Review Article - Interventional Cardiology (2010) Volume 2, Issue 2
Comparing MIDCAB surgery and stenting for isolated proximal left anterior descending stenosis
- Corresponding Author:
- Timothy Watson
Green Lane Cardiovascular Service, Auckland City Hospital Park Road, Auckland, New Zealand
Tel: +49 341 865 1428
Fax: +49 341 865 1461
E-mail: thielh@medizin.uni-leipzig.de
Abstract
Keywords
atherosclerosis, bypass, coronary artery disease, drug-eluting stent, left anterior descending coronary artery, percutaneous coronary intervention, restenosis
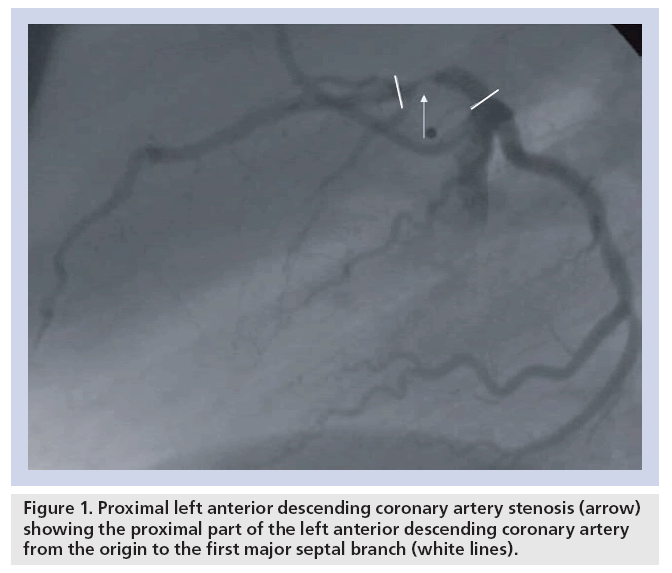
Single, double or triple vessel disease are defined according to the number of diseased coronary vessels. Of specific interest is the proximal left anterior descending (LAD) coronary artery, which can supply up to 50% of the left ventricular myocardium [1,2]. According to the American Heart Association/American College of Cardiology, the proximal LAD is defined as the part of the LAD from the origin of the left circumflex coronary artery to the first major septal branch (Figure 1) [3]. Significant stenosis of the proximal LAD coronary artery has worse prognosis than lesions at other locations owing to the large area of potentially jeopardized myocardium as demonstrated in autopsy studies [1,2]. Therefore, proximal LAD disease is considered high risk [4].
Figure 1: Proximal left anterior descending coronary artery stenosis (arrow) showing the proximal part of the left anterior descending coronary artery from the origin to the first major septal branch (white lines).
Established treatment options for isolated proximal LAD lesions are bypass surgery or percutaneous coronary intervention (PCI) with the use of stents. Bypass surgery can be performed either in a conventional manner with sternotomy or by minimally invasive direct coronary artery bypass (MIDCAB) surgery using the left internal mammary artery (LIMA) as bypass graft [5,6]. Both treatment strategies – surgery or PCI – effectively reduce symptoms [7,8]. However, randomized trials comparing both strategies in the era of baremetal stents revealed a significantly higher reintervention rate after stenting and similar results for mortality and reinfarction at mid- to long-term follow-up [9–12].
Stenosis location in the proximal LAD has been identified to be an independent risk factor for restenosis. Surgery with either balloon dilation or by PCI with the use of bare-metal stents still result in restenosis rates ranging from 33 to 44% [13–17]. The reason for the higher restenosis rate in the proximal LAD is widely unknown. However, the relative risk of restenosis appears to have been reduced by the introduction of drug-eluting stents (DES). In subanalyses of randomized studies involving stenoses at any location, similar results for restenosis reduction were shown in stenoses at other locations [18–20]. In this article, we evaluate the current techniques and the outcome of PCI with the use of stents – either bare-metal or des – compared with MIDCAB surgery for the treatment of LAD lesions.
Significant left anterior descending coronary artery stenosis
Coronary angiography is considered the standard tool to determine the severity of coronary artery disease. A typical cut-off value for significant proximal LAD stenosis is 70–75% luminal narrowing, and this has been used as inclusion criterion in multiple trials [14,21–23].
In randomized clinical trials, stenosis severity has been assessed by quantitative coronary angiography (QCA), whereas in clinical practice stenosis severity is assessed visually. However, the conventional coronary angiogram is only a lumenogram providing no information on the functional significance of coronary stenosis.
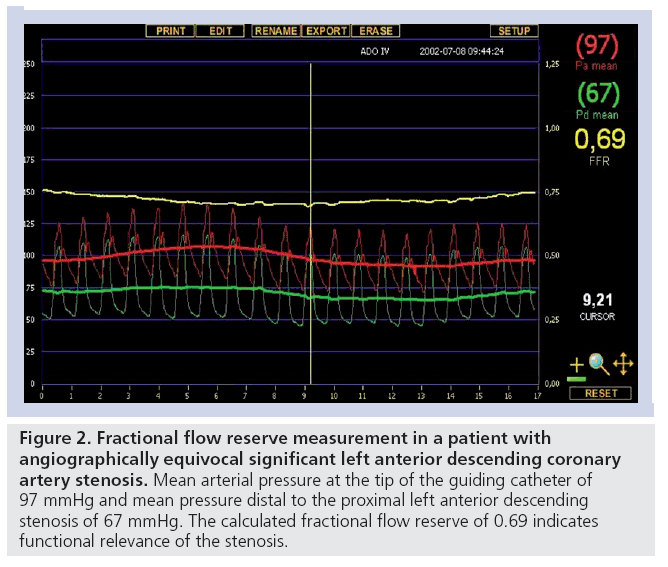
In clinical practice, noninvasive stress testing is an established option to determine the functional significance of coronary artery stenoses with imaging methods yielding higher sensitivity and specificity in comparison with conventional exercise testing [24–26]. Another option is invasive assessment of the fractional flow reserve (FFR). FFR can reliably identify flow-limiting stenosis in an epicardial coronary artery. It is independent of heart rate, blood pressure and left ventricular contractility and takes into account the contribution of collateral flow to myocardial perfusion [27]. An FFR value of less than 0.75 confirms that the stenosis being interrogated has the potential to induce reversible myocardial ischemia [27]. In patients with an angiographically equivocal proximal LAD stenosis, a strategy of revascularization versus medical therapy can be based on FFR measurements as shown in Figure 2.
Figure 2: Fractional flow reserve measurement in a patient with angiographically equivocal significant left anterior descending coronary artery stenosis. Mean arterial pressure at the tip of the guiding catheter of 97 mmHg and mean pressure distal to the proximal left anterior descending stenosis of 67 mmHg. The calculated fractional flow reserve of 0.69 indicates functional relevance of the stenosis..
Treatment
▪ Medical treatment
In earlier trials, conservative medical treatment was inferior to revascularization by PCI or bypass surgery with respect to symptom relief in the treatment of proximal LAD disease. However, these trials were not powered to detect differences in hard clinical end points [7,8]. In light of current trials comparing medical treatment with revascularization (mainly PCI), any differences in outcome with respect to death or infarction for single vessel disease are not likely to occur with revascularization [28]. Specific trials comparing isolated proximal LAD disease with medical therapy have not been performed and there are also no specific subanalyses from the randomized trials addressing high-risk proximal LAD stenosis.
Percutaneous coronary intervention
First performed in 1977 by Grüntzig, PCI became a central treatment option in interventional cardiology. Using arterial access, PCI is performed to restore the physiological blood flow in the coronary artery. Treatment of proximal LAD lesions does not differ from other locations and is considered a standard procedure. In some institutions, PCI of ostial LAD lesions is performed in surgical standby, ensuring higher safety in case of potential complications. In general, stenting with bare metal or DES is performed using the femoral approach. More recently, the radial approach is considered similarly effective with less bleeding complications [29]. However, many interventionalists feel more comfortable with the femoral approach, especially for proximal LAD disease, to handle potential complications more promptly.
Stenosis location in the proximal LAD has been identified to be an independent risk factor for restenosis following PCI with either plainold balloon dilation or the use of bare-metal stents [13–17]. The reason for the higher restenosis rate in the proximal LAD remains speculative. Interestingly, the relative risk of restenosis in the LAD as compared with other vessel locations appears to have been reduced by the introduction of DES [18–20].
If des are used, high-pressure stenting is considered the preferred strategy, whenever feasible. In trials and clinical practice angiographic success is usually defined as residual stenosis of less than 20% and Thrombolysis In Myocardial Infarction (TIMI)-flow grade 3 without signs of dissection [9,10,14,30].
During the procedure, unfractionated or lowmolecular heparin or alternatively bivalirudin are administered. After intervention, the sheath can be removed immediately with subsequent use of a closure device or use of compression systems until hemostasis. In patients undergoing PCI, clopidogrel (in addition to aspirin) is usually administered as a loading dose (300 or 600 mg loading-dose orally, followed by 75 mg per day for at least 12 months for DES and 4 weeks for bare-metal stents) the day before elective PCI.
In general, there are no specific contraindications for the treatment of proximal LAD lesions by PCI. However, chronic total occlusions or ostial lesions with extension of the stenosis to the left main stem are considered technically more challenging. These patients were usually excluded from randomized controlled trials [9,14,30].
The most frequent complications of PCI are related to the vascular access site (retroperitoneal hematoma, groin hematoma, pseudoaneurysm or arterial–venous fistula). Although rare, stent thrombosis is a major complication usually leading to infarction, which is inherited with high mortality [31]. This complication is predominant with the use of DES owing to the delayed endothelialization with potential late stent thrombosis. This persistent, potentially prothrombotic, substrate necessitates prolonged dual antiplatelet medication with aspirin and clopidogrel for at least 12 months [32].
Surgery
The second option for revascularization of the LAD is coronary artery bypass grafting. Interestingly, bypass surgery was first performed in 1964 on a beating heart without a cardiopulmonary bypass using a left anterior access with the LIMA anastomozed to the LAD [33]. However, in the following years the standard procedure for proximal LAD disease was the use of cardiopulmonary bypass via sternotomy. The disadvantages of this method are potential complications caused by trauma from the sternotomy and those induced by the cardiopulmonary bypass, such as atherosclerotic plaque emboli from cannulation of large arteries, increased inflammation and cognitive defects from the contact of patient blood with surfaces of the heart–lung machine [34,35].
▪ MIDCAB
These disadvantages of open bypass surgery led to the revival of beating heart surgery with the use of the LIMA, which in comparison with vein grafts has better patency rates at long-term followup [36]. In contrast to the initial bypass surgery strategy by Kolessov et al., a new minimized left anterolateral access was used, in particular for LAD revascularization [6]. This method is now established as MIDCAB surgery. Advantages of the new method are cosmetically better results and a lower complication rate mainly as a result of the avoidance of cardiopulmonary bypass and cardioplegia [6].
The technique of MIDCAB surgery has been described in detail elsewhere [6]. In brief, a left anterolateral minithoracotomy is performed through the fourth intercostal space. The LIMA is harvested under direct vision. After heparinization the LIMA is divided distally. Local immobilization of the anastomotic site is achieved with mechanical stabilizers. The anastomosis is usually performed using one running suture on the beating heart. Protamine is applied at the end of the procedure to neutralize 80% of the heparin dosage and then the wounds are closed in standard fashion.
The limited access is technically more challenging and was initially criticized as it was thought that this may impair the quality of the anastomosis and graft patency. However, in a meta-analysis involving 42 studies comparing conventional versus minimally invasive revascularization of the LAD by LIMA grafts, similar excellent patency rates of over 90% were observed [37]. General contraindications to perform bypass surgery and MIDCAB surgery in particular are extreme obesity, intramyocardial courses and extremely small peripheral LAD.
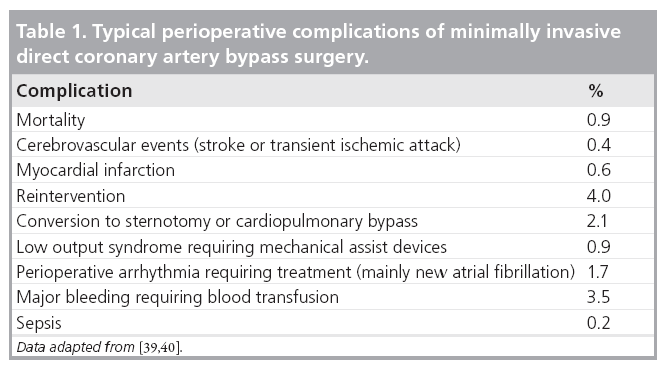
The typical complications that are observed after MIDCAB surgery are listed in Table 1 [10,14,38–40].
Overall, MIDCAB surgery has become a procedure with low mortality and low complication rates. However, the results are dependent on the case-load and the experience of the cardiac surgeon [40].
Comparison of MIDCAB surgery versus PCI plus stent implantation
▪ Bare-metal stents
In total, five randomized controlled trials compared MIDCAB surgery with bare-metal stenting [10,14,21,23,41–44]. The longest follow-up reported is 5.6 years for the largest trial from our own group [10]. The general description of the trials and the subject baseline demographics and clinical characteristics are summarized in Tables 2 & 3. All except one trial were singlecenter trials and were performed in Europe or Asia.
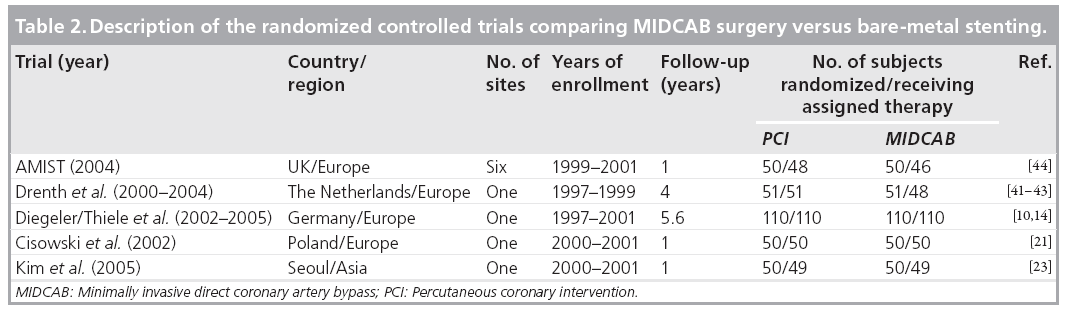
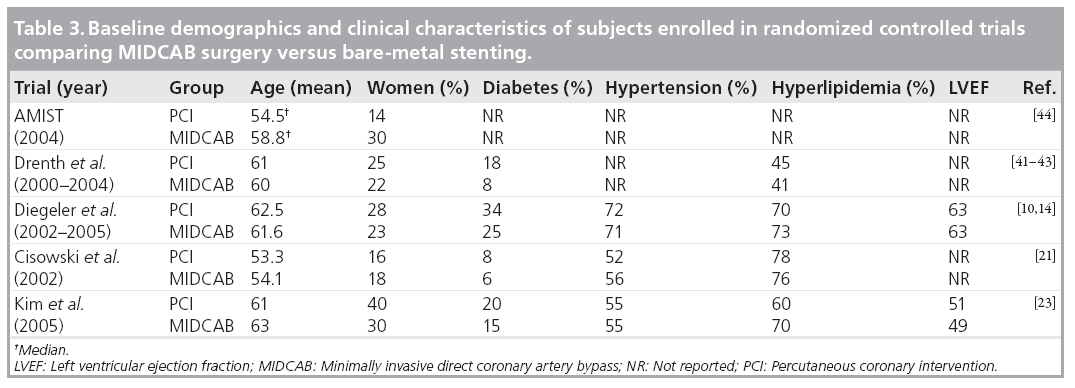
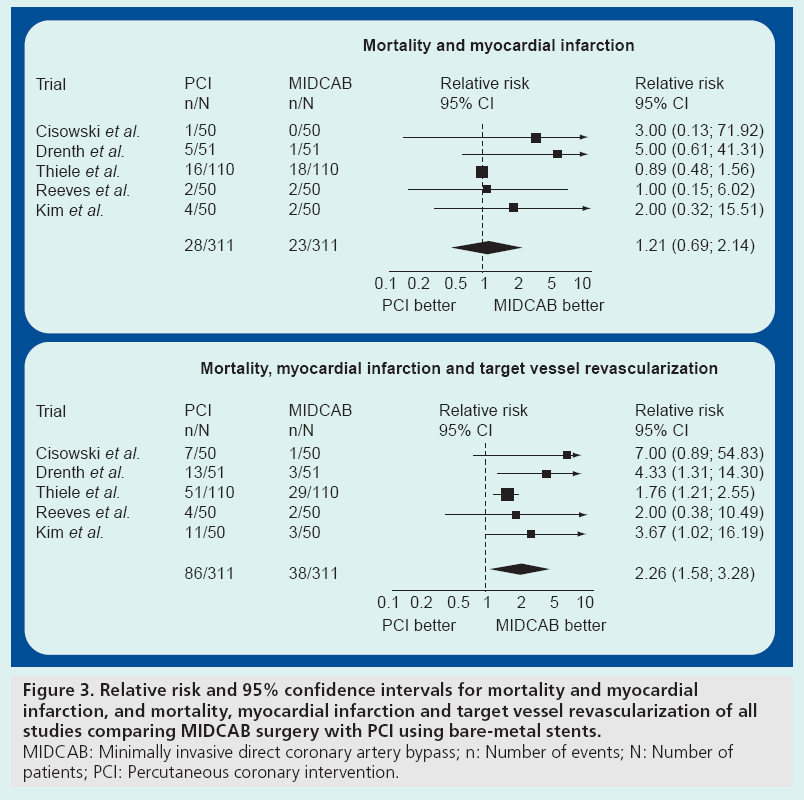
Altogether, there was only a small number of adverse events. Regarding major adverse cardiac events, there was no difference for mortality between the PCI versus MIDCAB groups. Similarly, the rate of myocardial infarction was not different. The combined results for death and myocardial infarction for the longest follow-up reported in each trial are shown in Figure 3A. However, in total there was a significantly higher rate of target vessel revascularization for the bare-metal stenting group in comparison with the MIDCAB group (22.0 vs 5.7%; p < 0.001). In most of the trials, target vessel revascularization was undertaken for documented symptomatic stenosis (>50%).
Figure 3: Relative risk and 95% confidence intervals for mortality and myocardial infarction, and mortality, myocardial infarction and target vessel revascularization of all studies comparing MIDCAB surgery with PCI using bare-metal stents. MIDCAB: Minimally invasive direct coronary artery bypass; n: Number of events; N: Number of patients; PCI: Percutaneous coronary intervention.
The higher restenosis rate was also the reason why patients in the MIDCAB group had fewer symptoms at follow-up in most of the trials. However, quality of life was not different in those trials assessing this end point [44]. The combined relative risks and 95% confidence intervals of major cardiac events including death, myocardial infarction and target vessel revascularization are displayed in Figure 3B.
▪ Drug-eluting stents
Drug-eluting stents decrease the risk of restenosis, in particular in proximal LAD disease, as shown in subanalyses of randomized trials comparing DES versus bare-metal stents for various locations [18–20]. At present, there is only one randomized controlled trial and limited evidence from registries and nonrandomized trials comparing DES and MIDCAB surgery for isolated proximal LAD coronary artery disease [30,45,46].
In another uncontrolled trial, 119 patients underwent PCI with DES and 70 patients MIDCAB surgery [22]. As shown by the imbalance between the number of patients assigned to PCI and MIDCAB, this trial was clearly not randomized. The rates of death and myocardial infarction were similar between the groups (stenting 1.7 vs MIDCAB 2.9%; p = 0.63). The need for revascularization was even lower (although not statically significant) in the DES group (1.7 vs 5.9%; p = 0.20) [22].
In the first randomized controlled trial comparing MIDCAB surgery with DES, 130 patients with isolated proximal LAD disease were included [30]. Sirolimus-eluting stents were used in the PCI group. The primary clinical end point was noninferiority of PCI as compared with surgery with respect to freedom from major adverse cardiac events within 12 months. Similar to previous trials, patients with symptomatic isolated stenosis of the LAD were included; excluded were patients with acute coronary syndromes requiring immediate intervention, additional valvular heart disease requiring treatment, previous interventional or surgical treatment for coronary artery or valvular disease, severe peripheral arterial disease, significant carotid stenosis requiring treatment, renal dysfunction requiring dialysis, any diseases with limited life-expectancy, overt congestive heart failure, upper gastrointestinal bleeding within the last 4 weeks, and contraindication to antiplatelet therapy. Angiographic exclusion criteria were total occlusions, involvement of the left main stem, stenosis of the first diagonal branch, intramyocardial course of the LAD, stenosis extending over a major diagonal branch (>1.5 mm) and stenosis at any other location requiring treatment.
Major adverse cardiac events occurred in 7.7% of patients after stenting, as compared with 7.7% after surgery (p = 0.03 for noninferiority). The individual components of the combined end point revealed mixed results. While PCI was noninferior with regard to death and myocardial infarction (1.5 vs 7.7%; noninferiority p < 0.001), noninferiority was not established for the difference in target vessel revascularization (6.2 vs 0%; noninferiority p = 0.21). At a median intermediate follow-up time of 43 months (interquartile range: 21–55) after stenting and 41 months (interquartile range: 21–51) after MIDCAB surgery, there was also no difference in the combined primary clinical end point (Figure 4). Clinical symptoms improved significantly in both treatment groups in comparison with baseline and the percentage of patients free from angina after 12 months was 81 versus 74% (p = 0.49). There was also similar improvement in both groups with respect to quality of life as assessed by standard questionnaires [30]. Periprocedural events and complications were more often encountered after MIDCAB surgery compared with after stenting (16.9 vs 3.1% of patients, respectively; p = 0.02) [30].
Figure 4: Kaplan–Meier curve at intermediate follow-up for the combined primary end point of major adverse cardiac events of the first randomized clinical trial comparing MIDCAB surgery with drug-eluting stenting. MIDCAB: Minimally invasive direct coronary artery bypass. Adapted from [10].
As shown in this recent trial, the restenosis rate was not zero and therefore did not satisfy the criteria for noninferiority [30]. The restenosis rate was very similar to registries, uncontrolled trials and subgroups of randomized trials, where the target vessel revascularization rate of the proximal LAD ranged from 0 to 7.9% with sirolimus- or paclitaxel-eluting stents [18–20,22,47]. Late lumen loss in this single center trial was 0.2 mm [30], which is similar to the rate observed in previous sirolimus-eluting stent trials and might be a reason why sirolimus-eluting stents are superior for the prevention of restenosis in comparison with paclitaxel-eluting stents [48,49]. In a registry including more than 2000 patients with proximal LAD disease, the best predictors for the prediction of target vessel revascularization using a multivariate analysis were multivessel disease, small stent diameter (≤2.75 mm) and the administration of GP IIb/IIIa antagonists. The target vessel revascularization rate according to the stent diameter was as high as 11.2% for 2.5 mm stents and as low as 4.0% for 3.5 mm stents [47]. Interestingly, other previously identified risk factors for restenosis, such as diabetes mellitus, ostial lesions or total stent length, did not influence outcome among this proximal LAD subgroup [47].
Stent thrombosis is a potentially important limitation of des . Whereas restenosis usually has a relatively benign clinical outcome, stent thrombosis is associated with increased risk of myocardial infarction and a high mortality [31]. DES must be considered a persistent potentially prothrombotic substrate and therefore prolonged dual antiplatelet medication for at least 12 months is recommended [32]. New more potent antiplatelets might partially overcome this problem; however, this needs further testing [50].
Costs & cost–effectiveness
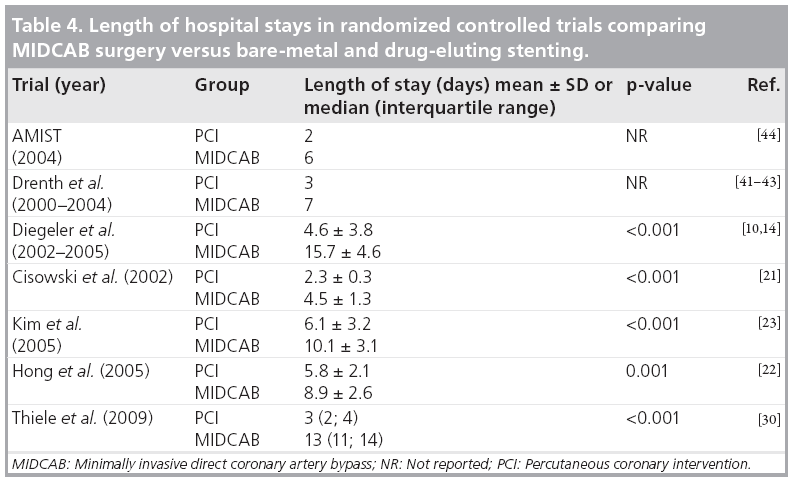
At present, there are no trials assessing cost and cost–effectiveness for the comparison of MIDCAB surgery with des . However, in most of the trials comparing bare-metal stenting with surgery there were significantly longer hospital stays and higher uses of hospital resources in the MIDCAB group. Table 4 summarizes the length of hospital stays in the current randomized clinical trials. Therefore, it is safe to say that costs are initially higher in the MIDCAB group. This is supported by a previous costeffective analysis comparing bare-metal stenting with MIDCAB surgery. Stenting was the dominant strategy in the first 2 years, being both more effective and less costly than bypass surgery. In the third year, bypass surgery still remained more expensive but became marginally more effective. However, by 5 years, the incremental cost–effectiveness ratio of £28042.95 (performed from a UK health service perspective) per quality adjusted life year began to turn in favor of MIDCAB surgery [51]. A detailed cost analysis has not been performed to date in the first randomized clinical trial comparing DES and MIDCAB surgery; although, preliminary data suggest much lower costs from the payer perspective for the DES procedure at 12-month follow-up (£6500 in comparison with £15,900 after surgery. More detailed analyses are required to further evaluate costs and cost–effectiveness at longer follow-up.
Future perspective
Although limited by moderate sample size, current studies with intermediate follow-up time have shown that both PCI, either with bare-metal or DES or MIDCAB surgery are safe treatment options for proximal high-grade LAD lesions. Both produce similar results with respect to survival and freedom from myocardial infarction. Whereas treatment with baremetal stents led to a significantly higher rate of target vessel revascularization of up to 35% at follow-up, the introduction of DES reduced this need for repeat intervention. Based on a limited number of patients, PCI with DES is noninferior to MIDCAB surgery at intermediate follow-up. Given the ease of therapy and short hospital stays, PCI with DES may therefore be considered the first-line treatment option in many patients.
However, nearly all trials comparing PCI with MIDCAB surgery excluded older patients over 75 years of age, those with acute myocardial infarction, extremely obese patients or those with severe left ventricular systolic dysfunction and relevant comorbidities. Furthermore, more complex lesions such as chronic total occlusions, bifurcations, in-stent restenosis and intramyocardial courses of the LAD were excluded. Thus, the conclusions about the comparative efficacy of PCI and MIDCAB surgery are limited for these patients. Moreover, although both the exact location and severity of a proximal LAD stenosis relate to prognosis, few of these trials strictly defined proximal LAD by these criteria prospectively.
Future studies are required to evaluate the comparative efficacy of PCI and MIDCAB surgery at longer follow-up and to what extent efficacy is affected by other key patient characteristics such as age, gender, diabetes, other comorbidities and also angiographic characteristics [47]. This will provide more patient-specific treatment decisions.
Until these data are available, the decision on how to optimally treat patients with proximal LAD disease is influenced by many factors such as patient preference, comorbidities, hospital or provider experience as well as anatomic variables (e.g., in-stent restenosis, lesion length, side branch involvement, ostial disease, left main involvement and calcification). Therefore, isolated proximal LAD lesions require an interdisciplinary approach with interdisciplinary patient information on both treatment options.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Long-term death & infarction after bare-metal stenting for proximal left anterior descending disease
▪ Death and infarction have a comparable incidence to minimally invasive direct coronary artery bypass grafting (MIDCAB) surgery.
▪ A longer follow-up is required.
Target vessel revascularization after bare-metal stenting for proximal left anterior descending disease
▪ Target vessel revascularization is required more often in comparison with MIDCAB surgery.
▪ Target vessel revascularization results in a lower rate of patients free from angina at intermediate and long-term follow-up.
Major adverse cardiac events (including target vessel revascularization) after drug-eluting stenting
▪ Drug-eluting stents are noninferior to MIDCAB surgery in a limited number of patients studied so far.
▪ A longer follow-up is required.
▪ Larger randomized clinical trials are required.
Symptom relief and quality of life after drug-eluting stenting
▪ There is a significant improvement after treatment.
▪ The results are similar to MIDCAB surgery.
Complications▪ Peri-interventional/perioperative complications after stenting are significantly lower in comparison with MIDCAB surgery.
Costs & cost–effectiveness of stenting versus MIDCAB surgery
▪ There are only limited data on costs and cost–effectiveness.
▪ Further studies are required.
Technical considerations
▪ Chronic total occlusions were not enrolled in randomized trials.
▪ Bifurcation lesions were not enrolled in randomized trials.
▪ In-stent restenosis lesions were not enrolled in randomized trials.
▪ Complex lesions were not enrolled in randomized trials.
▪ The length of dual antiplatelet therapy after drug-eluting stenting needs to be defined more clearly in future trials.
References
Papers of special note have been highlighted as:
▪ of interest
- Varnauskas E: Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N. Engl. J. Med. 319, 32–37 (1988).
- Klein LW, Weintraub WS, Agarwal JB et al.: Prognostic significance of severe narrowing of the proximal portion of the left anterior descending coronary artery. Am. J. Cardiol. 58, 2–46 (1986).
- Ryan TJ, Faxon DP, Gunnar RM et al.: Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (subcommittee on percutaneous transluminal coronary angioplasty). Circulation 78, 86–502 (1988).
- Califf RM, Armstrong PW, Carver JR, D’Agostino RB, Strauss WE: Task Force 5. Stratification of patients into high-, medium-, and low-risk subgroups for purposes of risk factor management. J. Am. Coll. Cardiol. 27, 64–147 (1996).
- Calafiore AM, Teodori G, Di Giammarco G et al.: Minimally invasive coronary artery bypass grafting on a beating heart. Ann. Thorac. Surg. 63, S72–S75 (1997).
- Diegeler A, Falk V, Matin M et al.: Minimally invasive coronary artery bypass grafting without cardiopulmonary bypass: early experience and follow-up. Ann. Thorac. Surg. 66, 22–25 (1998).
- Hueb WA, Bellotti G, de Oliveira SA et al.: The Medicine, Angioplasty or Surgery Study (MASS): a prospective, randomized trial of medical therapy, balloon angioplasty or bypass surgery for single proximal left anterior descending artery stenoses. J. Am. Coll. Cardiol. 26, 600–605 (1995).
- Parisi AF, Folland ED, Hartigan P: A comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease. Veterans Affairs ACME Investigators. N. Engl. J. Med. 326, 1–16 (1992).
- Jaffery Z, Kowalski M, Weaver WD, Khanal S: A meta-analysis of randomized control trials comparing minimally invasive direct coronary bypass grafting versus percutaneous coronary intervention for stenosis of the proximal left anterior descending artery. Eur. J. Cardiothorac. Surg. 31, 91–97 (2007).
- Thiele H, Oettel S, Jacobs S et al.: Comparison of bare-metal stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery. A 5 year follow-up. Circulation 112, 445–450 (2005).
- Kapoor JR, Gienger AL, Ardehali R et al.: Isolated disease of the proximal left anterior descending artery. JACC Cardiovascular Interventions 1, 83–91 (2008).
- Aziz O, Rao C, Panesar SS et al.: Metaanalysis of minimally invasive internal thoracic artery bypass versus percutaneous revascularisation for isolated lesions of the left anterior descending artery. BMJ 334, 17 (2007).
- Frierson JH, Dimas AP, Whitlow PL et al.: Angioplasty of the proximal left anterior descending coronary artery: initial success and long-term follow-up. J. Am. Coll. Cardiol. 19, 45–51 (1992).
- Diegeler A, Thiele H, Falk V et al.: Comparison of stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery. N. Engl. J. Med. 347, 61–66 (2002).
- Carrozza JP Jr, Kuntz RE, Levine MJ et al.: Angiographic and clinical outcome of intracoronary stenting: immediate and long-term results from a large single-center experience. J. Am. Coll. Cardiol. 20, 28–37 (1992).
- Fischman DL, Leon MB, Baim DS et al.: A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N. Engl. J. Med. 331, 96–501 (1994).
- Kastrati A, Schomig A, Elezi S et al.: Predictive factors of restenosis after coronary stent placement. J. Am. Coll. Cardiol. 30, 428–436 (1997).
- Dangas G, Ellis SG, Shlofmitz R et al.: Outcomes of paclitaxel-eluting stent implantation in patients with stenosis of the left anterior descending coronary artery. J. Am. Coll. Cardiol. 45, 186–192 (2005).
- Khattab AA, Hamm CW, Senges J et al.: Sirolimus-eluting stent treatment for isolated proximal left anterior descending artery stenoses. Z Kardiol. 94, 87–92 (2005).
- Sawhney N, Moses JW, Leon MB et al.: Treatment of left anterior descending coronary artery disease with sirolimus-eluting stents. Circulation 110, 74–79 (2004).
- Cisowski M, Drzewiecki J, Drzewiecki-Gerber A et al.: Primary stenting versus MIDCAB: Preliminary reportcomparison of two methods of revascularization in single left anterior descending coronary artery stenosis. Ann. Thorac. Surg. 74, S1334–S1339 (2002).
- Hong SJ, Lim DS, Seo HS et al.: Percutaneous coronary intervention with drug-eluting stent implantation vs minimally invasive direct coronary artery bypass (MIDCAB) in patients with left anterior descending coronary artery stenosis. Catheter Cardiovasc. Interv. 64, 5–81 (2005).
- Kim JW, Lim DS, Sun K, Shim WJ, Rho YM: Stenting or MIDCAB using ministernotomy for revascularization of proximal left anterior descending artery? Int. J. Cardiol. 99, 37–41 (2005).
- Ashley EA, Myers J, Froelicher V: Exercise testing in clinical medicine. Lancet 356, 592–597 (2000).
- Moir S, Haluska BA, Jenkins C, Fathi R, Marwick TH: Incremental benefit of myocardial contrast to combined dipyridamole-exercise stress echocardiography for the assessment of coronary artery disease. Circulation 110, 108–113 (2004).
- Underwood SR, Anagnostopoulos C, Cerqueira M et al.: Myocardial perfusion scintigraphy: the evidence. Eur. J. Nucl. Med. Mol. Imaging 31, 61–91 (2004).
- Pijls NH, De Bruyne B, Peels K et al.: Measurement of fractional flow reserve to assess the functional severity of coronaryartery stenoses. N. Engl. J. Med. 334, 703–708 (1996).
- Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM: Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis. Lancet 373, 11–18 (2009).
- Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR: Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am. Heart J. 157, 32–40 (2009).
- Thiele H, Neumann-Schniedewind P, Jacobs S et al.: Randomized comparison of minimally invasive direct coronary artery bypass surgery versus sirolimus-eluting stenting in isolated proximal left anterior descending coronary artery stenosis. J. Am. Coll. Cardiol. 53, 324–331 (2009).
- Cutlip DE, Baim DS, Ho KK et al.: Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation 103, 967–971 (2001).
- Grines CL, Bonow RO, Casey DEJ et al.: Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. J. Am. Coll. Cardiol. 49, 34–39 (2007).
- Kolessov VI: Mammary artery–coronary artery anastomosis as method of treatment for angina pectoris. J. Thorac. Cardiovasc. Surg. 54, 35–44 (1967).
- Diegeler A, Hirsch R, Schneider F et al.: Neuromonitoring and neurocognitive outcome in off-pump versus conventional bypass surgery. Ann. Thorac. Surg. 162–166 (2000).
- Paparella D, Yau TM, Young E: Cardio–pulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur. J. Cardiothorac. Surg. 21, 32–44 (2002).
- Cameron A, Davis KB, Green G, Schaff HV: Coronary bypass surgery with internalthoracic- artery grafts – effects on survival over a 15-year period. N. Engl. J. Med. 334, 216–219 (1996).
- Mack MJ, Magovern JA, Acuff TA et al.: Results of graft patency by immediate angiography in minimally invasive coronary artery surgery. Ann. Thorac. Surg. 68, 383–389; discussion 389–390 (1998).
- Thiele H, Diegeler A, Lauer B et al.: Minimally invasive bypass surgery versus stentimplantation in isolated proximal high lesions of the LAD in 200 patients. Circulation 102, II–II581 (2000).
- Holzhey DM, Jacobs S, Mochalski M et al.: Seven-year follow-up after minimally invasive direct coronary artery bypass: experience with more than 1300 patients. Ann. Thorac. Surg. 83, 8–14 (2007).
- Holzhey DM, Jacobs S, Walther T et al.: Cumulative sum failure analysis for eight surgeons performing minimally invasive direct coronary artery bypass. J. Thorac. Cardiovasc. Surg. 134, 63–69 (2007).
- Drenth DJ, Veeger NJ, Grandjean JG, Mariani MA, van Boven AJ, Boonstra PW: Isolated high-grade lesion of the proximal LAD: a stent or off-pump LIMA? Eur. J. Cardiothorac. Surg. 25, 67–71 (2004).
- Drenth DJ, Veeger NJ, Winter JB et al.: A prospective randomized trial comparing stenting with off-pump coronary surgery for high-grade stenosis in the proximal left anterior descending coronary artery: three-year follow-up. J. Am. Coll. Cardiol. 40, 955–960 (2000).
- Drenth DJ, Winter JB, Veeger NJ et al.: Minimally invasive coronary artery bypass grafting versus percutaneous transluminal coronary angioplasty with stenting in isolated high-grade stenosis of the proximal left anterior descending coronary artery: six months’ angiographic and clinical follow-up of a prospective randomized study. J. Thorac. Cardiovasc. Surg. 124, 30–35 (2002).
- Reeves BC, Angelini GD, Bryan AJ et al.: A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery. Health Technol. Assess. 8–41 (2004).
- Ben-Gal Y, Mohr R, Braunstein R: Revascularization of left anterior descending artery with drug-eluting stents: comparison with minimally invsive direct coronary artery bypass surgery. Ann. Thorac. Surg. 82, 67–71 (2006).
- Herz I, Moshkovitz Y, Hendler A et al.: Revascularization of left anterior descending artery with drug-eluting stents: comparison with off-pump surgery. Ann. Thorac. Surg. 79, 8–92 (2005).
- Khattab AA, Hamm CW, Senges J et al.: Incidence and predictors of target vessel revascularization after sirolimus-eluting stent treatment for proximal left anterior descending artery stenoses among 2274 patients from the prospective multicenter German Cypher Stent Registry. Clin. Res. Cardiol. 96, 79–84 (2007).
- Schomig A, Dibra A, Windecker S et al.: A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J. Am. Coll. Cardiol. 50, 373–380 (2007).
- Windecker S, Remondino A, Eberli FR et al.: Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N. Engl. J. Med. 353, 53–62 (2005).
- Wiviott SD, Braunwald E, McCabe CH et al.: Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 371, 353–363 (2008).
- Rao C, Aziz O, Panesar SS, Jones C et al.: Cost effectiveness analysis of minimally invasive internal thoracic artery bypass versus percutaneous revascularisation for isolated lesions of the left anterior descending artery. BMJ 334, 21 (2007).
▪ First trial reporting minimally invasive coronary artery bypass grafting (MIDCAB) surgery.
▪ Meta-analysis comparing MIDCAB surgery with percutaneous coronary intervention (PCI).
▪ Largest study of MIDCAB surgery versus bare-metal stenting with the longest follow-up available.
▪ Meta-analysis comparing surgery with PCI.
▪ Meta-analysis comparing MIDCAB surgery with PCI.
▪ First analysis of paclitaxel-eluting stent efficacy in left anterior descending disease.
▪ First analysis of sirolimus-eluting stent efficacy in LAD disease.
▪ First randomized study comparing MIDCAB surgery with drug-eluting stent implantation.
▪ Meta-analysis showing angiographically proven high rate of graft patency with MIDCAB surgery.
▪ Large registry assessing predictors for target vessel revascularization in proximal left anterior descending disease.