoing or irretrievable data, (3) use of bare-metal stents in the control group, and (4) no clinical outcome endpoint. The meta-analysis was performed per the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
The search included the following keywords: “bioresorbable vascular scaffold”, “drug-eluting stent”, “everolimus”; “randomized trial”, “mortality”, “clinical”. Two authors (RMP and AA) independently reviewed the search results, extracted potential articles, and assessed their eligibility. The Cochrane Collaboration risk-of-bias tool was used by two different authors (RMP and AA) to assess the quality of the included studies.
The primary outcome of this meta-analysis was mid-term all-cause mortality, which was defined as reported mortality at the longest reported follow-up, with the minimum being one year. Secondary outcomes included cardiovascular mortality, recurrent Myocardial Infarction (MI), Target Lesion Revascularization (TLR), stent thrombosis and device-oriented Target Lesion Failure (TLF) in the mid-term setting. Studies with mid-term follow-up periods of 2-5 years were included. In regards to stent thrombosis, both definite and probable were included. TLF was defined as the composite endpoint of cardiac death, target vessel MI, and TLR. We also collected the following characteristics of each study: first author’s name, year of publication, study abbreviation, enrollment year(s), single vs. multicenter and full manuscript vs. abstract, stent strategy, number of participants in each arm, follow-up duration and mean age. Additionally, the percentage of participants in respect to the specified study that had acute coronary syndrome, a female gender, diabetes mellitus, and BRS post-dilatation were noted. Statistical analysis was conducted using Review Manager (RevMan), version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark). The Mantel-Haenszel random-effects models were used to estimate the mean difference and the corresponding 95% Confidence Intervals (CI). Two-sided p values of <0.05 were considered as statistically significant. I2 statistics were used to assess statistical heterogeneity.
Results
Eleven RCTs were included with a total of 10,460 patients and a median-weighted follow-up of 2.66 years (Figure 1) [6-18]. Two of these studies, COMPARE ABSORB [12-13] and ISAR-Absorb MI [17,18], had manuscripts with newly published data. Of these 13 studies, 8 were full manuscripts, 4 were abstracts, and 1 short report (Table 1). Additionally, three of the included studies were recently published in 2020 (Table 1) [10,12,15]. The mean age across the included studies was 62.4 years and the percentages of patients with acute coronary syndrome were 37.9%, female gender was 23.8% and diabetes mellitus 26.6% (Table 2).
| Study | Authors | Article type | Publication year | Enrollment years | Study type | Stent strategy | |
|---|---|---|---|---|---|---|---|
| BRS | DES | ||||||
| ABSORB China | Gao, et al. [6] | Abstract | 2018 | 2015-2018 | Multi, RCT | EE-BRS | Co-Cr EES (XIENCE) |
| ABSORB II | Serruys, et al. [7] | Abstract | 2018 | 2015-2018 | Multi, RCT | EE-BRS | Co-Cr EES (XIENCE) |
| ABSORB III | Kereiakes, et al. [8] | Full Manuscript | 2019 | 2015-2018 | Multi, RCT | EE-BRS | Co-Cr EES (XIENCE) |
| ABSORB 4 | Stone, et al. [9] | Full Manuscript | 2018 | 2018 | Multi, RCT | EE-BRS | Co-Cr EES (XIENCE) |
| ABSORB Japan | Kozuma, et al. [10] | Full Manuscript | 2020 | 2013-2018 | Multi, RCT | EE-BRS | Co-Cr EES (XIENCE Prime) |
| AIDA | Tijssen, et al. [11] | Full Manuscript | 2018 | 2018 | Multi, RCT | EE-BRS | Co-Cr EES (XIENCE) |
| COMPARE ABSORB | Smits, et al. [12] | Full Manuscript | 2020 | 2018 | Multi, RCT | EE-BRS | Co-Cr EES (XIENCE) |
| Van Geuns, et al. [13] | Abstract | 2019 | |||||
| EVERBIO II | Arroyo, et al. [14] | Full Manuscript | 2017 | 2017 | Single, RCT | EE-BRS | PP-EES |
| Seo, et al. [15] | Full Manuscript | 2020 | 2020 | Multi, RCT | EE-BRS | EES | |
| TROFI II | Katagiri, et al. [16] | Short report | 2018 | 2015-2018 | Multi, RCT | EE-BRS | Co-Cr EES (XIENCE) |
| ISAR-Absorb MI | Byrne, et al. [17] | Full Manuscript | 2018 | 2018 | Multi, RCT | EE-BRS | EES |
| Wiebe, et al. [18] | Abstract | 2019 | 2018-2019 | Multi, RCT | EE-BRS | EES | |
Abbreviations: BVS: Bioresorbable; Co-Cr EES: Cobalt Chromium Everolimus-Eluting Stent; EE-BRS: Everolimus-Eluting Bioresorbable Stent; EES: Metallic Everolimus-Eluting Stent; PP-EES: Persistent Polymer Everolimus-Eluting Stent; RCT: Randomized Controlled Trial
Table 1: General characteristics of included studies.
| Study | BRS (n) | EES (n) | Follow-up (years) | Age (mean) | ACS (%) | Female gender (%) | Diabetes mellitus (%) | Post dilatation (BRS) |
|---|---|---|---|---|---|---|---|---|
| ABSORB China | 241 | 239 | 4 | 57.4 | 9.7 | 27.8 | 24.4 | 63 |
| ABSORB II | 335 | 166 | 5 | 61.2 | 2.5 | 22 | 24 | 61 |
| ABSORB III | 1322 | 686 | 5 | 63.6 | - | 29.6 | 32.1 | 65.5 |
| ABSORB 4 | 1300 | 1300 | 1 | 62.65 | 23.9 | 28.1 | 31.75 | 82.6 |
| ABSORB Japan | 266 | 134 | 5 | 67.2 | 16 | 22.6 | 36 | - |
| AIDA | 924 | 921 | 2 | 64.2 | 45.5 | 25.5 | 18 | - |
| COMPARE ABSORB | 822 | 800 | 2 | 62.1 | 12.8 | 22.1 | 35.35 | 90.7 |
| EVERBIO II | 78 | 80 | 2 | 65 | 30.5 | 21 | 23 | 34 |
| Seo et al | 171 | 170 | 1 | 62.5 | - | 21.7 | 31.1 | - |
| TROFI II | 95 | 96 | 3 | 58.7 | 100 | 17.9 | 16.8 | 50.5 |
| ISAR-Absorb MI | 173 | 89 | 2 | 62.5 | 100 | 23.6 | 20.5 | 56.6 |
Abbreviations: ACS: Acute Coronary Syndrome; BRS: Bioresorbable scaffold; EES: Metallic Everolimus-Eluting Stent
Table 2: Patient characteristics of included studies.
A pooled analysis of the data with mid-term follow-up period showed no significant difference in the primary outcome of all- cause mortality between everolimus BRS and EES groups (OR 0.99; 95% CI 0.77 to 1.26; p=0.92, I2=0%) (Figure 2). Additionally, cardiovascular mortality was determined to be not significant (OR 0.96; 95% CI 0.69 to 1.32; p=0.79, I2=0%) (Figure 2). However, MI (OR 1.38, 95% CI 1.16 to 1.65, p=0.0003, I2=0%), stent thrombosis (OR 2.91, 95% CI 1.95 to 4.35, p<0.00001, I2=0%), TLR (OR 1.27, 95% CI 1.06 to 1.54, p=0.01, I2=0%), and TVF (OR 1.22, 95% CI 1.08 to 1.39, p=0.002, I2=0%) were all determined to be significantly higher in the BRS as compared to the EES (Figure 3).
Figure 2: Forest plot of the rate of all-cause mortality, cardiovascular mortality, myocardial infarction.
Discussion
This updated meta-analysis with a median-weighted follow-up duration of 2.66 years demonstrated that mortality, both all-cause and cardiovascular, were insignificant between the BRS and EES groups. Furthermore, myocardial infarction, stent thrombosis, TLR, and TVF were significantly higher in the patients with BRS.
Recently, multiple RCTs have published their updated results with longer follow-up periods that compared BRS and EES in patients with coronary artery disease. Kozuma, et al. [10] reported the five-year follow-up results of the ABSORB Japan study. At 5 years, the 400 included patients had no significant differences between BRS and EES in the composite outcome of death/MI/ revascularization (BRS 29.1% vs. EES 26.8%, p=0.63), TVF (BRS 16.1% vs. EES 13.4%, p=0.48), TLF (BRS 11.0% vs. EES 7.9%, p=0.33). The author performed a separate analysis to compare the aforementioned clinical outcomes between years three to five of follow-up and no significant difference was found [10]. Van Geuns, et al [13]. published the two-year follow-up data of the COMPARE ABSORB trial on 1,670 patients. The study showed no difference in TLF between Absorb and Xience [13]. Similarly, Wiebe, et al. [18] updated the ISAR-Absorb MI trial to include two-year follow-up data. A total of 262 patients with acute MI were enrolled and illustrated that all-cause mortality, stent thrombosis, and TVF were comparable between BRS and EES [18]. Katagiri, et al. reported the three-year follow-up date of the TROFI II trial [16]. The updated data confirmed that stent thrombosis and TVF were low and insignificant between BRS and EES arms in patients with ST-segment elevation MI [16]. Finally, Seo, et al. [15] published a new RCT where 341 patients with diffuse long native coronary arterial disease were randomized to either BRS or EES. The inclusion criteria consisted of patients who required a device with a length longer than 28 millimeters. The study reported one year of follow-up data that revealed insignificant clinical differences of cardiovascular death, MI, device thrombosis, or TLR between BRS and EES. Unfortunately, this study had to terminate early after only one year as the manufacturer stopped supplying the BRS.
The etiology behind worse clinical outcomes of the BRS is likely multifactorial. A registry evaluating 36 patients with very late scaffold thrombosis, defined as thrombosis after one year of BRS implantation, showed that the most likely etiologies of very late scaffold thrombosis with BRS are, in descending order: scaffold discontinuity (42.1%), malpositioned (18.4%), neoatherosclerosis (18.4%), underexpansion or scaffold recoil (10.5%), uncovered struts (3.5%) [19]. Due to the concern of strut fracture from arterial dilatation, the stent can be malpositioned, which can lead to its increased risk of scaffold thrombosis [20]. Furthermore, BRS has characteristics of decreased radial strength, increased recoil, and thick struts that lead to poorer initial angiographic outcomes, increase the crossing profile, delay endothelialization, and alter local blood flow. The end result of these characteristics ultimately creates increased potential shear stress and stent thrombosis [20]. Patients may also react differently to the resorptive properties and biomechanics of the BRS. The resorption of BRS has been linked with increased inflammation and thrombogenesis [19]. Post-BRS implantation several proteins (including albumin, fibronectin, fibrinogen, and complement) can adhere to the poly- L-lactic-acid polymer of the stent and activate the complement system. This process eventually causes local foreign body reactions and hypersensitivity reactions [21]. Furthermore, after the resorption, there are likely anatomical arterial changes, which leads to flow disturbances and increased risk for thrombogenesis [22]. Additionally, the development of neoatherosclerosis at the remnant site of BRS has been reported [22]. Finally, the BRS is completely resorbed by 36-42 months after implantation [2]; therefore, the median-weighted follow-up period of 2.66 years in this meta-analysis is unable to definitively determine the arterial effects after the BRS is resorbed.
Some of the limitations of this study are inherent to the design of a meta-analysis, including compiling data from different trials and no standardized protocol for pre and post-procedural medications and stent implantation techniques. Despite an insignificant heterogeneity, there is a discrepancy in the baseline characteristics of the included patients. This meta-analysis included RCTs with a mid-term follow-up duration of 3-5 years, but there was one RCT by Seo, et al. [15] that was terminated after only one year due to production discontinuation by the manufacturer. Additionally, some studies only included a certain population, such as acute MI and long native coronary arterial disease.
Conclusion
Previously, RCTs have shown that the BRS has similar rates of side effects at one-year follow-up as the DES. This is contrary to the initial hypothesis that BRS would have significantly improved clinical outcomes compared to DES. Our meta-analysis with a mid-term follow-up period illustrated that there was no mortality (all-cause or cardiovascular) difference between the BRS and EES groups. However, MI, stent thrombosis, TLR and TVF were significantly increased for patients with BRS. Based on this data, we recommend that metallic DES remain as the gold standard form of treatment for coronary arterial disease in the short and mid-term. We also propose that the next generation of BRS be conducted with polymers that cause less inflammation and positive recoil. With these altered polymers and intrinsic self-expanding elasticity, RCTs should be conducted to determine the rate of MACE. Further RCTs with a follow-up duration of 4-5 years are needed to evaluate these findings and the effects on the arteries in the long-term setting after the BRS completely resorbs.
References
- Stefanini GG, Holmes DRJ. Drug-eluting coronary-artery stents. N Engl J Med. 368: 254-65 (2013).
- Jinnouchi H, Torii S, Sakamoto A, et al. Fully bioresorbable vascular scaffolds: Lessons learned and future directions. Nat Rev Cardiol. 16(5): 286-304 (2019).
- Byrne RA, Stefanini GG, Capodanno D, et al. Report of an ESC-EAPCI Task Force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention: Executive summary. Eur Heart J. 39(18): 1591-1601 (2018).
- Cassese S, Byrne RA, Ndrepepa G, et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: A meta-analysis of randomised controlled trials. Lancet. 387(10018): 537-44 (2016).
- Cassese S, Byrne RA, Juni P, et al. Mid-term clinical outcomes with everolimus-eluting bioresorbable scaffolds versus everolimus-eluting metallic stents for percutaneous coronary interventions: A meta-analysis of randomized trials. EuroIntervention. 25(7-8): 429-438 (2017).
- Gao R, Yang Y, Han Y, et al. ABSORB CHINA: Four Years clinical outcomes comparison of everolimus-eluting bioresorbable vascular scaffolds and metallic stents: Three-year clinical outcomes from the ABSORB China randomised trial. J Am Coll Cardiol. 72(13): Supple B128 (2018).
- Serryus P, Chevalier B. TCT-62 five-year clinical outcomes with everolimus-eluting bioresorbable scaffolds: Results from the randomized ABSORB II Trial. J Am Coll Cardiol. 72(13): Supple B128 (2018).
- Kereiakes DJ, Ellis SG, Metzger DC, et al. Clinical outcomes prior to and following complete everolimus-eluting bioresorbable scaffold resorption: Five-year follow-up from the ABSORB III trial. Circulation. 140(23): 1895-903 (2019).
- Stone GW, Ellis SG, Kereiakes DJ, et al. Blinded outcomes and angina assessment of coronary bioresorbable scaffolds: 30-day and 1-year results from the ABSORB IV randomised trial. Lancet. 392(10157): 1530-40 (2018).
- Kozuma K, Tanabe K, Hamazaki Y, et al. Long-term outcomes of absorb bioresorbable vascular scaffold vs. everolimus-eluting metallic stent: A randomized comparison through 5 Years in Japan. Circ J. 84(5): 733-741 (2020).
- Tijssen RYG, Kraak RP, Hofma SH, et al. Complete two-year follow-up with formal non-inferiority testing on primary outcomes of the AIDA trial comparing the absorb bioresorbable scaffold with the XIENCE drug-eluting metallic stent in routine PCI. EuroIntervent. 14(4): e426-e33 (2018).
- Smits PC, Chang CC, Chevalier B, et al. Bioresorbable vascular scaffold versus metallic drug-eluting stent in patients at high risk of restenosis: The COMPAREABSORB randomised clinical trial. Eurointervention. 16(8): 645-53 (2020).
- Van Geuns RJ, Chang CC, Chevalier B, et al. Bioresorbable scaffold vs. metallic drug-eluting stent in patients at high risk of restenosis (COMPARE-ABSORB trial): Very late device thrombosis while on extended DAPT. J Am Coll Cardiol. 74(13): Supple B128 (2019).
- Arroyo D, Gendre G, Schukraft S, et al. Comparison of everolimus and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds: Two-year clinical outcomes of the EVERBIO II trial. Int J Cardiol. 243: 121-5 (2017).
- Seo J, Ahn JM, Hong SJ, et al. Bioresorbable vascular scaffolds vs. drug-eluting stents for diffuse long coronary narrowings. Am J Cardiol. 125(11): 1624-30 (2020).
- Katagiri Y, Onuma Y, Asano T, et al. Three-year follow-up of the randomised comparison between an everolimus-eluting bioresorbable scaffold and a durable polymer everolimus-eluting metallic stent in patients with ST-segment elevation myocardial infarction (TROFI II trial). Eurointervention. 14(11): e1224-6 (2018).
- Byrne RA, Alfonso F, Schneider S, et al. Prospective, randomized trial of bioresorbable scaffolds vs. everolimus-eluting stents in patients undergoing coronary stenting for myocardial infarction: The intracoronary scaffold assessment a randomized evaluation of Absorb in myocardial infarction (ISAR-Absorb MI) trial. Eur Heart J 40(2): 167-76 (2019).
- Wiebe J, Schneider S, Cassese S, et al. Everolimus-eluting bioresorbable scaffolds vs. drug-eluting stents in patients with acute myocardial infarction: 2-year results of the randomized ISARAbsorb MI Trial. J Am Coll Cardiol. 74(13): Supple B11 (2019).
- Yamaji K, Raber L, Windecker S. What determines long-term outcomes using fully bioresorbable scaffolds-the device, the operator or the lesion? EuroIntervention. 12: 1684-7 (2017).
- Ormiston JA, Webber B, Ubod B, et al. An independent bench comparison of two bioresorbable drug-eluting coronary scaffolds (Absorb and DESolve) with a durable metallic drug-eluting stent (ML8/Xpedition). EuroIntervention. 11(1): 60-7 (2015).
- Kounis NG, Koniari I, Roumeliotis A, et al. Thrombotic responses to coronary stents, bioresorbable scaffolds, and the Kounis hypersensitivity-associated acute thrombotic syndrome. J Thorac Dis. 9(4): 1155-64 (2017).
- Cuculi F, Puricel S, Jamshidi P, et al. Optical coherence tomography findings in bioresorbable vascular scaffolds thrombosis. Circ Cardiovasc Interv. 8(10): e002518 (2015).