Research Article - International Journal of Clinical Rheumatology (2019) Volume 14, Issue 4
Comparison of alfacalcidol or prednisone effects as an add-on treatment to methotrexate in active rheumatoid arthritis
- Corresponding Author:
- Katarina Simic Pasalic
Institute of Rheumatology
University of Belgrade Medical School
Belgrade, Serbia
E-mail: simicpasalickatarina@gmail.com
Abstract
Objectives: To compare efficacy of alfacalcidol (1αD3) or prednisone as an add-on treatment for patients with active Rheumatoid Arthritis (RA). Methods: Sixty-seven RA patients on maximum tolerated stable methotrexate (MTX) dose, yet with moderate/high RA activity were included. Written consent, demographic data and blood samples for inflammatory biomarkers, calcium and lipid metabolism, cytokine testing were obtained, and disease activity was calculated (DAS28). They were randomly assigned to either three-month treatment with 1 μg (group A1), 2 μg (group A2), 3 μg (group A3) 1αD3 daily or prednisone (group C) 20 mg daily, for the first month and 10 mg afterwards, in addition to MTX. At the end of each treatment period, disease activity and laboratory tests were reassessed. Results: Out of 67 patients, 68% females, average age 56.24 ± 12.423, disease duration 7.71 ± 6.68 years, MTX dose 15.41 ± 3.28 mg/week, average RA activity DAS28 5.58 ± 0.905. Patients randomized to four different study treatments were fully comparable. After three-month treatment, we found highly significantly reduced disease activity in all groups as per DAS28 and patient visual assessment scale - PVAS (p<0.01). Group A2 and C in term of ΔDAS28 and ΔPVAS (p˂0.05) did not differ. In 1αD3 2 μg (A2) treated patients (N=19), C-Reactive Protein (CRP) (p˂0.01), erythrocyte sedimentation rate (ESR) and interleukin-6 (IL6) levels (p˂0.05) were significantly reduced, while High-Density Lipoprotein (HDL) increased (p˂0.05). Serum calcidiol in prednisone users (N=16) significantly decreased (p˂0.01). Conclusion: Addition of either 1 μg, 2 μg, 3 μg 1αD3 or prednisone to MTX for three months, led to a significant reduction of disease activity in active RAy.
Keywords:
rheumatoid arthritis • combination drug therapy • disease activity
Introduction
Rheumatoid Arthritis (RA) is a chronic systemic autoimmune inflammatory disease dominantly affecting synovial joints, of multifactorial aetiology [1]. Morbidity and mortality in RA are increased compared to general population. Cardio Vascular (CV) disease is the leading cause of death in RA patients [2]. Unremitting inflammation produces a proatherogenic state with elevation (x3-100) of proinflammatory cytokines, such are interleukin-6 (IL6), interleukin-1 (IL1), Tumor Necrosis Factor alpha (TNFα), C-Reactive Protein (CRP), but low total cholesterol and its fractions - “lipid paradox” [3-5]. Aggressive treatment of RA also treats co-morbidities [6]. Current strategy of treating RA to a target level of low disease activity or remission is based on anti-inflammatory actions of immunosuppressive agents, both disease modifying drugs (DMARD) synthetic or biologic and corticosteroids (CS) [6,7]. Despite anti-inflammatory, symptomatic and structural efficacy in RA, CS use is suggested to be as short as possible, due to its adverse effects [8-10].
Low serum vitamin D levels are linked to increased disease activity in RA [11]. Recently, 3 years follow up study of early onset RA (EORA) supported a role for calcidiol (25(OH)D3) levels as a clinical biomarker for the disease, and baseline 25(OH)D3 levels a potential predictor of disease severity for EORA patients [12]. Cells involved in innate and adaptive immune responses including macrophages, dendritic cells, T and B cells express vitamin D receptor (VDR) and can both produce and respond to hormone D (HD). VDR agonists including natural, HD, or synthetic VDR agonists seem primarily to inhibit dendritic cells and pathogenic proinflammatory T cells, an enhancement of innate immunity is coupled with multifaceted regulation of adaptive immunity [13].
Discovery of the immunomodulatory and anti-proliferative properties of HD prompted researchers to investigate the possibility of its use as therapeutic agent for autoimmune and malignant disease [14-16]. Throughout the last several decades there have been many attempts to synthesize hormone D analogues (VDR agonists) with the same biologic activity and even stronger anti-inflammatory properties, but lower blood calcium-increasing capacity [17]. Alfacalcidol (1αD3), synthetic steroidal VDR agonists, showed beneficial effects on disease activity and immunoregulation in patients with active rheumatoid and psoriatic arthritis [15,18- 20]. Hormone D deficiency in tissues, occurring in chronic inflammation, due to inhibition of α-hydroxylase, can be overcome by 1αD3 use. This is a consequence of conversion to HD in liver or other target organs, bypassing body`s own feed-back regulation [21,22].
The aim of this study was to compare the efficacy of alfacalcidol to prednisone treatment in active RA, as an add-on to standard methotrexate therapy.
Materials and methods
Study population and protocol
The open label intervention prospective study was approved by the Ethics Committee (decision No 29/1-7) and the Medicines and Medical Devices Agency (decision No 515-04-0544- 12- 2). Written informed consent was obtained from all patients prior to enrolment.
The study population consisted of 67 RA patients (46 females) on stable methotrexate (MTX 10- 25 mg/weekly) therapy of longer than 3 months duration prior to enrolment. RA diagnosis was established at least six months before the study, using ACR/EULAR 2010 criteria [23]. Patients were eligible for participation if RA was active (ESR-DAS28 was >3.2 on maximal tolerated stable MTX dose [24]. No other DMARD or CS treatment, nor D3 or calcium supplements use was allowed. Patients were advised to maintain a daily fluid intake of at least 1.5 l throughout the duration of the study. Patients were randomly assigned to administration of 1 μg/daily 1αD3 (group A1, N=17), 2 μg/daily 1αD3 (group A2, N=19), 3 μg/daily 1αD3 (group A3, N=16) treatment for three months or 20 mg prednisone daily for one month, followed by 10 mg prednisone daily for two months (group C, N=15), with an unchanged MTX dosing. Alfacalcidol was provided by investigator as gelatine capsules for oral administration (Alpha D3RTEVA, Serbia). Prednisone was provided by physician`s prescription. Alfacalcidol dosing was modified only in case of toxicity, i.e. disturbances in calcium levels in blood or urine (more than 2.65 mmol/l in blood or more than 0.3 g/DU in urine measured in two consecutive samples).
Study outcomes and safety parameters
Disease activity was assessed by ESR DAS 28 score, calculated from value of erythrocyte sedimentation rate (ESR), tender joint count (TJC) and swollen joint count (SJC) and the patients` assessment of disease activity (PVAS) based on answer to the question "Given the overall impact of your arthritis to you, how are you feeling today?" Rated on a scale of 100 mm, with 0 mm comparable to very good, and 100 mm comparable to very poor [24]. Inflammatory parameters ESR (Westergreen method), CRP (turbidimetry Gilford) were checked monthly, while total cholesterol (tC), HDL, low-density lipoprotein (LDL) (spectrophotometry-Ilab 300+), cytokines interleukin-4 (IL4), IL6, interleukin-10 (IL10) and TNFα (ELISA), 25(OH)D3 (ECL-Elecsys 2010) and parathormone (PTH) (FPIA-AxSym Abbot) were tested at the start and at the end of treatment period. Safety follow-up visits were conducted monthly. Patients were assessed for any relevant change in clinical status, weight, arterial pressure, electrocardiogram, haematology (Coulter HmX haematology analyser) and biochemistry tests glucose, creatinine, uric acid, alkaline phosphatase, albumin, liver function tests (LFT), calcium, ionized calcium and daily calciuria (spectrophotometry-Ilab 300+). At the end of the treatment period, clinical and laboratory data obtained were compared to baseline to assess efficacy and safety of the four different treatments in term of changes in disease activity, serum levels of inflammatory cytokines, lipids, calcium and vitamin D pre/post treatment and to each other.
Statistical analysis
Statistical analysis was performed using the SPSS 20 package, data was presented as mean ± SD (min-max). Subgroup changes of variables pre and post treatment were analysed by paired t-test or Wilcoxon test. The subgroup differences were assessed by independent t-test, ANOVA or chi square test, as appropriate, and least significant difference (LSD) method post hoc; p<0.05 considered as significant
Results
Out of 67 RA patients included, 46 (68%) were females, average age was 56.24 ± 12.423 (23-83), disease duration 7.71 ± 6.68 (1-33) years, MTX dose 15.41 ± 3.28 (10-25) mg/weekly and MTX use of 5.67 ± 5.899(0.5-15) years, mean disease activity ESRDAS28 was 5.58 ± 0.905(3.22- 7.55). Among 67 patients, DAS28 between 3.2 and 5.1, was noted in 20 patients (29.8%), while it was over 5.1 in 47 (70,2%). The patients randomized to four different treatment arms for three months were fully comparable (Table 1). All patients completed study.
| Variable | A1 | A2 | A3 | C | p |
|---|---|---|---|---|---|
| N (%M) | 17/29.4% | 19/31.5% | 16/31.2% | 15/33.3% | 0.881 |
| M/F ratio | 5/12 | 7/12 | 5/11 | 4/11 | 0.926 |
| Age (y) | 57.94 ± 12.279 | 53.79 ± 12.012 | 53.06 ± 10.909 | 60.67 ± 13.751 | 0.257 |
| RA (y) | 9.82 ± 8.149 | 7.63 ± 7.12 | 5.63 ± 4.334 | 7.53 ± 6.791 | 0.374 |
| MTX (y) | 7.82 ± 2.112 | 6.71 ± 1.567 | 5.31 ± 2.223 | 6.89 ± 4.114 | 0.232 |
| MTX (mg/w) | 14.09 ± 1.988 | 14.45 ± 3.533 | 16.87 ± 3.476 | 16.66 ± 2.937 | 0.061 |
| DAS28 | 5.28 ± 0.874 | 5.81 ± 0.891 | 5.73 ± 0.869 | 5.86 ± 0.789 | 0.058 |
| ESR (mm/h) | 28.18 ± 20 | 39.84 ± 23.735 | 42.88 ± 28.98 | 43.73 ± 15.895 | 0.188 |
| CRP (mg/l) | 8.25 ± 14.876 | 28.41 ± 28.162 | 22.42 ± 26.322 | 23.55 ± 24.92 | 0.093 |
| 25(OH)D3 (ng/ml) | 31.88 ± 13.573 | 28.97 ± 9.914 | 28.02 ± 14.118 | 34.02 ± 15.741 | 0.237 |
| PTH (pg/ml) | 41.49 ± 19.635 | 31.2 ± 9.382 | 42.74 ± 11.657 | 40.49 ± 15.49 | 0.075 |
| IL6 (pg/ml) | 34.343 ± 8.867 | 113.297 ± 50.528 | 126.96 ± 79.403 | 81.980 ± 78.597 | 0.495 |
| IL10 (pg/ml) | 5.09 ± 0.762 | 14.61 ± 8.648 | 17.99 ± 13.526 | 24.66 ± 15.551 | 0.631 |
| IL4 (pg/ml) | 9.94 ± 4.932 | 4.85 ± 0.625 | 4.94 ± 0.603 | 8.6 ± 1.12 | 0.417 |
| TNFα (pg/ml) | 3.38 ± 0.325 | 3.4 ± 0.634 | 3.05 ± 0.303 | 3.29 ± 0.307 | 0.927 |
| tChol (mmol/l) | 5.45 ± 1.044 | 5.11 ± 0.967 | 5.28 ± 1.025 | 5.65 ± 0.927 | 0.429 |
| LDL (mmol/l) | 3.37 ± 0.933 | 3.06 ± 0.88 | 3.15 ± 0.417 | 3.36 ± 1.11 | 0.722 |
| HDL (mmol/l) | 1.46 ± 0.358 | 1.51 ± 0.464 | 1.54 ± 0.824 | 1.68 ± 0.585 | 0.551 |
| Ca (mmol/l) | 2.42 ± 0.080 | 2.36 ± 0.112 | 2.38 ± 0.128 | 2.33 ± 0.097 | 0.889 |
| gluc.(mmol/l) | 4.32 ± 1.1 | 4.03 ± 0.987 | 4.72 ± 1.05 | 4.09 ± 0.65 | 0.661 |
| RF (n%+/N) | 15(88.23)/17 | 13(68.42)/19 | 14(87.5)/16 | 12(80)/15 | 0.567 |
| ACPA (n%+/N) | 3(17.64)/17 | 7(36.84)/19 | 4(25)/16 | 4(26.66)/15 | 0.588 |
| PsDMARD (n%+/N) | 8(47.05)/17 | 12(63.15)/19 | 11(68.75)/16 | 9(60)/15 | 0.978 |
| PbDMARD (n%+/N) | 1(5.88)/17 | 1(5.26)/19 | 1(6.25)/16 | 1(6.66)/15 | 0.998 |
| BMI (kg/m2) | 22.2 ± 1.87 | 21.9 ± 2.34 | 23.1 ± 2.76 | 22.7 ± 2.98 | 0.542 |
| Comorb.(n%+/N) | 6(35.29)/17 | 9(47.36)/19 | 6(37.5)/16 | 7(46.66)/15 | 0.690 |
A1: Group treated with 1µg 1αD3; A2: Group treated with 2µg 1αD3; A3: Group treated with 3µg 1αD3; C: Group treated with prednisone; N: Number of patients; M: Men; F: Women; y: Years; RA: Rheumatoid Arthritis; MTX: Methotrexate Weekly Dose; MTX (y): Duration of MTX Treatment; DAS28: RA Activity; ESR: Erythrocyte Sedimentation Rate; CRP: C Reactive Protein; 25(OH)D3: Serum Level of Vitamin D; PTH: Serum Parathormone Level; IL6: Serum Interleukin 6 Level; IL10: Serum Interleukin 10 Level; IL4: Serum Interleukin 4 Level; TNFα: Serum Tumor Necrosis Factor Alpha; tChol: Serum Total Cholesterol Level; LDL: Serum Low Density Lipoprotein Level; HDL: Serum High Density Lipoprotein Level; Ca: Serum Calcium Level; gluc: Serum Glucose Level; RF: Rheumatoid Factor Positive; ACPA: Anti-Citrullinated Protein Antibodies Positive; psDMARD: Previous Synthetic DMARD Treatment; pbDMARD: Previous Biologic DMARD Treatment; BMI: Body Mass Index; comorb.: Additional Chronic Disease Present.
Table 1. Comparison of demographic, clinical and disease characteristics among study subgroups (A1, A2, A3 and AC).
Influence on disease activity
Clinical efficacy indices showed improvement in terms of ESR DAS28 in all therapeutic regimens, highly statistically significant reduction between pre and post treatment was achieved in group A1 (5.28 ± 0.874 vs. 4.16 ± 1.029, p<0.01), A2 (5.81 ± 0.891 vs. 4.32 ± 1.032, p<0.01), A3 (5.74 ± 0.869 vs. 4.44 ± 0.861, p<0.01), along with group C (5.86 ± 0.789 vs. 4.13 ± 0.913, p<0.01, t- test).
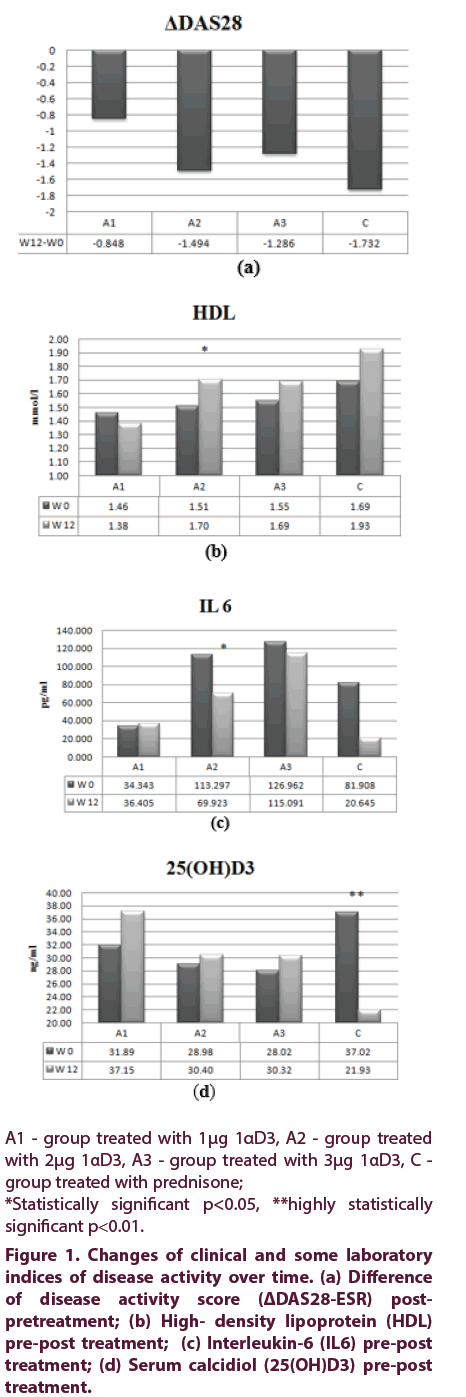
To further explore treatment efficacy, EULAR DAS28 response model was utilized to compare changes of DAS28 achieved (ΔDAS28) in the four different treatment groups (Figure 1a). There was no difference in ΔDAS28 of A2 group versus C group (p=0.437, post-hoc LSD). Also, there was no significant difference in number of patients with good DAS28 response in subgroups A2, A3 and C (p=0.532, χ2). Moderate DAS28 response (ΔDAS28>1.2) was achieved in 6 patients (33%) of A1 group and in 11 patients in each of the other groups: A2, A3 and C (comprising 58%, 69% and 71% of the group, respectively). There were no non-responders (ΔDAS28 ≤0.6) in A2 and C groups, but one non-responder in each of other two treatment groups (A1 and A3) [25].
Figure 1: Changes of clinical and some laboratory indices of disease activity over time. (a) Difference of disease activity score (ΔDAS28-ESR) postpretreatment; (b) High- density lipoprotein (HDL) pre-post treatment; (c) Interleukin-6 (IL6) pre-post treatment; (d) Serum c``alcidiol (25(OH)D3) pre-post treatment.
Influence on inflammatory and biochemical parameters
Significant reduction in ESR was observed in A2 (39.84 ± 23.375 vs. 31.83 ± 21.920, p>0.05) and C treatment groups (43.83 ± 15.895 vs. 27.8 ± 18.044, p<0.01, paired t-test), no differences when compared them to each other (ΔESR 8.01 ± 22.647 vs. 16.03 ± 16.969, p=NS, ANOVA). CRP level was significantly lowered, comparing pre and post treatment in A2 (28.42 ± 28.162 vs. 10.03 ± 11.682, p<0.01, paired t-test).
Serum levels of total cholesterol and LDL were elevated at the end of treatment in A2, A3 and C groups and HDL, also, which was significantly higher later in the group of patients treated with 2 μg 1αD3 (1.51 ± 0.464 vs. 1.7 ± 0.611, p<0.05, paired t test), shown in Figure 1b, compared to baseline level. Atherogenic index (tC/HDL ratio) in A2 group changed as well (from baseline 3.39 to 3.12 after treatment).
Interleukin-6 in serum significantly decreased post treatment in 2 μg 1αD3 treated patients (113.29 ± 220.249 vs. 69.92 ± 161.589, p<0.05, paired t-test), Figure 1c. This was the only treatment arm producing raised serum IL4, IL 10 and anti TNFα post treatment, yet not at the level of statistical significance. Compared to baseline, serum 25(OH)D3 levels became elevated in all 1αD3 treated patients, whilst in patients treated with prednisone it significantly decreased (34,02 ± 15,741 vs. 21.93 ± 10.896, p<0.01, paired t test), at the end of study (Figure 1d).
Influence on safety indices
Patients tolerated all study treatments well, without any serious clinical or laboratory adverse event observed. Serum calcium and ionized calcium remained unchanged, in all treatment groups, however, daily calciuria was significantly raised in those treated with 1αD3 2 μg (0.179 ± 0.0687 vs. 0.265 ± 0.090, p<0.01) and in 3 μg 1αD3, with a upper limit normal exceeding 0.3 g/DU (0.133 ± 0.0466 vs. 0.321 ± 0.1093, p<0.01, paired t-test) noted in latter. Fasting glucose increased pre compared to post treatment in C group by 1,7 mmol/l (about 40% compared to baseline), yet not above ULN, in contrast to 1αD3 treated patients, in whom about a 2% lower glucose was recorded at the end of treatment.
Discussion
A large amount of preclinical data supporting VDR analogue anti-inflammatory capacity exists [26]. Several studies have confirmed favourable therapeutic potential of 1αD3 in autoimmune diseases, chronic arthritis, osteoporosis and sarcopenia [15,18,27-29]. One of the most cited works in this area was an open label trial of 1αD3 2 μg use for 12 weeks in active RA patients (N=19), that showed significantly fewer swollen joints and improvement in two symptom scale scores, the Ritchie and Lee indices [15]. In addition, our study supports the evidence of 1αD3 treatment efficacy in RA, with highly significant improvement of disease activity assessed by ESR-DAS28, in all 1αD3 treated patients. This was comparable to prednisone treatment effect. All patients reported highly statistically significant improvement, assessed by patient reported outcome (PRO) as PVAS. Although subjective, PROs are shown to be less susceptible to placebo effect, positively correlated with inflammatory parameters and considered reliable measures for RA treatment response analysis [30]. Subjective improvement data obtained in our study fulfils PRO reliability criteria in all 1αD3 treated patients, as ΔPVAS was over 25 mm, which was defined as the threshold [31].
Corticosteroid use can overcome RA symptoms as morning stiffness and pain, which is partly explained by the influence to circulating IL6 in the morning [32]. In our study, even though prednisone treated patient achieved the higher ESRDAS28 reduction (-1.73), only 2 μg 1αD3 treatment led to statistically significant reduction of IL6 (113.2 vs. 69.9 pg/ml), together with significant ESR (-8) and CRP reduction (-18.52 mmol/l). Serum levels of IL4 were lowered, except in A2 group, and IL10 showed trend to increase in A1(5.095 vs.5,33 pg/ml) and A2 (14.61 vs. 25.76 pg/ml). Circulating TNFα was elevated the most in A2 group (3.402 vs. 6.827 pg/ml) post treatment, implicating that 1αD3 anti-inflammatory actions spares suppression of this cytokine. Complete suppression of TNFα is linked to increased susceptibility to infections under bDMARD [33]. Our findings have some analogy with Zold’s study, who published correction of derailed immunoregulation by 1αD3 treatment, in 21 undifferentiated connective tissue disease patients treated with 0,5 μg, 1 μg or 1,5 μg 1αD3. At the end of 5 weeks’ study, lowering of circulating interferon gamma (IFγ), IL6, interleukin-12 (IL12), interleukin-23 (IL23), interleukin-17 (IL17), TNFα, elevation of IL10, also correction of serum vitamin D deficiency was noted [27].
In this study, prednisone treatment (on average 13.3 mg daily, three months), resulted in highly significant reduction of serum level of 25(OH) D3. On the contrary, all the 1αD3 treatment regimens used, resulted in increased levels of serum 25(OH)D3 and lower serum PTH, which is known to be related with improved muscle mass and strength and better physical performance [34,35]. Recent multi-centre survey to assess 25(OH)D3 status in RA patients (N=625) provided evidence that low serum concentrations may influence RA activity outcome measure (DAS28) and PROs – rheumatoid arthritis impact of disease (RAID) and health assessment questionnaire (HAQ) [36]. In our study both DAS28 and HAQ improved significantly in 2 μg 1αD3 patients.
Circulating level, composition, functionality of HDL is damaged in chronic inflammation leading to derailed anti-antioxidant actions in autoimmune diseases [37]. Anti-inflammatory treatment with CS, sDMARD, bDMARD treatment is proved to correct undesirable serum lipid state in inflammatory rheumatic diseases [38]. Chen et al analysed serum lipid levels in 92 consecutive active RA patients under bDMARD treatment and found significantly raised serum levels of HDL after 6 months’ treatment with anti TNFα agents, without change in atherogenic index (AI) [39]. This was also confirmed in a recent meta-analysis of RA anti-TNFα users [40]. Anti IL6 treatment led to an increase of tC, LDL, without changes in AI or HDL [39]. In one case - control study, three months of tocilizumab treatment in active RA patients led to significant increase in serum tC, LDL, HDL compared to control group of sDMARD treated RA patients [41]. In our study, increase of serum tC, LDL and HDL in patients treated with 2 μg and 3 μg 1αD3 and CS, at the end of treatment, yet HDL elevation was statistically significant only in 2 μg 1αD3 treated patients. There was improvement of AI (3.39 to 3.12) in the same group. Although none of our patients in 2 μg treated group achieved remission post treatment (mean ESRDAS28 was 4,32), we got results very similar to those obtained in study of Boers, which reported that patients in remission or in well controlled RA, had increase in HDL and reduction of AI compared to patients with active disease [42].
Hypercalcemia as an adverse effect is rare in 1αD3 use, as shown in post-marketing surveillance of 13550 osteoporotic Japanese patients [43]. We treated patients with high doses of 1αD3, without any resultant hypercalcemia.
Conclusions
The potential of 1αD3 for disease and metabolic modulation in active RA, derived from our findings can be a starting point for further work in the field. Double-blind placebo-controlled trials are required to validate our findings. The main drawback of our study was the absence of a placebo group, which was based on ethical considerations. Lack of tight long term follow up is also a study limitation. We have shown for the first time that in vitamin D replete, active RA patients, alfacalcidol (2 μg daily) was equally effective in disease activity reduction as prednisone (mean 13.3 mg/daily) and improved metabolic indices of inflammation, with a good safety profile.
Acknowledgements
Special thanks to all patients for their participation and compliance. We are very grateful to Katarina Gosic MrPh, Snezana Jovicic MrPh PhD and Slavica Stekovic, lab. nurse, for contribution in laboratory investigations and Cheryl Thomson MD, for English language assistance.
Conflicts of interest
None
References
- Lipsky PE. Rheumatoid arthritis. In: Kasper D, Fauci A, Braunwald E, Hauser S, Lango D, Jameson J (eds.) Harrison’s Principles of Internal Medicine (16thedition). New York: McGraw-Hill, 1968–1977 (2005).
- Avina-Zubieta JA, Thomas J, Sadatsafavi M et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann. Rheum. Dis. 71(9), 1524–1529 (2012).
- Cutolo M, Otsa K, Uprus M et al. Vitamin D in rheumatoid arthritis. Autoimmun. Rev. 7(1), 59–64 (2007).
- Wallberg-Jonsson S, Johansson H, Ohman ML et al. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J. Rheumatol. 26, 2562–2571 (1999).
- Robertson J, Peters MJ, McInnes IB et al. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat. Rev. Rheumatol. 9(9), 513–523 (2013).
- Smolen JS. Treat to target: rationale and strategies. Clin. Exp. Rheumatol. 30, S2–S6 (2012).
- Smolen J, Landevé R, Bijlsma J et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease modifying drugs: 2016 update. Ann. Rheum. Dis. 76(6), 960–977 (2017).
- Gorter SL, Bijlsma JW, Cutolo M et al. Current evidence for the management of rheumatoid arthritis with glucocorticoids: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 69(6), 1010–1014 (2010).
- van der Goes MC, Jacobs JWG, Boers M et al. Monitoring adverse events of low-dose glucocorticoid therapy: EULAR recommendations for clinical trials and daily practice. Ann. Rheum. Dis. 69(11), 1913–1919 (2010).
- Duru N, van der Goes MC, Jacobs JWG et al. EULAR evidence-based recommendations on the management of medium to high-dose glucocorticoid therapy in rheumatic diseases. Ann. Rheum. Dis. 72(12), 1905–1913 (2013).
- Cutolo M, Otsa K, Laas K et al. Circannual vitamin D serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin. Exp. Rheumatol. 24, 702–704 (2006).
- Quintana-Duque MA, Caminos JE, Varela-Nariño A et al. The Role of 25-Hydroxyvitamin D as a Predictor of Clinical and Radiological Outcomes in Early Onset Rheumatoid Arthritis. J. Clin. Rheumatol. 23(1), 33–39 (2017).
- Adorini A, Penna G. Control of autoimmune diseases by vitamin D endocrine system. Nat. Clin. Pract. Rheumatol. 4(8), 404–412 (2008).
- De Luca HF. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 80(6), 1689–1696 (2004).
- Andjelkovic, Z. Vojinovic J. Pejnovic N et al. Disease modifying and immunoregulatory effects of high oral dose 1α(OH)D3 in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 17, 59–62 (1999).
- Antico A, Tampoia M, Tozzoli R et al. Can supplementation with vitamin D reduce the risk of modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun. Rev. 12(2), 127–136 (2012).
- De Luca HF. Vitamin D: the vitamin and the hormone. Fed. Proc. 33, 2211–2219 (1974).
- Yamauchi Y, Tsunematsu T, Konda S et al. A double-blind trial of alphacalcidol onpatients with rheumatoid arthritis (RA). Ryumachi. 29, 11–24 (1989).
- Hein G, Oelzner P. Vitamin D metabolites in rheumatoid arthritis: findings - hypotheses- consequences. Z. Rheumatol. 59(S1), 28–32 (2000).
- Gaal J, Lakos G, Szodoray P et al. Immunological and clinical effect s of alphacalcidol in patients with psoriatic arthropathy: results of an open follow up pilot study. Acta. Derm. Venereol. 89(3), 140–144 (2009).
- Nordin BEC, Need AG, Morris HA et al. The special role of “hormonal” forms of vitamin D in the treatment of osteoporosis. Calcif. Tissue. Int. 65(4), 307–310 (1999).
- Schacht E, Richy F, Reginster JY. The therapeutic effects of alfacalcidol on bone strength, muscle metabolism and prevention of falls and fractures. J. Musculoskelet. Neuronal. Interact. 5, 273–284 (2005).
- Aletaha D, Neogi T, Silman JA et al. 2010 rheumatoid arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 69, 1580–1588 (2010).
- Smolen JS, Breedveld FC, Schiff MH et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford). 42(2), 244–257 (2003).
- Fransen J, van Riel PLCM. The Disease Activity Score and the EULAR response criteria. Clin. Exp. Rheumatol. 23, 93–99 (2005).
- Vojinovic J. Vitamin D receptor agonists' anti-inflammatory properties. Ann. N Y Acad. Sci. 1317(1), 47–56 (2014).
- Zold E, Szodoray P, Nakken B et al. Alfacalcidol treatment restores derailed immune - regulation in patients with undifferentiated connective tissue disease. Autoimmun. rev. 10(3), 155–162 (2011).
- Ringe JD, Dorst A, Faber H et al. Superiority of alfacalcidol over plain vitamin D in the treatment of glucocorticoid induced osteoporosis. Rheumatol. Int. 24(2), 63–70 (2004).
- Schacht E. Rationale for treatment of involutional osteoporosis in women and for prevention and treatment of corticosteroid induced osteoporosis with alfacalcidol. Calcif. Tissue. Int. 65(4), 317–327 (1999).
- Strand V, Cohen S, Crawford B et al. Leflunomide Investigators Groups. Patient-reported outcomes better discriminate active treatment from placebo in randomized controlled trials in rheumatoid arthritis. Rheumatology (Oxford). 43(5), 640–647 (2004).
- Studenic P, Stamm T, Smolen JS et al. Reliability of patient–reported outcomes in rheumatoid arthritis patients: an observational prospective cohort. Rheumatology. 55(1), 41–48 (2016).
- Kirwan JR, Buttgereit F. Symptom control with low-dose glucocorticoid therapy for rheumatoid arthritis. Rheumatology (Oxford). 51(Suppl 4), 14–20 (2012).
- Galloway JB, Mercer LK, Moseley A et al. Risk of skin and soft tissue (including shingles) in patients exposed to anti-tumor necrosis factor alpha therapy: results from British Society for Rheumatology Biologics Register. Ann. Rheum. Dis. 72(2), 229–234 (2013).
- Schact E, Richy F. Reductions of falls in elderly. The central role of alfacalcidol in a multi-dimensional paradigm. Internet. J. Epidemiol. 7(1) (2008).
- Visser M, Deeg DJ, Lips P. Low vitamin D and high paratyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): Longitudinal Aging Study Amsterdam-LASA. J. Clin. Endocrinol. Metab. 88(12), 5766–5772 (2003).
- Vojinovic J, Tincani A, Sulli A et al. European multicentre pilot survey to assess vitamin D status in rheumatoid arthritis patients and early development of a new Patient Reported Outcome questionnaire (D-PRO). Autoimmun. Rev. 16(5), 548–554 (2017).
- Duffy D, Rader DJ. Update on strategies to increase HDL quantity and function. Nat. Rev. Cardiol. 6(7), 455–463 (2009).
- Choy E, Ganeshalingam K, Semb AG et al. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology (Oxford). 53(12), 2143–2154 (2014).
- Chen DY, Chen YM, Hsieh TY et al. Significant effects of biologic therapy on lipid profiles and insulin resistance in patients with rheumatoid arthritis. Arthritis. Res. Ther. 7, 17–52 (2015).
- Daïen CI, Duny Y, Barnetche T et al. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann. Rheum. Dis. 71(6), 862–868 (2012).
- Kawashiri SY, Kawakami A, Yamasaki S et al. Effects of the anti-interleukin-6-receptor antibody tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol. Int. 31(4), 451–456 (2011).
- Boers M, Nurmohamed MT, Doelman CJ et al. Influence of glucocorticoid and disease activity on total and high-density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann. Rheum. Dis. 62(9), 842–845 (2003).
- Orimo H, Schacht E. The D-hormone analog alfacalcidol: the pioneer beyond the horizon of osteoporosis treatment. J. Rheumatol. 19, 4–10 (2005).