Device Evaluations - Interventional Cardiology (2012) Volume 4, Issue 1
Clinical review of the Resolute zotarolimus-eluting stent for the treatment of coronary artery disease
- Corresponding Author:
- Laura Mauri
Harvard Clinical Research Institute & Harvard Medical School
Boston, MA, USA
Tel: +1 617 732 8936
Fax: +1 617 525 9452
E-mail: lmauri1@partners.org
Abstract
Keywords
drug-eluting stents n restenosis n zotarolimus-eluting stent
Percutaneous treatment of epicardial coronary artery disease (CAD) with coronary stents has become a fundamental facet of CAD management. Coronary stenting is a more common revascularization strategy compared with coronary artery bypass graft surgery, with over 600,000 procedures performed last year in the USA alone, and over 2 million procedures annually are estimated worldwide [1,2]. While bare-metal stents (BMS) decreased the risk of acute vessel closure associated with balloon angioplasty, rates of restenosis requiring repeat procedures remained elevated at 10–30% at 1 year [3,4]. Restenosis following stenting is caused by vascular injury and inflammation in the stented vessel, leading to neointimal hyperplasia [5]. Drug-eluting stents (DES) deliver pharmacologic agents with antiproliferative properties directly to the vessel intima, thereby reducing neointimal hyperplasia caused by arterial injury. The first DES were introduced in 2003 – the sirolimus-eluting stent (SES; Cypher®, Cordis Corporation, NJ, USA) and the paclitaxel-eluting stent (Taxus™ Liberté™, Boston Scientific, MA, USA; Table 1), and were associated with a reduced risk of target vessel revascularization (TVR) by 75–80% compared with BMS [6,7]. Early studies of these DES were conducted in native, de novo, single coronary artery stenoses; however, up to 70% of patients undergoing percutaneous coronary intervention (PCI) with a DES have comorbid conditions or complex lesions that put them at higher risk for restenosis [2,8]. Additionally, a possible increased risk for late (30 days to 1 year) and very late (>1 year) stent thrombosis (ST) has been observed with DES compared with BMS [9,10]. Safety, particularly regarding the risk of ST, in more complex patients and lesions treated beyond the scope of randomized trials, has been a concern for all stent types. The US FDA convened an advisory panel meeting in 2006 to evaluate the safety of DES. Pooled analysis of randomized DES versus BMS trials did not reveal an increased hazard of Academic Research Consortium (ARC)-defined ST with DES use over 4-year follow-up, but concerns regarding late ST, particularly in the broader population, remained. Since that time, additional data have been acquired in observational datasets to evaluate the safety of these stents in unrestricted patient populations. Current guidelines recommend thienopyridine therapy in addition to aspirin (dual antiplatelet therapy [DAPT]) for 12 months after treatment with DES. Guidelines from the European Society of Cardiology, which recommend 6–12 months of DAPT following stenting.
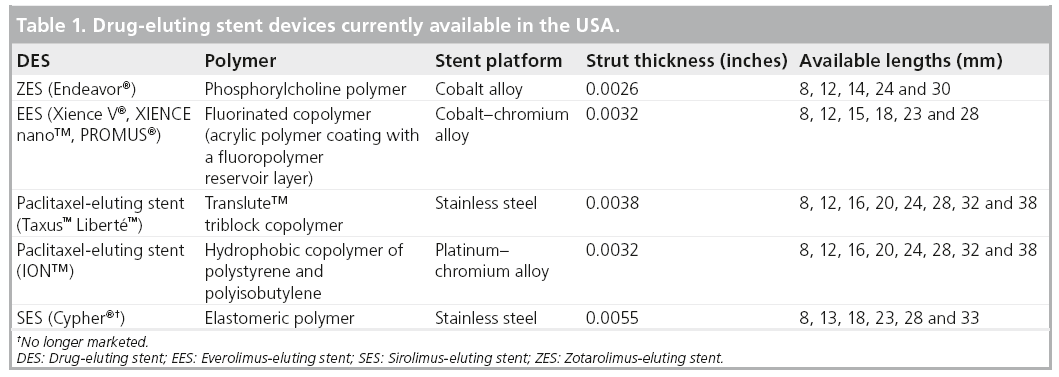
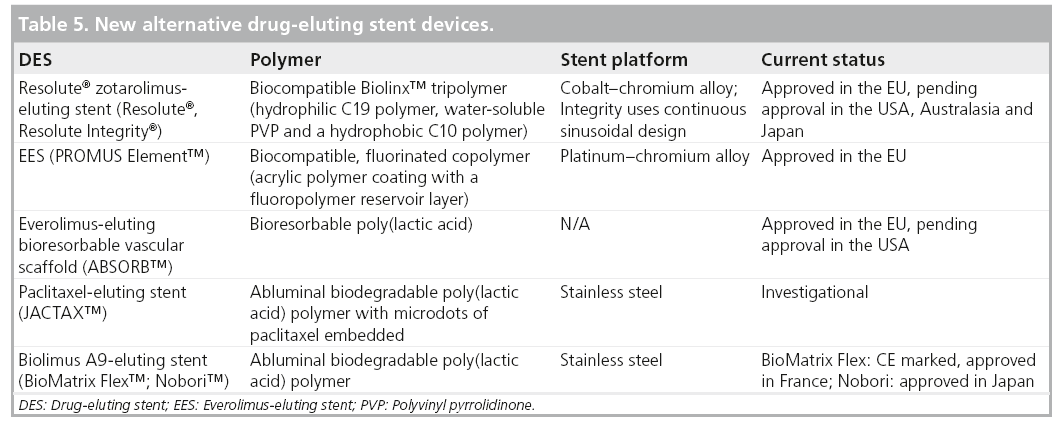
New DES have focused on refining the stent design, polymer chemistry and local pharmacologic effect in order to improve clinical outcomes and enhance safety in both simple and complex patients. In 2008, two DES were approved for use in the USA, a zotarolimus-eluting stent (ZES; the Endeavor® ZES [E-ZES], Medtronic Cardiovascular, Inc., Ca, USA) and an everolimus-eluting stent (EES; Xience V® EES, Abbott Laboratories, Abbott Park, IL, USA, and PROMUS®, Boston Scientific, MA, USA; Table 1). The new Resolute® ZES (R-ZES; Medtronic Cardiovascular, Inc., Santa Rosa, California) is CE marked in Europe and pending FDA approval in the USA.
As a result of heightened attention to safety and efficacy in broad patient populations, randomized trials to evaluate DES have gradually become more inclusive [11]. In addition, pivotal trials such as those required for FDA approval have begun to allow treatment of smaller vessels and multivessel disease, in order to include more complex patient and lesion subsets. The RESOLUTE clinical trials program discussed here is an example of the current approach to obtain a core set of lesion-specific data, while also examining clinical outcomes in a broad population.
Overview of market & unmet clinical needs
It is currently estimated that 70% of stents implanted are DES [12]. Many of these cases fall into a higher risk, complex or off-label category where the efficacy of individual DES is less well-defined. Furthermore, whether stent type affects ST risk is not well understood. It is possible that variation in drug, polymer and stent characteristics could translate into differences in the risk of this rare event. ST risk, particularly late-ST risk, is a factor to be optimized in the development of new DES.
In pooled data from the E-ZES clinical trial program, the cumulative 5-year incidence of definite or probable ST was favorably compared with BMS, while target lesion revascularization (TLR) was significantly lower (7.0 vs 16.5%; adjusted hazard ratio [HR]: 0.42; p < 0.001) [13]. A large, multicenter, prospective trial designed to compare the incidence of clinical events, including ST, in patients receiving an E-ZES or a SES has recently completed enrolment [14]. The study has enrolled a more broadly inclusive population of patients with multivessel disease and acute myocardial infarction (MI) and will provide important long-term safety data. The R-ZES was designed to provide the same efficacy and safety as the E-ZES, with improved efficacy in more complex lesions and patients.
Introduction to R-ZES device
▪▪ Device description
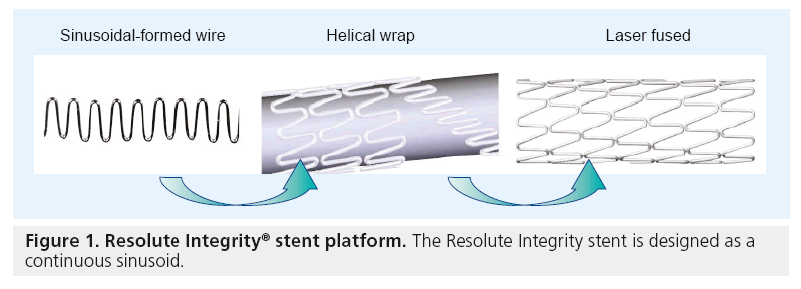
The R-ZES was developed with the same antiproliferative drug (zotarolimus) and platform as the E-ZES that is currently available in the USA, but uses a new biocompatible tripolymer (Biolinx™) to prolong drug elution [15]. The R-ZES Endeavor stent platform is a thin-strut (0.0036”) cobalt–chromium alloy mounted on a low-profile balloon. A new Integrity® stent platform, engineered with a continuous sinusoid geometry (Figure 1). The Integrity platform provides improved flexibility and smoother tracking for reduced vessel trauma. The Integrity platform is currently used in the R-ZES in most European countries and is likely to be used in the USA and Japan once the device receives regulatory approval in those countries. Both R-ZES devices have identical stent dimensions, drug-dose and drug-elution profiles.
Polymer
The proprietary BioLinx polymer is a blend of three polymers – a hydrophilic C19 polymer, a water-soluble polyvinyl pyrrolidinone (PVP) and a hydrophobic C10 polymer. Despite the hydrophobic nature of the C10 component, the higher concentration of the outer hydrophilic PVP component makes this an overall hydrophilic polymer, with biocompatibility proven in preclinical studies [16]. The PVP component promotes the initial drug burst from the stent surface. The C10 hydrophobic polymer allows the slow, controlled elution of the remaining drug. The drug elution characteristics are such that 50% of the zotarolimus in the stent is eluted within the first week after deployment. The remaining drug continues to elute out to 180 days, which may prove more effective in higher restenosis risk lesions [17,18].
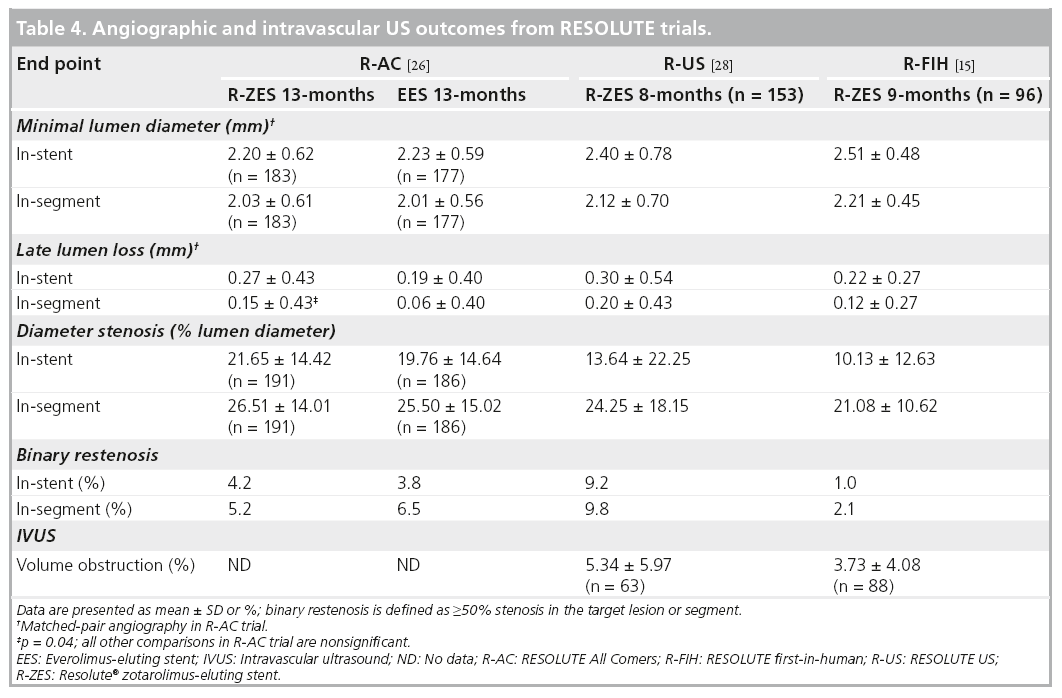
Two RESOLUTE substudies have highlighted the low neointimal obstruction associated with the durable tripolymer of the R-ZES [19,20]. Intravascular ultrasound (IVUS) results from the f irst-in-man RESOLUTE trial, reported a low (3.7%) neointimal obstruction at 9 months in 88 patients [19]. The authors postulated that the substantially longer drug elution time of the R-ZES likely accounts for this result. Optical coherence tomography was used to compare the tissue coverage of the R-ZES and the fluoropolymer-coated EES in patients treated at prespecified optical coherence tomography sites in the RESOLUTE All Comers (R-AC) trial [19].This substudy evaluated 30 R-ZES patients and 28 EES patients with 13-month followup. No differences in neointimal hyperplasia volume (R-ZES: 15.9 mm3; EES: 18.7 mm3; p = 0.274) or neointimal volume obstruction (R-ZES: 12.5%; EES: 15.0%; p = 0.157) were found between the groups. Incomplete stent apposition was low for both stents (R-ZES: 1.8%; EES: 1.4%; p = 0.569) and strut coverage was similar at 92.6% for R-ZES and 94.2% for EES [20].
Zotarolimus
Zotarolimus (formerly ABT-578) is a cytostatic analog made by substitution of the native hydroxyl group at position 42 in rapamycin with a tetrazole ring [21]. This drug is highly lipophilic with an octanol–water partition coefficient greater than sirolimus and paclitaxel. This lipophilic nature prevents rapid dissolution of the drug into the circulation and allows slower elution from hydrophobic components of stent polymers, thereby facilitating enhanced drug levels in the arterial wall around the stent. The lipophilicity of zotarolimus also facilitates crossing of the cell membrane to reach the drugs site of action [21,22].
The mechanism of action of zotarolimus is inhibition of proliferation of smooth muscle cells that occurs in response to expansion of stents against the artery wall. This proliferation is initiated by the release of several growth factors, including PDGF isoforms, b-FGF and thrombin [23,24]. Zotarolimus inhibits events that occur downstream of the binding of these growth factors to cell surface receptors. Zotarolimus binds to an intracellular immunophilin, FKBP-12. This complex secondarily binds to mTOR, a key protein kinase which phosphorylates and inactivates proteins associated with translation. This process results in the arrest of the cell cycle in the G1 phase and secondarily prevents cell proliferation [21]. Research using a porcine coronary artery model of restenosis, confirmed that zotarolimus bound to FKBP-12 inhibited proliferation of smooth muscle cells and endothelial cells, and further demonstrated that neointimal formation at 28 days was significantly inhibited with a ZES [25].
▪▪ Preclinical studies
Monocyte adhesion studies have shown that that activated monocytes do not adhere to the BioLinx polymer and preclinical studies further support the noninflammatory nature of the tripolymer blend [17,26]. Inflammation scores from porcine studies of the R-ZES show equivalent biocompatibility compared with the E-ZES. Scanning electron microscope studies show endothelialization as soon as 28 days out and by 180 days there is confluent endothelialization [24].
Clinical profile
▪▪ Overview of RESOLUTE program
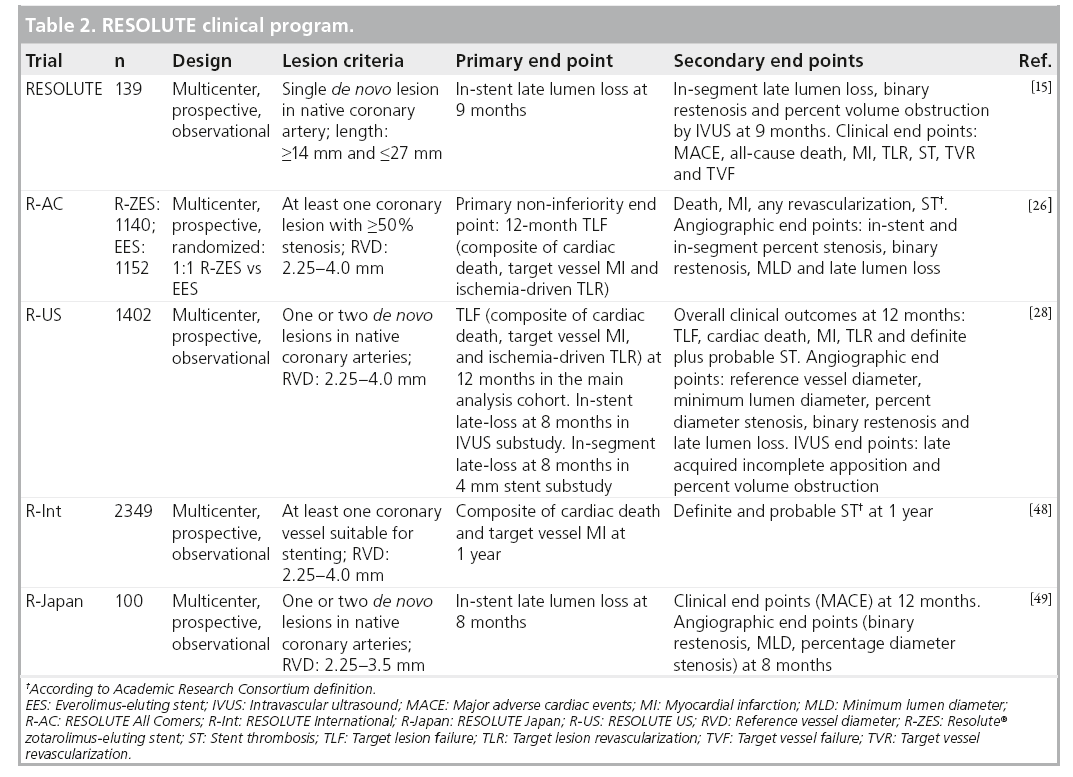
The RESOLUTE clinical program consists of the following multicenter, prospective studies: RESOLUTE [15] – a single-arm first-in-human study of 139 patients; R-AC [27] – a randomized, controlled trial comparing R-ZES and EES in a 1:1 randomization; RESOLUTE International (R-Int) [28] – an observational study of 2200 patients with minimal exclusion criteria; and RESOLUTE US (R-US) [29] – an observational trial of 1241 patients compared with historical controls (Table 2). The RESOLUTE trials were designed using similar end point definitions, adjudication processes and data collection procedures, allowing consistent interpretation and comparison of results.
RESOLUTE first-in-human trial
RESOLUTE was a multicenter, prospective, first-in-human study of 139 patients with de novo native coronary lesions with reference vessel diameters ≥2.5 and ≤3.5 mm, and lesion length ≥14 and ≤27 mm [15]. Exclusion criteria included patients with MI within 72 h of PCI, left ventricular ejection fraction <30%, left main or ostial lesions, severe calcification by angiography and bifurcation lesions. The first 30 patients were included in a 4-month quantitative coronary angiography and IVUS follow-up evaluation, and the next 100 patients underwent quantitative coronary angiography and IVUS evaluation at 9 months postprocedure. Patients were prescribed DAPT (aspirin 75-mg daily plus clopidogrel 300-mg loading dose and 75-mg daily thereafter) for 6 months and aspirin to continue indefinitely. The primary and secondary end points of the trial are described in Table 2. The 9-month in-stent late lumen loss was 0.22 ± 0.27 mm and TLR rates were 0.0, 0.8 and 1.5% at 9, 12 and 24 months, respectively. In comparison, the 1-year late lumen loss was 0.18 mm for the Xience V EES at 1 year [30], 0.09 mm for the Cypher SES at 8 months [31], 0.31 for the Taxus paclitaxel-eluting stent at 8 months [31] and 0.61 mm for the E-ZES at 1-year follow-up [32]. There were no definite and probable ST events through 24-month follow-up. These results are sustained at 4-year follow-up with a cumulative TLR rate of 2.3% and 0.0% ST [33]. This feasibility study provided the safety and efficacy data for the R-ZES in de novo coronary lesions as the groundwork for subsequent larger studies in a broader patient population.
RESOLUTE All Comers
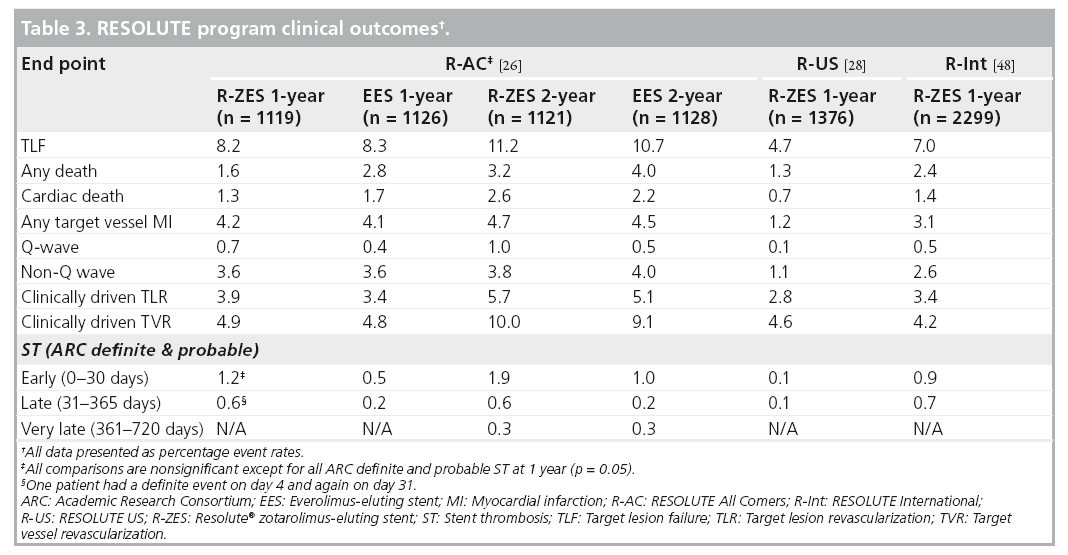
The R-AC trial randomized 2300 patients in 17 European sites in a 1:1 fashion to the R-ZES and EES, with 13‑month planned angiographic follow-up in 230 patients in each arm [27]. Enrolled patients had at least one coronary lesion 2.25–4.0 mm in diameter, with >50% stenosis and minimal exclusion criteria. Approximately two-thirds of the patients were considered to be complex, defined by the following criteria; placement of a stent in a patient with one or more of the following clinical or lesion characteristics: renal insufficiency, ejection fraction of <30%, occurrence of acute MI within the previous 72 h, >1 lesion per vessel, ≥2 vessels with stents, a lesion measuring >27 mm, bifurcation, bypass grafts, in-stent restenosis, unprotected left main artery, lesions with thrombus or total occlusion. DAPT was prescribed for 6 months. The primary non-inferiority end point of target lesion failure (TLF) was met (R-ZES 8.2% vs EES 8.3%, pnon-inferiority < 0.001). There were no significant differences in cardiac death, target vessel MI or ischemia-driven TLR (Table 3). These results were consistent across all relevant subgroups, including patients with diabetes mellitus (DM), multivessel disease, bifurcation lesions, long lesions, overlapping stents, left main lesions and bypass graft disease. There was a higher rate of ARC-defined definite or probable ST in the R-ZES patients (1.6 vs 0.7%; p = 0.05) which is primarily accounted for by early events (0–30 days). 1-year definite ST rate was higher in the R-ZES arm (1.2 vs 0.3%; p < 0.05) and the rate of ARC-definite/probable ST at 1 year was 1.6 versus 0.7% in the R-ZES versus EES patients, respectively (rate difference 0.9; 95% CI: 0.0 and 1.8, respectively; p = 0.05). However, this did not translate into increased rates of clinical end points such as cardiac death or target vessel MI at 1-year follow-up.
Prespecified 2-year clinical outcomes of the R-AC trial demonstrated sustained safety and efficacy for the R-ZES and EES [34]. There was no difference in patient-related outcomes (any death, MI or revascularization) or stentrelated outcomes (TLF, cardiac death, target vessel MI or ischemia driven TLR). There were three additional very late (between 1 and 2 years follow-up) ST events in each treatment arm, with no associated mortality.
RESOLUTE US
The R-US trial studied 1402 patients with 1- or 2-vessel CAD at 116 centers across the USA [29]. Lesions were suitable for 2.25- to 4.0-mm stents. The study enrolled 1242 patients in the clinical cohort. A subset of this group, with 2.5- to 3.5-mm stents and a single lesion, comprised the main analysis cohort (n = 1001). The remaining 241 patients were treated with a 2.25 mm stent and/or had two lesions treated. A separate angiographic cohort enrolled 160 patients. The main analysis cohort compared R-ZES treatment outcomes with historical controls derived from the ENDEAVOR clinical program (ENDEAVOR II, ENDEAVOR IV, ENDEAVOR PK and ENDEAVOR II Continued Access trials) [35–37]. The patients in the main analysis cohort were more complex (smaller mean reference vessel diameter and more DM) than historical control patients treated with the E-ZES. The primary end point of 12-month rate of TLF for the main analysis cohort was 3.7% compared with 6.5% for E-ZES controls (propensity score adjusted one-sided 95% CI upper limit: -1.3%; pnon-inferiority < 0.001; psuperiority = 0.002). The 12-month rates of cardiac death, MI and TLR in the main analysis cohort were 0.4, 1.3 and 2.0%, respectively. Overall clinical outcomes for patients with 2.25–4.0 mm stents at 12-months are shown in Table 2. The in-stent binary restenosis rate was 9.2% for the R-ZES stent group; this did not translate into an increased rate of clinical events at 12-month follow-up. There were two ST events – an early probable ST and a definite ST after 30 days – both in 2.25 mm stents. The rate of DAPT was 93.3% at 12-months in the R-US trial.
RESOLUTE International
The R-Int trial enrolled 2349 patients (3147 lesions) from 88 centers [27]. The only requirement for enrollment was the intent to implant at least one R-ZES (2.25–4.0 mm stent diameter). There were no exclusions for complex patient or lesion characteristics and no restriction on the number of lesions treated. DAPT was recommended for 6 months and continuation of DAPT was at physician discretion thereafter. More than two-thirds of the patients met the prespecified off-label or complex criteria (the same complex criteria as used in R-AC). Chronic total occlusions were present in 6.3% of patients, bifurcation in 18.2% of lesions and multivessel treatment was performed in 14% of patients. The rate of DM was 30.5% and 46% of patients had an acute coronary syndrome with 27% presenting with an MI. The composite primary end point of cardiac death and target vessel MI at 1 year was 4.3%. Most of the events (2.9%) occurred before 30 days postprocedure and were more likely to be a periprocedural MI (3.1%) rather than cardiac death (1.4%).
Clinically driven 1-year rates of TLR and TVR were similar to results observed in the R-AC trial (Table 2). ARC-defined definite and probable ST at 1 year was 0.9% with most events occurring early (0.7%) and three events (0.1%) occurring after 30 days.
RESOLUTE pooled safety
The RESOLUTE clinical program has studied the R-ZES in over 5100 patients, including a large proportion of patients with complex clinical and lesion characteristics. Consistent efficacy was shown across all the clinical trials. However, low frequency events such as ST are more difficult to interpret in individual trials that are underpowered to determine significant differences between stent types. Since the RESOLUTE trials were designed with similar event definitions, adjudication and data management methodology, the data from these trials could be pooled for analysis of rare events [38]. Propensity scores were used to adjust for between-patient variation across comparisons. Safety end points analyzed from the R-ZES pooled data set were death, cardiac death, MI and ST, which were compared with the R-AC trial data for the patients receiving the EES. Safety outcome data for a cohort of patients without complex clinical or lesion characteristics who underwent single lesion treatment, was also compared with data from patients who had undergone PCI with a BMS in the ENDEAVOR II randomized trial. The BMS treated patients were prescribed 3 months of DAPT and all R-ZES patients received 6 months of DAPT.
All safety end points were similar between the R-ZES and EES patients. The cumulative 2-year rate of cardiac death and MI was 5.4% for the R-ZES group and 6.2% for the EES group (adjusted HR for R-ZES vs EES: 0.873; 95% CI: 0.65 and 1.17, respectively; p = 0.37) [38]. TLR rates were 5.0 and 5.2% for R-ZES and EES, respectively (adjusted HR: 0.985; 95% CI: 0.71 and 1.36, respectively; p = 0.93). Importantly, the 2-year cumulative ARC-defined definite and probable ST rates were 1.0% for both R-ZES and EES patients (adjusted HR: 1.335; 95% CI: 0.66 and 2.71, respectively; p = 0.42).
Death, cardiac death and MI were also similar between the R-ZES subset and BMS historical control patients. The 2-year rate of cardiac death and MI was 4.3% for the R-ZES subset and 5.8% for the BMS control group (adjusted HR for R-ZES vs BMS; 0.880; 95% CI: 0.55 and 1.40, respectively; p = 0.59). 2-year TLR and ST rates were significantly lower for the R-ZES patients compared with the BMS patients. TLR rates at 2 years were 3.8% for R-ZES and 14.2% for BMS (adjusted HR; 0.194; 95% CI: 0.13 and 0.29, respectively; p = 0.19). The ARC-defined definite and probable ST rates were 0.3% in the R-ZES subset and 1.3% in the BMS patients (adjusted HR: 0.206; 95% CI: 0.06 and 0.71, respectively; p = 0.01).
▪▪ Angiography & IVUS outcomes
The R-AC trial was powered for non-inferiority testing of 13-month in-stent percent diameter stenosis [26]. The prespecified criterion for non-inferiority of this angiographic end point was met with 21.65 ± 14.42% in the R-ZES group and 19.76 ± 14.64% in the EES group (pnon-inferiority= 0.04). In-stent and in-segment binary restenosis rates were low in both R-ZES and EES groups (Table 4).
A subset of patients within the RESOLUTE first-in-human and R-US trials underwent angiographic and IVUS analysis at 8 or 9 months after stent placement [15,28]. Angiographic end points in the less complex, single-lesion patients enrolled in the first RESOLUTE trial, were 10.13% instent diameter stenosis and 1.0% in-stent binary restenosis and 13.64% and 9.2%, respectively, in the R-US trial. There were six cases of late incomplete apposition, defined as failure of the stent to completely appose the vessel wall that was not observed post stent placement (one required TLR), in the first-in-human trial and one case (1.7%) of late incomplete apposition in the R-US trial (Table 4).
▪▪ RESOLUTE outcomes in high-risk patients
Clinical studies have identified patients at higher risk for restenosis and stent thrombosis. DM, recent acute MI, chronic renal insufficiency, left main coronary artery or ostial disease and chronic total occlusions are known risk factors for poorer outcomes following DES placement, and many of these characteristics are excluded from registration studies. Recent studies have expanded eligibility to include more complex patient and lesion characteristics in order to more fully understand appropriate treatment options for these patients. The R-AC and the R-Int trials both enrolled a high proportion of complex patients and demonstrated encouraging safety and efficacy in higher risk populations.
A substudy of the R-AC trial examined the impact of patient and lesion complexity on outcomes after R-ZES versus EES treatment [39]. The complex subgroup included patients presenting with acute MI within 72 h (43.2%) and patients with left ventricular ejection fraction <30%. The mean SYNTAX score was 16.6 ± 9.4 in the complex R-ZES patients, compared with 11.2 ± 7.9 in the simple R-ZES patients. Approximately a fourth of R-ZES and EES patients in the complex group had at least one bifurcation or at total occlusion. The target vessel was a bypass graft in 3.7% of patients in the complex group. Overall event rates were higher in complex versus noncomplex patients (n = 1520) but were similar between the two treatment arms (R-ZES vs EES) with no significant difference in risk between complex and noncomplex patients (p = 0.43 for interaction on TLF at 1 year). Additionally, ARC-defined definite plus probable ST was not significantly different between R-ZES and EES in the complex and simple subgroups.
Diabetes mellitus
DM is a predictor of poor prognosis in patients with CAD. Multiple studies have shown poor outcomes, with increased rates of in-stent restenosis, repeat revascularization, ST and death in patients with DM undergoing PCI [40–43]. Studies have reported DES TLR rates as high as 18% among patients with DM and as high as 35% in patients who are insulin- dependent [44]. Revascularization rates are even greater in patients undergoing multivessel PCI [45].
Approximately one-third of the patients enrolled in R-US presented with DM and 9.6% were insulin-dependent [29]. This subgroup was included in a prespecified analysis of outcomes in those treated with 2.5- to 3.5-mm R-ZES. The rate of TLF at 12 months was 4.3% (cardiac death: 0.5%; MI: 0.8%; TLR: 3.0%), similar to the overall trial population, confirming the consistent efficacy of R-ZES in this high-risk patient group.
Bifurcation lesions
Bifurcations are complex, technically challenging coronary lesions [46]. Appropriate stent selection is an important consideration, yet sufficient clinical data for various DES options are lacking. The R-ZES has recently been investigated in a three-center Italian registry of patients with >70% stenosis at a major bifurcation point, main vessel diameter >2.5 mm and side-branch diameter >2.0 mm. PCI was performed using a provisional T-stenting approach [47]. The primary end point of the study was freedom from major adverse cardiac events at 9 months. The secondary end point was procedural success defined as post-PCI stenosis <20% in the main vessel and thrombolysis in myocardial infarction 3 flow in the main vessel and side-branch. A total of 180 consecutive patients were enrolled – 29% had DM and 62.8% presented with acute coronary syndrome. The target bifurcation was in an unprotected left main artery in 16.7% of patients. The procedural success rate was 98.3% and major adverse cardiac event-free survival at 9 months was 97.8%. No definite, probable or possible stent thromboses occurred during the study.
How R-ZES fits into the field of medical devices
As a group of medical devices, DES are an example of what was a breakthrough technology in 2003, becoming a subject of more mature product development and investigation over a rapid time frame during the past decade (Table 5). The interactions between stent design, polymer chemistry and drug-delivery are better understood and allow for more rational selection of these components in the design of new devices. Safety concerns surrounding very late ST with use of DES have lingered and BMS have generally been looked upon as the gold standard for safety. DES have provided the standard for efficacy given the significant reduction in TVR associated with their use. More recent trials have neutralized the safety concerns. For instance, pooled data from the RESOLUTE trials comparing over 6000 patients treated with R-ZES with a contemporary DES (Xience EES) and BMS were recently presented at the 2011 Euro- PCR meeting in France [38]. Clinically driven TVR at 3 years was lower in the DES group. Interestingly, ST rates were lower in the DES group compared with the BMS group. Similar and low rates of target vessel failure and ST were observed for both DES types over a 3-year follow-up. The R-US clinical trial showed a significant reduction in target vessel failure in the R-ZES stent group compared with historical controls of the E-ZES stent, despite inclusion of 2.25 mm lesions and a greater proportion of patients with DM in this study. These recent trials represent a paradigm shift in the perception of DES safety and suggest that DES use in complex is reasonable.
Conclusion
The R-ZES has an innovative biocompatible polymer that allows for a sustained release of zotarolimus over a longer duration. Introduction of new DES such as the R-ZES hold the promise of improved performance based on a unique combination of a biocompatible polymer, proven antiproliferative drug and a modern flexible stent design. This combination appears to be very effective and safe for both the average and the complex coronary lesion or patient. While results are available to 2 years of follow-up, ongoing follow-up to 5 years in this program will help define the long-term safety profile of this new stent.
Future perspective
As DES technology has progressed over the past decade, it has become clear that different drug, polymer and stent characteristics yield devices that are not interchangeable. While the success of early DES facilitated the treatment of a larger proportion of coronary lesions, allowing more patients to have relief of angina with a less invasive method than coronary artery bypass surgery, future device evaluation will require successive evaluation of novel devices in studies ranging from proof-of-principle to broadly inclusive designs, to observational, population-based studies. While at its core, PCI is a mechanical procedure, pharmaceutical and materials science has had a profound impact on the long-term success of these procedures. In order to allow continued innovation while preserving safety, creative device evaluation programs are essential.
Financial & competing interests disclosure
L Mauri receives institutional research support from Cordis, Medtronic, Abbott Vascular, Boston Scientific, Eli Lilly, Daiichi Sankyo, Bristol-Myers Squibb and Sanofi-Aventis and is a consultant to Medtronic and Cordis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Executive summary
Device description
▪▪ The Resolute® zotarolimus-eluting stent (R-ZES) combines the antiproliferative drug, zotarolimus, with a biocompatible tripolymer on a thin-strut cobalt–chromium stent platform.
▪▪ Zotarolimus is a cytostatic rapamycin analog that inhibits smooth muscle proliferation and reduces neointimal hyperplasia.
▪▪ Zotarolimus concentration is 1.6 μg/mm2 stent length.
▪▪ A cobalt–chromium alloy is used in the original Endeavor® Resolute stent and the new Resolute Integrity® stent.
▪▪ The Integrity bare-metal stent platform is improved by the use of a continuous sinusoidal design to improve flexibility and tracking.
▪▪ The Biolinx™ polymer is a blend of a hydrophilic C19 polymer, water-soluble polyvinyl pyrrolidinone and a hydrophobic C10 polymer.
▪▪ The polymer blend provides an initial drug burst followed by slow elution of zotarolimus over 180 days.
▪▪ The polymer is biocompatible with a hydrophilic surface.
Clinical outcomes
▪▪ Clinically driven target lesion revascularization (TLR) rates in patients with single de novo lesions range from 0.7–2.0% and 3.4–3.9% in more complex patient populations.
▪▪ R-ZES target lesion failure (TLF) is noninferior to the everolimus-eluting stent (EES; 8.2 vs 8.3%; p < 0.001).
▪▪ No significant differences in cardiac death, target vessel myocardial infarction (MI) or ischemia-driven TLR in RESOLUTE All Comers (R-AC) trial at 1 and 2 years. Results were consistent across all relevant subgroups.
▪▪ Low rates of definite and probable stent thrombosis (ST) across trials at 1 year (0.0% RESOLUTE first-in-human; 1.6% R-AC; 0.1% RESOLUTE US [R-US]; 0.9% RESOLUTE International).
▪▪ Pooled analysis of adverse events showed similar cumulative incidence of cardiac death or MI (5.4% for R-ZES and 6.2% for EES) and definite or probable ST (1.0% for both R-ZES and EES).
Angiographic outcomes
▪▪ Late lumen loss ranges from 0.22 ± 0.27 mm to 0.30 ± 0.54 mm.
▪▪ Rates of in-stent binary restenosis: 1.0% (RESOLUTE first-in-human), 4.2% (R-AC) and 9.2% (R-US).
▪▪ In-stent percent diameter stenosis: 10.1%.
Outcomes in complex patients
▪▪ 66.3% of patients in R-AC were complex; event rates were higher in both R-ZES and EES complex lesion groups but similar across stent types:
– TLF: 8.9% for R-ZES and 9.7% for EES (p = 0.66);
– TLR: 4.4% for R-ZES and 4.0% for EES (p = 0.80);
– Death or MI: 5.6% for R-ZES and 7.4% for EES (p = 0.17);
– Definite or probable ST (overall): 1.7% for R-ZES and 0.9% for EES (p = 0.26);
– In-stent binary restenosis: 5.1% for R-ZES and EES (p = 1.00).
▪▪ Diabetes mellitus: 34.4% of patients in R-US – similar outcomes with TLF of 4.3% among patients with and without diabetes.
▪▪ Bifurcation lesions: R-ZES was safe and effective in treatment of bifurcation lesions in a registry of 180 patients. At 9 months the rate of freedom from major adverse coronary events was 97.8% and there were no ST events.
▪▪ Resolute Integrity ZES approved for use in EU, Israel, India, Columbia, Bosnia, Serbia and Macedonia; Investigational in USA, Canada, Australia and Japan.
▪▪ Endeavor Resolute ZES approved in Australia, EU, Israel, Latin America, South Africa and India; investigational in the USA, Canada and Japan.
References
- Roger VL, Go AS, Lloyd-Jones DM et al. Heart disease and stroke statistics: 2011 update: a report from the American Heart Association. Circulation 123(4), e18–e209 (2011).
- Kirtane AJ, Gupta A, Iyengar S et al. Safety and efficacy of drug-eluting and bare-metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation 119(25), 3198–3206 (2009).
- Stettler C, Wandel S, Allemann S et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370(9591), 937–948 (2007).
- Abbott JD, Voss MR, Nakamura M et al. Unrestricted use of drug-eluting stents compared with bare-metal stents in routine clinical practice: findings from the National Heart, Lung and Blood Institute Dynamic Registry. J. Am. Coll. Cardiol. 50(21), 2029–2036 (2007).
- Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation 105(25), 2974–2980 (2002).
- Moses JW, Leon MB, Popma JJ et al. Sirolimuseluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315–1323 (2003).
- Stone GW, Ellis SG, Cox DA et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- Curfman GD, Morrissey S, Jarcho JA, Drazen JM. Drug-eluting coronary stents – promise and uncertainty. N. Engl. J. Med. 356(10), 1059–1060 (2007).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxeleluting coronary stents. N. Engl. J. Med. 356(10), 998–1008 (2007).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 52(14), 1134– 1140 (2008).
- Windecker S, Serruys PW, Wandel S et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomized non-inferiority trial. Lancet 372(9644), 1163–1173 (2008).
- King SB 3rd, Smith SC Jr, Hirshfeld JW Jr et al. 2007 focused update of the ACC/AHA/ SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J. Am. Coll. Cardiol. 51(2), 172–209 (2008).
- Mauri L, Massaro JM, Jiang S et al. Long-term clinical outcomes with zotarolimus-eluting versus bare-metal coronary stents. JACC Cardiovasc. Interv. 3(12), 1240–1249 (2010).
- Camenzind E, Wijns W, Mauri L et al. Rationale and design of the Patient Related OuTcomes with Endeavor versus Cypher stenting Trial (PROTECT), randomized controlled trial comparing the incidence of stent thrombosis and clinical events after sirolimus or zotarolimus drug-eluting stent implantation. Am. Heart J. 158(6), 902–909 (2009).
- Meredith IT, Worthley S, Whitbourn R et al. Clinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trial. JACC Cardiovasc. Interv. 2(10), 977–985 (2009).
- Udipi K, Melder RJ, Chen M et al. The next generation Endeavor Resolute Stent: role of the BioLinx Polymer System. EuroIntervention 3(1), 137–139 (2007).
- Carter A, Ozdil F, Virmani R, Wilcox JN. In vivo performance of a novel copolymer system for extended release of zotarolimus in a next generation drug-eluting stent. Presented at: Transcatheter Cardiovascular Therapeutics Symposium. Washington, DC, USA, 22–27 October 2006.
- Meredith IT, Worthley S, Whitbourn R et al. The next-generation Endeavor Resolute stent: 4-month clinical and angiographic results from the Endeavor Resolute first-in-man trial. EuroIntervention 3(1), 50–53 (2007).
- Waseda K, Ako J, Yamasaki M et al. Short- and mid-term intravascular ultrasound analysis of the new zotarolimus-eluting stent with durable polymer: results from the RESOLUTE trial. Circulation J. 74(10), 2097–2102 (2010).
- Gutierrez-Chico JL, van Geuns RJ, Regar E et al. Tissue coverage of a hydrophilic polymer-coated zotarolimus-eluting stent vs a fluoropolymer-coated everolimus-eluting stent at 13-month follow-up: an optical coherence tomography substudy from the RESOLUTE All Comers trial. Eur. Heart J. 32(19), 2454–2463 (2011).
- Burke SE, Kuntz RE, Schwartz LB. Zotarolimus (ABT-578) eluting stents. Adv. Drug Deliv. Rev. 58(3), 437–446 (2006).
- Brugaletta S, Burzotta F, Sabate M. Zotarolimus for the treatment of coronary artery disease: pathophysiology, DES design, clinical evaluation and future perspective. Expert Opin. Pharmacother. 10, 1047–1058 (2009).
- Segev A, Aviezer D, Safran M, Gross Z, Yayon A. Inhibition of vascular smooth muscle cell proliferation by a novel fibroblast growth factor receptor antagonist. Cardiovasc. Res. 53(1), 232–241 (2002).
- Badimon L, Meyer BJ, Badimon JJ. Thrombin in arterial thrombosis. Haemostasis 24(2), 69–80 (1994).
- Garcia-Touchard A, Burke SE, Toner JL, Cromack K, Schwartz RS. Zotarolimuseluting stents reduce experimental coronary artery neointimal hyperplasia after 4 weeks. Eur. Heart J. 27(8), 988–993 (2006).
- Udipi K, Chen M, Cheng P et al. Development of a novel biocompatible polymer system for extended drug release in a next-generation drug-eluting stent. J. Biomed. Mat. Res. 85(4), 1064–1071 (2008).
- Serruys PW, Silber S, Garg S et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N. Engl. J. Med. 363(2), 136–146 (2010).
- Neumann FJ, Belardi JA. One-year outcomes of patients with the zotarolimus-eluting coronary stent: RESOLUTE International Registry. Eurointervention (2011) (In press).
- Yeung AC, Leon MB, Jain A et al. Clinical evaluation of the Resolute zotarolimus-eluting coronary stent system in the treatment of de novo lesions in native coronary arteries: the RESOLUTE US clinical trial. J. Am. Coll. Cardiol. 57(17), 1778–1783 (2011).
- Tsuchida K, Piek JJ, Neumann FJ et al. One-year results of a durable polymer everolimus-eluting stent in de novo coronary narrowings (the Spirit First trial). EuroIntervention 1(3), 266–272 (2005).
- Morice MC, Colombo A, Meier B et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: The Reality trial – a randomized controlled trial. JAMA 295(8), 895–904 (2006).
- Meredith IT, Worthley S, Whitbourn R et al. The next-generation endeavor resolute stent: 4-month clinical and angiographic results from the endeavor resolute first-in-man trial. EuroIntervention 3(1), 50–53 (2007).
- Meredith IT. TCT-238: 4-year clinical outcomes from the RESOLUTE first-in-man trial. J. Am. Coll. Cardiol. 56(13), B55 (2010).
- Silber S, Windecker S, Vranckx P, Serruys PW. Unrestricted randomised use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the Resolute All Comers trial. Lancet 377(9773), 1241–1247 (2011).
- Fajadet J, Wijns W, Laarman GJ et al. Randomized, double-blind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trial. Circulation 114(8), 798–806 (2006).
- Leon MB, Mauri L, Popma JJ et al. A randomized comparison of the ENDEAVOR zotarolimus-eluting stent versus the TAXUS paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the Endeavor IV trial. J. Am. Coll. Cardiol. 55(6), 543–554 (2010).
- Schultheiss HP, Grube E, Kuck KH et al. Safety of direct stenting with the Endeavor stent: results of the Endeavor II continued access registry. EuroIntervention 3(1), 76–81 (2007).
- Mauri L, Leon M, Yeung A et al. Pooled analysis of the Resolute programme clinical safety results. Presented at: EuroPCR 2011. Paris, France, 17–20 May 2011.
- Stefanini GG, Serruys PW, Silber S et al. The impact of patient and lesion complexity on clinical and angiographic outcomes after revascularization with zotarolimus- and everolimus-eluting stents: a substudy of the RESOLUTE All Comers Trial (a randomized comparison of a zotarolimus-eluting stent with an everolimus-eluting stent for percutaneous coronary intervention). J. Am. Coll. Cardiol. 57(22), 2221–2232 (2011).
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology and management. JAMA 287(19), 2570–2581 (2002).
- Stein B, Weintraub WS, Gebhart SP et al. Influence of diabetes mellitus on early and late outcome after percutaneous transluminal coronary angioplasty. Circulation 91(4), 979–989 (1995).
- Donahoe SM, Stewart GC, McCabe CH et al. Diabetes and mortality following acute coronary syndromes. JAMA 298(7), 765–775 (2007).
- Elezi S, Kastrati A, Pache J et al. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J. Am. Coll. Cardiol. 32(7), 1866–1873 (1998).
- Mehran R, Dangas GD, Kobayashi Y et al. Short- and long-term results after multivessel stenting in diabetic patients. J. Am. Coll. Cardiol. 43(8), 1348–1354 (2004).
- Banning AP, Westaby S, Morice MC et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J. Am. Coll. Cardiol. 55(11), 1067–1075 (2010).
- Stankovic G, Darremont O, Ferenc M et al. Percutaneous coronary intervention for bifurcation lesions: 2008 consensus document from the fourth meeting of the European Bifurcation Club. EuroIntervention 5(1), 39–49 (2009).
- Tommasino A, Burzotta F, Sciahbasi A et al. Procedural and clinical evaluation of the novel zotarolimus-eluting resolute stent in patients with unselected bifurcated coronary stensis treated by provisional approach: a multicenter registry. J. Invas. Cardiol. 23, 50–54 (2011).
- TCT-282: one year outcomes of patients with Resolute zotarolimus eluting stent: results of the RESOLUTE International Registry. J. Am. Coll. Cardiol. doi:10.1016/j. jacc.2010.08.306 (2012).
- Saito S. Medtronic Resolute® drug-eluting stent shows superiority to Taxus® DES in study of patients with coronary artery disease. Presented at: The 2011 Annual Metting of the Japanese Association of Cardiovascular Intervention and Therapeutics. Osaka, Japan, July 2011.
▪ Prospective observational study of the Resolute® zotarolimus-eluting stent compared with historical cohorts derived from the ENDEAVOR trials. Demonstrated safety and efficacy of the Resolute stent.
▪ Randomized clinical trial comparing the Resolute zotarolimus-eluting stent with the Xience V® everolimus-eluting stent in an inclusive cohort with more complex coronary disease. A 2-year follow-up on patient and stent-level end points demonstrated no difference between the two drug-eluting stent types.