Review Article - Interventional Cardiology (2012) Volume 4, Issue 4
Clinical strategy for treating renal artery stenosis and contemporary tactics for renal artery stenting
- Corresponding Author:
- Aravinda Nanjundappa
Department of Surgery, Robert C. Byrd Health Sciences Center
West Virginia University School of Medicine Charleston Division
3100 MacCorkle Ave SE, Charleston, WV 25304, USA
Tel: +1 304 347 1371
Fax: +1 304 344 5536
E-mail: dappamd@yahoo.com
Abstract
Keywords
angioplasty, atherosclerotic, hypertension, ischemic,ephropathy, renal artery stenosis, renal function, stent, stenting
It has been almost eight decades since the landmark work by Goldblatt et al. on renovascular hypertension and 35 years since the first renal artery balloon angioplasty [1,2]. Renal artery balloon angioplasty was initially limited by vessel recoil and prohibitive restenosis rates but these tactical issues were overcome with the advent of the endovascular architectural support provided by a stent [3–5]. We now know that clinical recurrence of restenosis and need for target vessel revascularization ranges from 10 to 30% after stenting and thus, catheter intervention now seems a durable and less-invasive alternative to surgery [6–8]. The tactical approach to renal intervention was enhanced by the availability of stents and improved catheter designs. Unfortunately, the strategic fundamentals remain controversial as the literature is populated with poorly designed prospective trials and observational data [9,10]. The goal of this invited review is to summarize the current literature and provide a template for the individual operator to develop their own renal stenting strategy and tactics until definitive data become available. For the purposes of this analysis, we define ‘strategy’ as a high-level view of the clinical condition and the risk:benifit of intervention versus medical therapy, whereas ‘tactics’ are more related to the execution of treatment and accordingly focus on technique and required experience.
Renal artery stenosis treatment strategy
Defining the optimal treatment strategy for renal artery stenosis (RAS) requires a fundamental understanding of the disease prevalence and pathophysiology. End-organ knowledge is essential in the strategic decision process fundamentals and is not intuitive. In addition, knowledge of the natural history and procedural risks is critical for considering an individual patient risk:benifit analysis. Finally, the operator training and center outcomes must be considered at the strategic level if intervention is to be contemplated.
▪ Prevalence of RAS
RAS is second only to sleep apnea as a cause for secondary hypertension and may be present in 1–6% of all hypertensive patients [11].
Atherosclerotic disease is the leading cause of RAS in the USA, although nonatherosclerotic diseases may account for as many as many as 60% of RAS seen in India and the far east [12–14]. Fundamental knowledge of this condition is important for practicing interventional cardiologists, since incidental RAS is seen in 36% of patients with coronary artery disease, and the finding is more common in the presence of three-vessel and/or left main disease [15,16].
Atherosclerotic RAS (ARAS) is a frequently missed disease entity and becomes much more prevalent with increased age, as reflected by the finding of significant ARAS in over 40% of patients older than 75 years of age during autopsy [17,18]. In addition to age, ARAS is seen in 39–59% of patients with peripheral arterial disease. Population-based studies suggest that ARAS increases with age, elevated lipid levels and systolic blood pressure, while there seemed to be no association with ethnicity [19]. Unsuspected RAS is seen in 24% of patients aged 45–75 years who present with renal insufficiency (serum creatinine ≥2.00 mg/dl) [20]. RAS was found in 50% of patients over 50 years old with who had end-stage renal artery disease [21]. RAS is common in patients with peripheral arterial disease and its prevalence is closely associated with atherosclerosis elsewhere in the body [22,23].
▪ Natural history
Initial enthusiasm regarding renal stenting was driven by the perception that surgical intervention was poorly tolerated in these advanced atherosclerosis patients and concern regarding disappointing medical therapy results due to the unfavorable natural history. For example, in 14,000 patients with abdominal angiography performed during left heart catheterization at Duke University (NC, USA), incidental ARAS was seen in 1659 patients and significant progression was observed in 11.1% in an average of 2.5 years [24]. Eugene Standness and his team conducted landmark duplex studies in the 1990s that prospectively followed 295 stenotic renal vessels for 33 months; frequent progression was observed, although occlusion occurred in only 3% [25]. The factors predicting progression were: >60% RAS at baseline, elevated systolic blood pressure and diabetes mellitus [25]. Some natural history studies suggested that the ‘wait-and-see’ approach for patients with RAS may lead to dialysis but there are no prospective randomized trials to confirm that intervention will change this course [26,27].
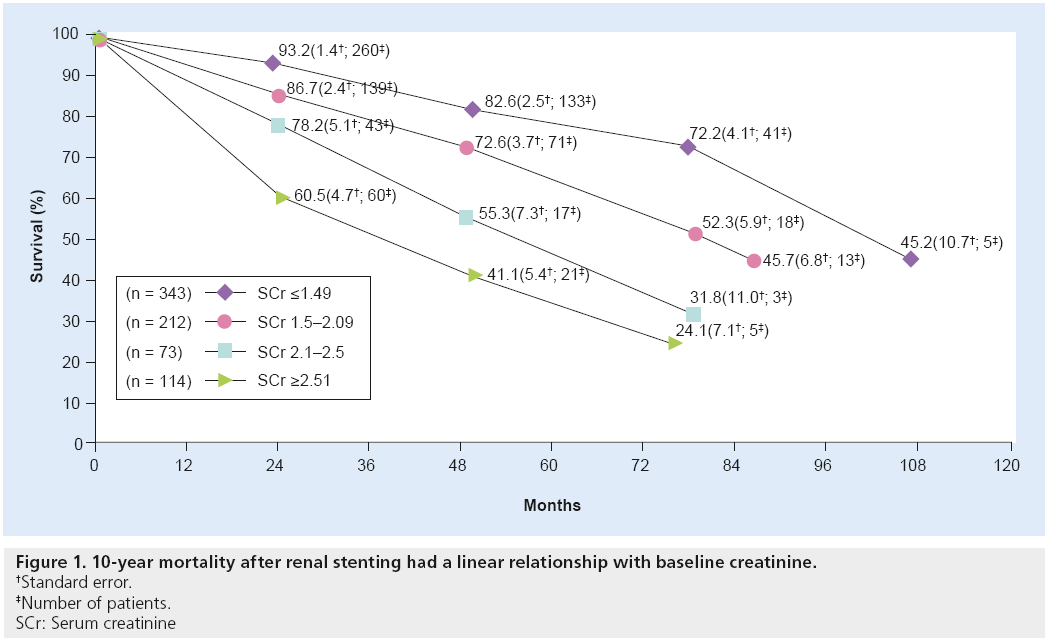
In 3987 patients undergoing abdominal angiography in a study by Conlon et al., the 4-year survival rate was 89% in those without RAS, but only 57% in individuals with RAS. However, the change in outcome may not have been impacted by the RAS as much as the fact that RAS is a marker of advanced vascular disease. There was a graded effect of RAS on survival, with those having stenosis >75% having lower survival than those with >50% RAS [28]. In the SOCRATES series, the 10-year mortality rate after renal stenting had a linear relationship with baseline creatinine (Figure 1) [29]. There is no clear correlation between glomerular filtration rate (GFR) and the extent of RAS, although a high mortality rate is noted in this specific subset of ARAS and renal insufficiency [30].
▪ End-organ pathophysiology
RAS leads to ischemia, which triggers release of renin. Renin facilitates the conversion of angiotensin I to angiotensin II, which leads to vasoconstriction and the release of aldosterone [31]. The volume changes and the renin–angiotensin system blockade depend on the presence of a functioning kidney. It is believed that the unilateral hemodynamically significant RAS begins with euvolemic hyper-reninemic-induced hypertension, as the contralateral healthy kidney compensates for the sodium retention [32,33]. The renin levels then tend to retreat as the disease progresses and the patient has severe hypertension with increased volume and paradoxically normal renins. In the final or third phase of renovascular hypertension, elevated blood pressure persists despite restoration or flow to the renal artery due to irreversible vascular and renal parenchyma damage. This concept of irreversible damage was demonstrated by histology in both stenotic and nonstenotic kidneys [34]. The nonstenotic kidney may be damaged by hypertensive nephrosclerosis, recurrent ischemia, oxidative stress, cytokines such as TNF and interstitial fibrosis. Chade et al., from the Mayo clinic (MN, USA), had shown that hypercholesterolemia plays a distinct detrimental role in renal function in individuals with renal artery stenosis [34]. The oxidative stress, along with inflammatory and growth changes, were seen at the tubular and glomerular level. Currently there are no definitive predictors of patients with RAS who have reached an irreversible stage of disease. In our experience, severe cortical thinning, significant renal atrophy and parenchymal vascular pruning/ branch vessel disease may be anatomic clues, particularly in patients with a low trans-stenotic gradient and slow baseline flow.
Strategy of medical therapy versus intervention
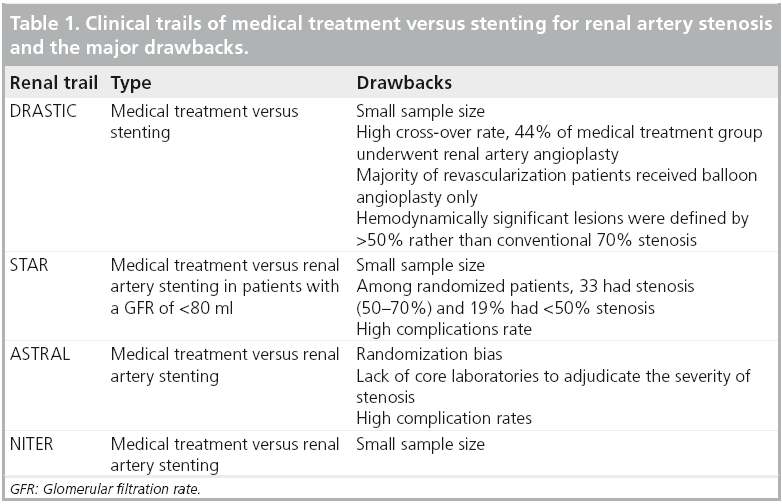
The last 3 decades have witnessed thousands of renal artery interventions performed worldwide [35]. Recently, the procedure has come under scrutiny in response to randomized trials, suggesting there may be limited benefit to stenting [36]. Unfortunately, all these trials had critical flaws limiting the clinical applicability (Table 1). These recent randomized studies did suggest that renal stenting should be avoided in patients with borderline lesions and in azotemic patients without hypertension. The most interesting inclusion criteria in the largest, most quoted trial (ASTRAL) were to randomize the patient when the physician was uncertain as to whether renal stenting would be beneficial [37]. Bhatt et al. conducted a meta-analysis of group of patients with significant RAS and hypertension and/or chronic renal insufficiency. This group consisted of patients who were randomized to medical therapy plus RAS versus medical therapy alone. The outcomes, such as hypertension, serum creatinine, mortality, congestive heart failure and renal function, were compared between the two arms. This metaanalysis included all randomized clinical trials that randomized RAS patients, in a prospective manner, to RAS or medical treatment. A total of six trials were included: EMMA, SNRASCG, DRASTIC, ASTRAL, STAR and NITER. The overall change in systolic blood pressure from baseline was analyzed and a 1.2-mm Hg (95% CI: p = 0.32) difference between the RAS and medical therapy arms was noted. An overall decrease in diastolic pressure of 1.6 mm of Hg with RAS compared with medical therapy was not significant (95% CI: p = 0.23). The RAS resulted in a significant reduction in the number of antihypertensive medications compared with medical therapy: 2.67 versus 2.92 (p < 0.001). The serum creatinine showed a reduction of 0.14 mg/dl with RAS at the end of follow-up compared with medical therapy (2.0 vs 2.2 mg/dl [p = 0.06]), which was not statistically significant. All-cause mortality occurred in 14.9% in the RAS arm versus 15.4% in the medical therapy arm (p = 0.76). The incidence of congestive heart failure was 9.8 versus 12.2% (p = 0.20) in the RAS and medical therapy arms, respectively. The worsening of renal function occurred in 11.5 versus 12.6% in RAS versus medical therapy (p = 0.54). The meta-analysis concluded that in patients with significant RAS and hypertension and/or chronic renal insufficiency, RAS was not associated with change in systolic and diastolic blood pressure, or serum creatinine. However, there was a significant reduction in the number of antihypertensive medications at the end of follow-up. There was no significant difference in all-cause mortality, congestive heart failure, stroke and change in renal function between the two arms [38].
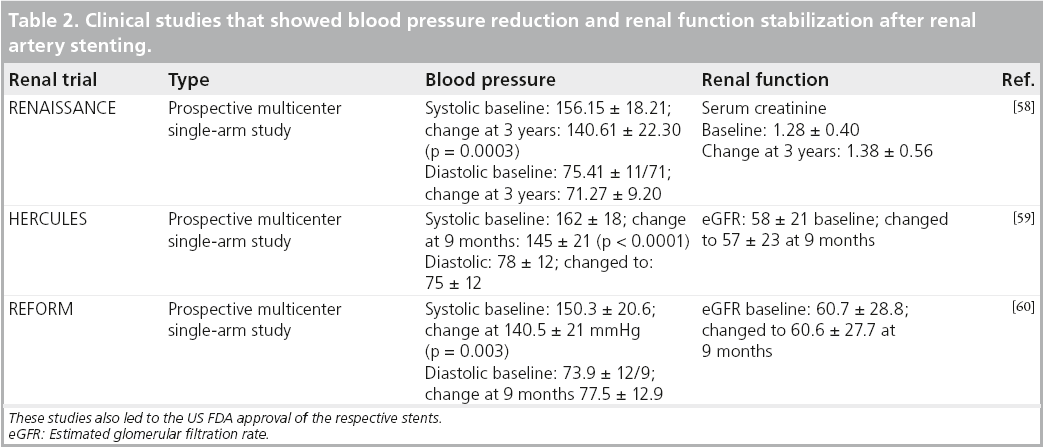
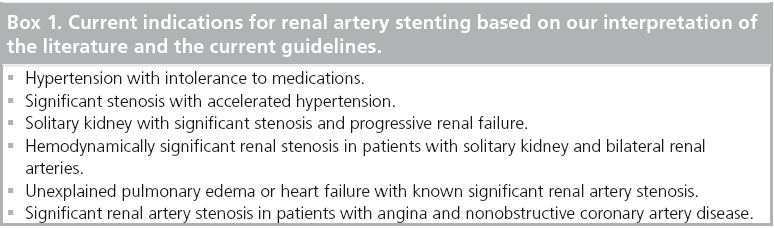
In most academic centers, the borderline lesions/indications and normotensive patients were not undergoing interventions, so these recent studies should not change practice patterns. There are many studies that have confirmed blood pressure decreases and stabilizing and/or that azotemia improves after stenting, but these had only historical controls (Table 2). Based on our interpretation of the literature and the current guidelines, the current indications for renal stenting are detailed in Box 1.
The patients who benefit from renal artery stenting are [39]:
▪ Those with asymptomatic stenosis;
▪ Those with asymptomatic bilateral or solitary viable kidney with a hemodynamically significant RAS: class IIb, level of evidence C;
▪ In patients with asymptomatic unilateral hemodynamically significant RAS in viable kidney, percutaneous revascularization is not clinically proven: class IIb, level of evidence C;
▪ Patients with hypertension;
▪ Patients with accelerated hypertension, resistant hypertension, malignant hypertension and uncontrolled hypertension intolerant to medications and hemodynamically significant RAS: class IIa, level of evidence B;
▪ Those who require preservation of renal function;
▪ Patients with hemodynamically significant bilateral RAS or a functioning solitary kidney with RAS and progressive chronic kidney disease: class IIa, level of evidence B;
▪ Patients with unilateral hemodynamically significant RAS and chronic renal failure: class IIb, level of evidence C;
▪ Those with congestive heart failure and unstable angina;
▪ Patients with hemodynamically significant RAS and unexplained f lash pulmonary edema: class I, level of evidence B;
▪ Patients with hemodynamically significant RAS and unstable angina: class IIa, level of evidence B.
We believe that the CORAL trail will provide more answers to the dilemmas and controversies of renal artery stenting [40]. However, this trial may also be limited by selection bias and heterogeneity of technique and operator experience.
▪ Best medical therapy
All patients with ARAS should undergo aggressive secondary prevention and risk factor reduction, similar to atherosclerotic lesions in other vascular beds [41,42]. These patients will rarely have a ‘hypertension cure’ post-renal artery revascularization. The subgroup of ischemic nephropathy among ARAS carries a dismal prognosis. A subgroup of 83 patients among 683 dialysis patients had concomitant ARAS and the survival at 10 years was 5% [43]. The use of angiotensin-converting enzyme (ACE) inhibitors in patients with bilateral RAS or RAS in a solitary kidney can lead to an irreversible decline in renal function, especially after unexpected dehydration in older patients with congestive heart failure [44,45]. The renin–angiotensin system is the main controller of intravascular volume. In patients with essential hypertension, the renal vascular tone is elevated and ACE inhibitors can augment the renal blood flow. This results in salt and water excretion resulting in reduction of blood pressure. In patients with renal artery stenosis, the angiotensin-mediated mechanism can improve afferent arteriolar dilatation and increase post-glomerular resistance. Suppression of such angiotensin in bilateral RAS or RAS with solitary kidney leads to reduction of glomerular filtration and can induce renal failure. Lower mortality rates and improved survival were seen in long-term follow-up of RAS treated with ACE inhibitors [46]. The recent CORAL trial prospectively randomized patients with RAS to optimal medical treatment versus optimal medical treatment plus renal artery stenting. The optimal medical treatment l included the use of angiotensin receptor blockers and aspirin. Patients in the CORAL trial received guidelinedriven treatment of blood pressure, LDL and HbA1c. Patients were further advised on smoking cessation. The primary outcomes that will be analyzed at 5 years will be cardiac or renal death, stroke and myocardial infarction, hospital admission for heart failure, progressive renal failure and hemodialysis [47].
Tactics to enhance interventional results
There is very little clinical feedback during renal artery intervention. The patient may have back or abdominal pain during balloon inflation and this can be an important point for operator feedback regarding balloon/stent sizing and/or potential rupture. Pain that persists or increases after balloon deflation should alert the physician to possible rupture and, in such cases, an angiogram should be completed before the balloon is retracted from the stent. Once the balloon is pulled free of the stent, the wire bias and/or poor balloon re-wrap may make advancement back across the stent impossible. Loss of balloon access and inability to quickly tamponade the rupture could result in catastrophic hemorrhage before catheter exchanges or covered stent deployment can be performed.
Unfortunately, there is generally no feedback regarding poor procedural technique and atheroembolism during renal intervention. Whereas during coronary intervention we get immediate feedback with pain and arrhythmias, and during a carotid artery intervention one sees neurological deficits or seizures, renal embolization rarely causes pain. Thus, poor techniques, such as scraping the guide and clumsy aggressive device movements, may cause significant, longterm, permanent renal injury while the operator assumes he or she has completed the case without incident. When the operator defines success as an open vessel and expanded stent, they falsely assume that no harm has been done. In our opinion, this is one of the key reasons for such a disparity in renal artery stent outcomes.
The peri-renal aorta is often diseased and fragile. Pathologic studies have shown that in the fourth, fifth and sixth decades of life, the peri-renal aorta often contains complicated plaques with early and late pathological intimal thickening, fibroatheromas and thin-cap fibroatheromas (cap thickness <155 microns), ruptured plaques, healed ruptures and fibrotic calcified plaques. In addition, there is often significant macrophage infiltration, particularly in the shoulder regions of the fibrous caps [48]. In the most advanced cases, you will find severe medical necrosis and plaque hemorrhage and, in these cases, the catheter may sink several millimeters into soft friable material with very little force.
We believe meticulous procedural technique is critical to the outcome of the individual patient and also must be standardized in clinical trials owing to the apparent narrow risk:benifit ratio of the procedure. The following suggested techniques (tactics) are based on our experience in hundreds of patients, review of the literature and individual anecdotal experience based on selective post-mortem studies. It should be noted that these suggestions are based on our experience and understanding of the disease and are not defined by rigorous evidence-based studies; thus, they must be considered in that context.
▪ Suggestions for tactical success Improve accuracy
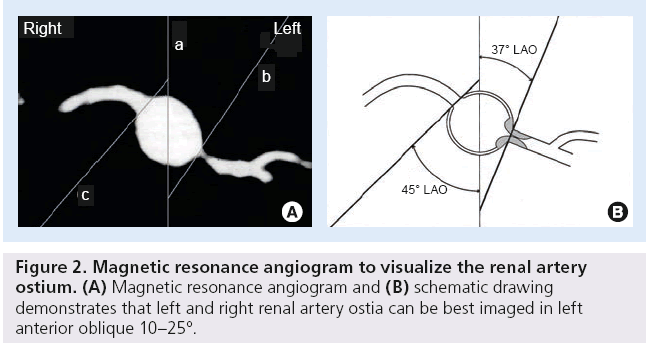
Geographic miss can cause unnecessary device manipulation, and expose patients and staff to excess fluoroscopy while also increasing contrast exposure in a population of patients that are often sensitive to contrast-induced renal injury. This complication can be minimized by a 3D understanding of the renal anatomy and well thought out access planning. The critical step here is to have the appropriate image intensifier angulations so that the ostium is not eclipsed by contrast reflux into the aorta. We showed, many years ago, that the right and left renal ostia are both best imaged in the left anterior oblique position (Figure 2) [49]. However, significant variation can occur, so using the baseline coronary computed tomography angiogram or magnetic resonance angiogram axial views (when available) to actually calculate the angle is ideal. Alternatively, using test injections during baseline imaging to find the ideal image angle may be helpful.
Reduce atheroembolization
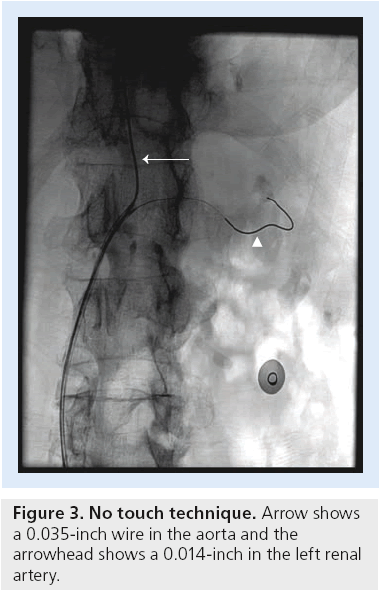
As detailed above, the peri-renal aorta can be very hostile so guide or sheath engagement must be carried out in the least traumatic way. Our team developed a dual-wire technique for access with a guide catheter from the femoral approach nearly 20 years ago. At that time, renal stenting required a 9-French guide via femoral access or a 6-French brachial 90-cm sheath approach to allow the high-profile hand-mounted stents to advance around the catheter bend without slipping back off the balloon. Through time, the femoral approach replaced the brachial access as the standard for our group, and most of our cases in the early 1990s were done with a double-wire technique. This technique was born and developed after a patient died from mesenteric embolization following renal stenting. Subsequently, Feldman and his team eloquently described a series of patients in whom the double-wire technique was used, at which time he coined the term ‘no-touch’ [50]. The procedure is pictured in Figure 3. It is critical to use the appropriate guide catheter and wires for this technique and we recommend a short transition stiff wire such as the Spartacore (Abbott Laboratories, IL, USA) to help prevent guide rotation and guide bouncing while also enhancing stent tracking. The alternative approach to avoid scraping of the aorta is to use a ‘telescoping technique’ to access the renal artery and perform stenting [50]. We use a special-ordered guide that is designed for the notouch technique called the Bates’ guide (available from multiple companies; note: we receive no financial remuneration for this product). The Bates’ 2 curve is best for standard aortas while the 1 curve is best for contralateral access and small aortas. Finally, the 3 curve is needed in unwound aortas, severe aortic angulations and in aneurysmal disease. Utilization of radial artery for vascular access and subsequent renal interventions may reduce the vascular access site-related complications in the future [51]. Selective use of embolic protection devices may also be reasonable in high-risk patients. The RESIST trial was a prospective randomized study designed to evaluate the impact of a distal filter and the GP IIb/IIIa inhibitor abciximab (Eli Lilly, IN, USA) during RAS [52]. Overall, 100 patients who underwent RAS were randomized to distal filter, distal filter plus abciximab, abciximab alone or control. The end point was change in GFR from baseline to 1 month. There was a 10% decrease in GFR in control patients and with abciximab only and a 12% decrease (p = 0.05) in GFR with the distal filter alone. The group with a distal filter and abciximab showed a 9% increase (p = 0.01) in GFR. This trial was not powered to provide conclusive evidence but suggests that further studies may be appropriate.
Reduce restenosis
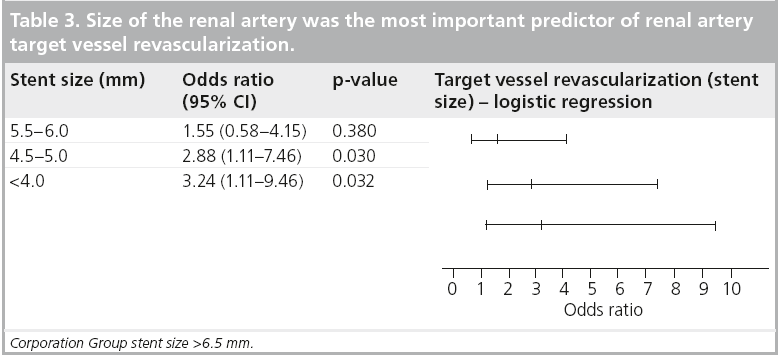
The GREAT trail evaluated the role of baremetal stents versus drug-eluting stents in the renal arteries. Despite a blood pressure improvement in both arms, there was no difference in the angiographic outcomes in terms of restenosis, target vessel revascularization, worsening of renal function and major adverse events [53]. We often consider a coronary drug-eluting stent in renal lesions <4.5 mm in size, since the renal artery size was the number one indicator of target lesion revascularization in our series. However, there are currently no level 1 data on drug-eluting stents in RAS, and this is considered ‘off-label’ in most countries (Table 3) [54].
Predictors of outcomes of renal artery stenting
The predictors that can improve outcome of renal artery stenting have been published in various studies. Lessar et al. investigated the accuracy of resting and hyperemic translesional pressure gradients, intravascular ultrasound and angiography in 62 patients who underwent RAS in predicting improvement in hypertension. A hyperemic systolic gradient (HSG) of >21 mm Hg had a sensitivity, specificity and accuracy of 82, 84 and 84%, respectively, in predicting improvement in hypertension after RAS. Improvement in hypertension was seen in 84% of patients with HSG >21 mm Hg versus 36% of patients with a HSG <21 mm Hg at 1 year (p < 0.01) [55]. Baseline estimated GFR (eGFR) as a predictor of improvement in hypertension has demonstrated mixed results. In one study, 61 patients with baseline elevated serum creatinine of >1.8 mg/dl underwent RAS. One of the end points considered among responders to RAS is an improvement in eGFR by 20%, when compared to baseline eGFR. 27% of patients were considered responders at 2 years. The rate of decline in renal function at baseline was an independent predictor of improved renal function after RAS (odds ratio: 3.4; 95% CI: 1.6–7.5; p = 0.0019) [56]. In a retrospective study by Beck et al., 129 patients (179 renal arteries) underwent RAS for renovascular hypertension. A baseline eGFR of <40 ml/min/1.73 m2 (odds ratio: 1.6; 95% CI: 1.0–2.9; p = 0.02) was an independent predictor of failure to achieve improvement in hypertension [57].
Future perspective
We learned from recent randomized trials that renal stenting should not be performed in patients with borderline indications and/or in centers with high complication rates. In addition, the risk of atheroembolization during renal artery access and intervention has likely been underestimated. The outcomes of renal artery stenting could be dramatically impacted by procedural techniques and operator experience. We believe that in the future, the following events will further illuminate the best strategy and tactics:
▪ The development of more sensitive markers of renal cell injury will provide needed feedback regarding ideal technique and use of protection devices. Renal protection may become mandatory when specific renal protection systems specifically designed for renal artery anatomy are available, but this will require randomized data;
▪ Catheter designs and stent profiles will continue to improve and further reduce risk, and may allow transition to radial access for improved access trajectory;
▪ We believe that geographic miss can be reduced if not eliminated by ideal image intensifier obliquity and adequate operator experience as detailed above. However, ostial location systems may improve ease of use. The unintended consequence of injury to the often hostile contiguous peri-renal aorta is a theoretical concern with all these devices;
▪ Once data-driven procedural technique guidelines are available and clinical, as well as anatomic, patient selection criteria are well defined, a threshold for center outcomes must then be declared. We currently believe that renal stenting should not be carried out in centers that have a higher than 3% major adverse event rate, but this must be confirmed using statistically valid metrics in randomized trials;
▪ Additional data will likely illuminate which patients (if any) would benefit from drugeluting stents or covered stents. It may be that small renal vessels (<5 mm) should receive drug-eluting stents while covered stents should be reserved for large-vessel restenosis. Trials are needed to see if the theoretical advantage of covered stents in reducing embolization and restenosis is not countered by the higher-profile and late-thrombosis risk.
Conclusion
Renal artery stenting improves blood pressure in patients with recalcitrant hypertension and may stabilize or improve renal function in select patients. Randomized data would be helpful to confirm current recommended anatomic and clinical indications. However, the ideal ‘wellcontrolled’ trial may be elusive since randomization of critical lesions in patients known to benefit may not occur in all sites, leaving a critical selection bias flaw. For today, stenting should be reserved for patients with clear indications and only be conducted in experienced centers with acceptable outcomes.
Executive summary
▪ Renal artery stenosis (RAS) represents an important manifestation of systemic atherosclerosis.
▪ The three cardinal cardiovascular manifestations of RAS are secondary hypertension, pulmonary edema and renal insufficiency.
▪ Management of renal artery stenosis continues to evolve and remains controversial.
▪ Risk factor management to address atherosclerosis is pivotal in patients with RAS.
▪ Percutaneous revascularization should be considered in RAS patients with uncontrolled hypertension (140/90) on three anti-hypertensives, flash pulmonary edema and progressive renal insufficiency.
▪ Renal artery stenting in multiple US FDA-approved clinical trials have demonstrated reduction in systolic blood pressure and stabilization of renal function.
▪ Comparative studies and randomized trials are essential to understand the patients who may benefit from renal artery revascularization and standardize procedural techniques.
▪ We believe renal artery stenting is safe and effective when performed for appropriate indications in patients with significant RAS.
▪ Renal artery stenting should be performed by appropriately trained operators at centers with acceptable clinical outcomes.
Financial and competing interests disclosure
A Nanjundappa has no disclosures to make. MC Bates is Founder and patent holder of Nexeon MedSystem and Presidio Medical, and Board member and consultant for CeloNova Biosciences. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J. Exp. Med. 59, 347–348 (1934).
- Grüntzig A, Kuhlmann U, Vetter W, Lütolf U, Meier B, Siegenthaler W. Treatment of renovascular hypertension with percutaneous transluminal dilatation of a renal-artery stenosis. Lancet 1(8068), 801–802 (1978).
- Martin LG, Cork RD, Kaufman SL. Long-term results of angioplasty in 110 patients with renal artery stenosis. J. Vasc. Interv. Radiol. 3, 619–626 (1992).
- Plouin PF, Chatellier G, Darne B. et al. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 31, 823–829 (1998).
- van de Ven PJ, Kaatee R, Beutler JJ et al. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trial. Lancet 353(9149), 282–286 (1999).
- Dorros G, Jaff M, Mathiak L et al. Four-year follow-up of Palmaz–Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation 98, 642–647 (1998).
- White CJ, Ramee SR, Collins TJ et al. Renal artery stent placement: utility in lesions difficult to treat with balloon angioplasty. J. Am. Coll. Cardiol. 30, 1445–1450 (1997).
- Cambria RP. Surgery: indications and variables that affect procedural outcome, as well as morbidity and mortality. J. Invasive Cardiol. 10, 55–58 (1998).
- Sarac TP. Influence and critique of the ASTRAL and CORAL trials. Semin. Vasc. Surg. 24(3), 162–166 (2011).
- Rabbia C, Pini R. Evidence-based medicine in renal artery stenting. J. Cardiovasc. Surg. (Torino) 51(5), 755–763 (2010).
- Simon N, Franklin SS, Bleifer KH, Maxwell MH. Clinical characteristics of renovascular hypertension. JAMA 220(9), 1209–1218 (1972).
- Kalra PA, Guo H, Kausz AT et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 68(1), 293–301 (2005).
- Plouin PF, Perdu J, La Batide-Alanore A, Boutouyrie P, Gimenez-Roqueplo AP, Jeunemaitre X. Fibromuscular dysplasia. Orphanet. J. Rare Dis. 2, 28 (2007).
- Cheung CM, Hegarty J, Kalra PA. Dilemmas in the management of renal artery stenosis. Br. Med. Bull. 73–74, 35–55 (2005).
- Stancanelli B, Maugeri E, Nicosia A et al. Coronary heart disease extension as a predictor of atherosclerotic renal artery stenosis. J. Nephrol. 21(3), 421–425 (2008).
- Crowley JJ, Santos RM, Peter RH et al. Progression of renal artery stenosis in patients undergoing cardiac catheterization. Am. Heart J. 136(5), 913–918 (1998).
- Schwartz CJ, White TA. Stenosis of the renal artery: an unselected necropsy study. BMJ 2, 1415–1421 (1964).
- Choudhri AH, Cleland JGF, Rowlands PC et al. Unsuspected renal artery stenosis in peripheral vascular disease. BMJ 301, 1197–1198 (1990).
- Hansen KJ, Edwards MS, Craven TE et al. Prevalence of renovascular disease in the elderly: a population-based study. J. Vasc. Surg. 36(3), 443–451 (2002).
- O’Neil EA, Hansen KJ, Canzanello VJ, Pennell TC, Dean RH. Prevalence of ischemic nephropathy in patients with renal insufficiency. Am. Surg. 58(8), 485–490 (1992).
- Scoble JE, Maher ER, Hamilton G, Dick R, Sweny P, Moorhead JF. Atherosclerotic renovascular disease causing renal impairment – a case for treatment. Clin. Nephrol. 31(3), 119–122 (1989).
- Missouris CG, Buckenham T, Cappuccio FP, MacGregor GA. Renal artery stenosis: a common and important problem in patients with peripheral vascular disease. Am. J. Med. 96, 10–14 (1994).
- Olin JW, Melia M, Young JR et al. Prevalence of atherosclerotic renal artery stenosis in patients with atherosclerosis elsewhere. Am. J. Med. 88(1), N46–N51 (1990).
- Crowley JJ, Santos RM, Peter RH et al. Progression of renal artery stenosis in patients undergoing cardiac catheterization. Am. Heart J. 136(5), 913–918 (1998).
- Caps MT, Perissinotto C, Zierler RE et al. Prospective study of atherosclerotic disease progression in the renal artery. Circulation 98(25), 2866–2872 (1998).
- Scoble JE. Is the ‘wait-and-see’ approach justified in atherosclerotic renal artery stenosis? Nephrol. Dial. Transplant. 10(5), 588–589 (1995).
- Fehr T, Rickli H, Müller J, Wüthrich RP, Ammann P. Kidney at risk: 11‑year course of renal artery stenosis. Nephrol. Dial. Transplant. 18(2), 443–444 (2003).
- Conlon PJ, Little MA, Pieper K, Mark DB. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 60, 1490–1497 (2001).
- Bates MC, Campbell JE, Stone PA, Jaff MR, Broce M, Lavigne PS. Factors affecting long-term survival following renal artery stenting. Catheter. Cardiovasc. Interv. 69(7), 1037–1043 (2007).
- Suresh M, Laboi P, Mamtora H, Kalra PA. Relationship of renal dysfunction to proximal arterial disease severity in atherosclerotic renovascular disease. Nephrol. Dial. Trans. 15(5), 631–636 (2000).
- Goldblatt H, Lynch J, Hanzal R, Summerville W. Studies on experimental hypertension I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J. Exp. Med. 59, 347–379 (1934).
- Kaplan NM. Renal vascular hypertension. In: Clinical Hypertension (7th Edition). Lippincott Williams & Wilkins, MD, USA, 301–321 (1998).
- Ploth DW. Renovascular hypertension. In: The Principles and Practice of Nephrology (2nd Edition). Jacobson H, Striker G, Klahr S (Eds). BC Decker, PA, USA, 379–386 (1995).
- Chade AR, Rodriguez-Porcel M, Grande JP et al. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 106(9), 1165–1171 (2002).
- Murphy TP, Soares G, Kim M. Increase in utilization of percutaneous renal artery interventions by medicare beneficiaries, 1996–2000. AJR Am. J. Roentgenol. 183(3), 561–568 (2004).
- Henry M, Benjelloun A, Henry I, Polydorou A, Hugel M. Renal angioplasty and stenting: is it still indicated after ASTRAL and STAR studies? J. Cardiovasc. Surg. (Torino) 51(5), 701–720 (2010).
- George JC, White CJ. Renal artery stenting: lessons from ASTRAL (Angioplasty and Stenting for Renal Artery Lesions). JACC Cardiovasc. Interv. 3(7), 786–787 (2010).
- Kumbhani DJ, Bavry AA, Harvey JE et al. Clinical outcomes after percutaneous revascularization versus medical management in patients with significant renal artery stenosis: a meta-analysis of randomized controlled trials. Am. Heart J. 161(3), 622-630.e1 (2011).
- Hirsch AT, Haskal ZJ, Hertzer NR et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/ Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J. Am. Coll. Cardiol. 47, 1239–1312 (2006).
- Cooper CJ, Murphy TP, Matsumoto A et al. Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am. Heart J. 152(1), 59–66 (2006).
- Hirsch AT, Cirqui MH, Treat-Jacobson D et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 286(11), 1317–1324 (2001).
- Safian RD, Textor SC. Renal artery stenosis. N. Engl. J. Med. 344(6), 431–442 (2001).
- Mailloux LU, Napolitano B, Bellucci AG, Vernace M, Wilkes BM, Mossey R. Renal vascular disease causing end-stage renal disease, incidence, clinical correlates, and outcomes: a 20-year clinical experience. Am. J. Kidney Dis. 24(4), 622–629 (1994).
- Burnier M, Waeber B, Nussberger J, Brunner HR. Effect of angiotensin converting enzyme inhibition in renovascular hypertension. J. Hypertens. Suppl. 7(7), S27–S31 (1989).
- Hollenberg NK. Renal hemodynamics in essential and renovascular hypertension. Influence of captopril. Am. J. Med. 31, 76(5B), 22–28 (1984).
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensinconverting- enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 342(3), 145–153 (2000).
- Murphy TP, Cooper CJ, Dworkin LD et al. The cardiovascular outcomes with renal atherosclerotic lesions (CORAL) study: rationale and methods. J. Vasc. Interv. Radiol. 16(10), 1295–1300 (2005).
- Van Dijk RA, Virmani R, von der Thüsen JH, Schaapherder AF, Lindeman JH. The natural history of aortic atherosclerosis: a systematic histopathological evaluation of the peri-renal region. Atherosclerosis 210(1), 100–106 (2010).
- Bates MC, Crotty B, Kavasmaneck C, Campbell JR. Renal artery angiography: ‘the right ipsilateral oblique’ myth. Catheter. Cardiovasc. Interv. 67(2), 283–287 (2006).
- Feldman RL, Wargovich TJ, Bittl JA. No-touch technique for reducing aortic wall trauma during renal artery stenting. Catheter. Cardiovasc. Interv. 46(2), 245–248 (1999).
- Christopher J, White MD. Advances in interventional cardiology: optimizing outcomes for renal artery intervention. Circ. Cardiovasc. Interv. 3, 184–192 (2010).
- Cooper CJ, Haller ST, Colyer W et al. Embolic protection and platelet inhibition during renal artery stenting. Circulation 117(21), 2752–2760 (2008).
- Zähringer M, Sapoval M, Pattynama PM et al. Sirolimus-eluting versus bare-metal low-profile stent for renal artery treatment (GREAT Trial): angiographic follow-up after 6 months and clinical outcome up to 2 years. J. Endovasc. Ther. 14(4), 460–468 (2007).
- Bates MC, Rashid M, Campbell JE, Stone PA, Broce M, Lavigne PS. Factors influencing the need for target vessel revascularization after renal artery stenting. J. Endovasc. Ther. 13(5), 569–577 (2006).
- Leesar MA, Varma J, Shapira A et al. Prediction of hypertension improvement after stenting of renal artery stenosis: comparative accuracy of translesional pressure gradients, intravascular ultrasound, and angiography. J. Am. Coll. Cardiol. 53(25), 2363–2371 (2009).
- Modrall JG, Timaran CH, Rosero EB et al. Predictors of outcome for renal artery stenting performed for salvage of renal function. J. Vasc. Surg. 54(5), 1414–1421.e1 (2011).
- Beck AW, Nolan BW, De Martino R et al. Predicting blood pressure response after renal artery stenting. J. Vasc. Surg. 51(2), 380–385 (2010).
- Rocha-Singh K, Jaff M, Kelley EL. Renal Artery Stenting with NonInvasive Duplex Ultrasound Follow-up: 3-year results from the RENAISSANCE renal stent trial. Catheter. Cardiovasc. Interv. 72, 853–862 (2008).
- Jaff MR, Bates M, Sullivan T et al.; on behalf of the HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter. Cardiovasc. Interv. doi:10.1002/ccd.24449 (2012) (Epub ahead of print).
- Bersin RM. 9-month results of the REFORM study (a prospective, single-arm, multicenter clinical study of the safety and effectiveness of the Formula™ balloonexpandable stent for treatment of renal artery stenosis. Catheter Cardiovasc. Interv. doi:10.1002/ccd.24481 (2012) (Epub ahead of print).