Research Paper - Imaging in Medicine (2017) Volume 9, Issue 5
Comparison of CT findings of between MDR-TB and XDR-TB: A propensity score matching study
Hyejin Cheon*Department of Radiology, Kyungpook national university hospital, 130 Dongdeok-ro, Jung-gu, Daegu 41944, Korea
- Corresponding Author:
- Hyejin Cheon
Department of Radiology
Kyungpook national university hospital
130 Dongdeok-ro, Jung-gu, Daegu 41944, Korea
E-mail: sugerin82@gmail.com
Abstract
Purpose: The purpose of this study was to compare the CT findings between MDR-TB and XDR-TB groups using by propensity score matching.
Materials and methods: This retrospective study was approved by the institutional review board, and the requirement to obtain informed consent was waived. From October 2008 to March 2016, 72 MDR-TB patients and 25 XDR-TB were enrolled in this study. A 2:1 MDR-TB group/XDR-TB group matching was done by using propensity score matching. Computed Tomography (CT) findings and demographic factors were compared before and after propensity score matching.
Results: After propensity matching, 50 MDR-TB patients and (mean age, 42.9 ± 6.1 [standard deviation]; age range, 46-91 years) 25 XDR-TB patients (mean age, 44.4 ± 9.7 [standard deviation]; age range, 36-77 years) were included this study after 2:1 propensity score matching. Before matching, among independent variables for propensity matching, the anti-tuberculous treatment history was significantly different between two groups (p=0.017). After matching, statistically significant radiologic finding between MDR- and XDR-TB were the cavity wall thickness (P<0.001) and cavity size (P=0.041). The mean thickness of cavities was 8.0 mm in MDR-TB group and 11.5 mm in XDR-TB group respectively. The mean size of cavities was 21 mm in MDR-TB group and 36 mm in XDR-TB group, respectively. Other CT findings were not significantly different between two groups.
Conclusion: The cavity wall thickness and size of cavity were significantly different between MDR-TB and XDR-TB groups after 2:1 propensity score matching.
Keywords
Multidrug-resistant tuberculosis ▪ Extensive-resistant tuberculosis ▪ Computed Tomography
Introduction
Multidrug-resistant tuberculosis (MDR-TB) is an emerging life-threatening disease and is caused by Mycobacterium tuberculosis strains with resistance to at least isoniazid and rifampin. Extensively drug-resistant tuberculosis (XDR-TB) is defined as a train resistant to any type of fluoroquinolone and at least one of the three following injectable drugs: amikacin, capreomycin or kanamycin in addition to isoniazid and rifampin [1]. According to a World Health Organization (WHO) report [2], about 3.2% of all new tuberculosis cases are multidrug resistant (MDR-TB).
Comparing with patients with drug-sensitive TB, non-AIDS patients with MDR-TB are younger and have more frequent history of TB treatment and show more cavitary lung lesions on CT [3]. Although clinical findings of XDRand MDR-TB have been widely reported [4-6], to the best of our knowledge, the radiologic comparison of MDR- and XDR-TB has rarely been reported. Thus, the purpose of our study was to evaluate the radiologic findings of MDRand XDR-TB and to compare the findings with two groups in non-AIDS patients.
Materials and Methods
■ Baseline characteristics
This study was approved by our institutional review board by the Masan National Tuberculosis Hospital (Masan, Korea), which waived the requirement for informed consent. A retrospective searching of electronic medical record system from October 2008 to March 2016, 107 patients with MDR-TB and 31 patients with XDR-TB patients were identified by computer searching disease that developed in patients with no history of anti-tuberculous chemotherapy or a history of less than one month of chemotherapy. Among them, after exclusion of HIV-seropositive patients, 102 MDR-TB patients and 29 XDR-TB patients were identified. After exception of the patients with lack of the availability of chest CT scan, 72 MDR-TB disease that developed in patients with no history of anti-tuberculous chemotherapy (n=30) or developed in patients who had a treatment history only with the use of firstline drugs (n=42) and 25 XDR-TB disease that developed in patients with no history of anti-tuberculous chemotherapy (n=4) or developed in patients who had a treatment history only with the use of first-line drugs (n=21) were included in this study. The mean time interval between MDR-TB isolation and the initial chest CT scans was 19 ± 6.1 (range, 3-67 days) and mean time interval between XDR-TB isolation and CT was 22 ± 9.1 (range, 1-39 days), respectively. The time interval between specimen isolation and CT scan was not statistically different between MDR- and XDR-TB patient groups respectively (p=0.538, using by Mann-Whitney U test).
■ Statistical analysis
Because patients were not randomized, we matched patients on the basis of their propensity to minimize the effect of potential confounders on selection bias by using binary logistic regression. Independent variables entered into the propensity model included age, sex, previous anti-tuberculous treatment, smoking history, diabetes mellitus, hypertension, alcoholic history. 2:1 matching between the groups was accomplished by using the nearest-neighbor matching method [7]. After adjustment for these factors, demographic factors and CT findings were compared between two groups. Categorical data were compared by using the x2 test or the Fisher exact test. Comparison of quantitative variables for two groups was performed by using either a t-test or a Mann- Whitney test. A P values less than 0.05 were considered to indicate a statistically significant difference. Statistical analysis was performed by using IBM SPSS statistics software for windows version 22.0 (SPSS, Chicago, Ill).
■ Imaging technique and analysis
All examinations were performed with a 4-detector spiral CT scanner (Asteion; Toshiba Medical, Tokyo, Japan) or a 16-section MDCT scanner (Somatom Sensation 16; Siemens Medical Solutions, Erlangen, Germany), using a dedicated chest CT protocol. None of the patients were administered an intravenous injection of contrast medium. Helical scan date were acquired using 16 detector rows and a beam collimation of 3 mm (16*3.0 mm), a gantry rotation time of 0.5 s, a section reconstruction thickness of 3.0 to 5.0 mm, an image reconstruction interval of 3.0 mm and an effective tube current-time product of 200 mAs and 120 kVp.
Chest CT scans were reviewed by one radiologist with 7 years ‘experience who had no knowledge of the patients’ clinical information and diagnosis. All the CT images were reviewed at a workstation (HP Z800; Hewlett-Packard Development Company, USA) that had a spatial resolution of 1536*2048 (PGL21; WIDE, Korea) with the PACS (PiViewSTAR; Infinitt, Seoul, Korea). Both mediastinal (window width, 400 HU; window level, 20 HU) and lung parenchyma (window width, 1,500 HU; window level, -800 HU) window images were available on the PACS systems for analysis. The assessment of pulmonary parenchyme included as follows: tree-in-bud pattern, cavity (presence, number, wall thickness), consolidation, number of involved lobes. The presence or absence of pleural effusion and mediastinal lymphadenopathy were also assessed. The treein- bud pattern was defined as centrilobular branching structures that resemble a budding tree. The cavity was defined as gas-filled of fluidfilled space, seen as a lucency or low attenuated area within pulmonary consolidation or mass. To measure the wall thickness of cavities, the CT images were magnified two or three times and by using electronic caliper, three portion of wall thickness were measured at largest portion of cavity. The mean cavity wall thickness was calculated by dividing the sum of three portion of cavity wall thickness.
Results
■ Patients demographics
TABLE 1 shows the patient characteristics for the two groups. MDR-TB group patients had significantly more history of previous antituberculous treatment than did XDR-TB group patients (P=0.017). After matching, using by Pearson’s Chi-square test, anti-tuberculous treatment history was not significantly different between two groups (P=0.521). Other variables such as mean age, sex ratio, history of smoking, diabetes mellitus, hypertension, alcoholics, HIV were not significantly different between two groups before and after propensity score matching.
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Variable | MDR-TB (n=72) | XDR-TB (n=25) | P value | MDR-TB (n=50) | XDR-TB (n=25) | P value |
| Mean age (y)* Sex Male Female Smoking Never Current Former Anti-tuberculous treatment history Absent Less than 6 months More than 6 months Diabetes mellitus Absent Presence Hypertension Absent Present Alcoholic history Absent Presence HIV Absent Presence |
41.4 ± 1350 (69.4) 22 (30.6)6 (8.3) 27 (37.5) 39 (54.2) 2 (44.4) 30 (41.7) 10 (13.9)67 (93.1) 5 (6.9)22(30.6) 50 (69.4)30 (71.4) 42 (58.3)69 (95.8) 2 (4.2) |
44.4 ± 9.718 (72) 7 (28)3 (12) 12 (48) 10 (40) 2 (8) 7 (28) 16 (64)21(84) 4 (16)7 (28) 18 (72)10 (40) 15 (60)25 (100) 0 (0) |
0.2680.810 0.312 0.017 0.938 0.810 0.884 0.396 | 42.9 ± 6.137 (74) 13 (26)4 (8) 20 (40) 16 (32) 2 (4) 19 (38) 29 (58)40 (80) 10 (20)15 (30) 35 (70)17 (34) 33 (66)50 (100) 0 (0) |
44.4 ± 9.718 (72) 7 (28)3 (12) 12 (48) 10 (40) 2 (8) 7 (28) 16 (64)21(84) 4 (16)7 (28) 18 (72)10 (40) 15 (60)25 (100) 0 (0) |
0.5300.854 0.987 0.521 0.675 0.858 0.610 1.000 |
Note: Unless otherwise indicated, data is number of patients, with percentages in parentheses
* Data are mean ± standard deviate
Table 1: Baseline patient characteristics before and after 2:1 propensity matching between MDR-TB and XDR-TB groups.
Finally, 50 MDR-TB patients and (mean age, 58.4±6.1 [standard deviation]; age range, 46-91 years) 25 XDR-TB patients (mean age, 57.8 ± 9.7 [standard deviation]; age range, 36-77 years) were included this study after 2:1 propensity score matching. After matching, all variables were not significantly different between two groups.
■ Comparison of CT findings between MDR-TB and XDR-TB after 2:1 propensity score matching
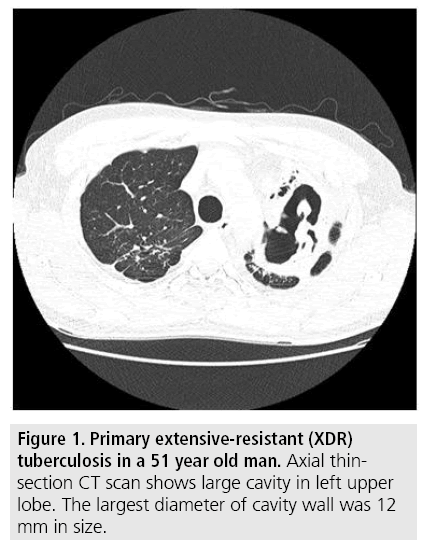
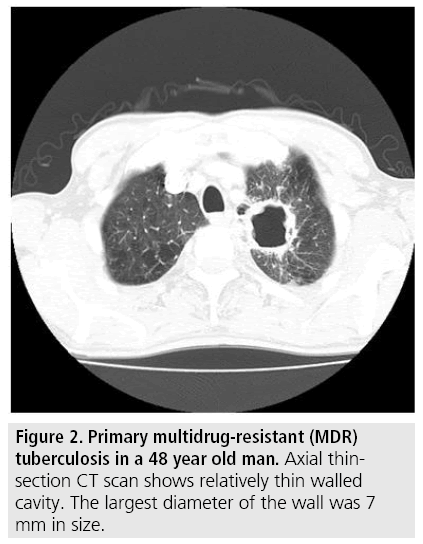
A total of 75 patients (50 MDR-TB patients and 25 XDR-TB patients) were matched by applying 2:1 propensity score matching. The comparison of CT findings between MDR-TB and XDR-TB before and after 2:1 propensity score matching is summarized in TABLE 2. Before propensity score matching, mean wall thickness of cavity was significantly different between two groups (MDR-TB:8.3 mm, XDRTB: 11.5 mm, P=0.001) (FIGURES 1 and 2) and other variables were not significantly different between two groups. After 2:1 propensity score matching, not only for the mean wall thickness of cavity, but also for the mean size of cavity was additionally significantly different between two groups (MDR-TB: 21 mm, XDR-TB: 36 mm). After propensity score matching, other variables such as presence of cavities, number of cavities, number of involved lobes, signs of treein- bud pattern, consolidation, pleural effusion, lymphadenopathy were not significantly different between two groups.
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| CT findings | MDR-TB (n=72) | XDR-TB (n=25) | P value | MDR-TB (n=50) | XDR-TB (n=25) | P value |
| Cavities Presence Number of cavities* Mean wall thickness* Mean size of cavity* Tree-in-bud sign Involved lobes* Consolidation Pleural effusion Lymphadenopathy |
57 (79.1) 2.9 8.3 mm 24 mm 70 (97.2) 2.9 58 (80.6) 9 (25) 6 (8.3) |
19 (76) 3.3 11.5 mm 36 mm 22 (88) 3.3 20 (80) 3 (24) 1 (4) |
0.845 0.455 0.001 0.075 0.211 0.753 0.997 1.000 0.756 |
37 (74) 3.0 8.0 mm 21 mm 46 (92) 2.6 39 (78) 10 (20) 4 (8) |
19 (76) 3.3 11.5 mm 36 mm 22 (88) 3.3 20 (80) 3 (24) 1 (4) |
0.901 0.522 0.001 0.041 0.465 0.401 0.821 0.444 0.622 |
Data are presented as No.(%) of patients unless otherwise specified
* Mean value
Numbers in parenthesis are percentages. MDR-TB=Multidrug-Resistant Tuberculosis; XDR-TB=Extensively Drug-Resistant Tuberculosis
Table 2: Comparison of CT findings before and after 2:1 propensity matching between MDR-TB and XDR-TB groups.
Figure 1: Primary extensive-resistant (XDR) tuberculosis in a 51 year old man. Axial thinsection CT scan shows large cavity in left upper lobe. The largest diameter of cavity wall was 12 mm in size.
Figure 2: Primary multidrug-resistant (MDR) tuberculosis in a 48 year old man. Axial thinsection CT scan shows relatively thin walled cavity. The largest diameter of the wall was 7 mm in size.
Discussion
Anti-TB drug resistance is a major public health care problem that threatens the success of TB control. The major concerns of drug resistance are fear regarding the spread of drugresistant organisms and the ineffectiveness of chemotherapy in patients infected with the resistant organisms. Imaging findings of MDRTB do not quite differ from those of drugsensitive TB. Multiple cavities and calcified granulomas, however, are more common in patients with MDR-TB [8]. In South Korea, TB remains a major public health problems and an economic burden. MDR-TB strains occurred in 3% of new patients and in 14% of previously treated cases [9] and about 5-15% of MDRTB cases were confirmed as having XDR-TB [10-14]. Cha et al. [11] suggested that there was no difference in imaging findings in terms of frequency and extent of each parenchymal abnormality between XDR-TB patients and MDR-TB patients except for pleural thickening. In our study, however, the cavity wall thickness and size of cavity were significantly different between MDR-TB and XDR-TB groups.
Cavities are usually formed when an area of caseous necrosis liquefies and communicates with the airway. The thickened lining of cavity tends to reduce the penetration of the anti-TB drug from bloodstream. Moreover, elevated bacillary titers in cavities increase the probability of establishing drug resistant bacterial populations [15,16]. Thus, it is believed that cavities are the biologic foundation for MDRand XDR-TB. Based on this background, we expected that thicker walls were expected the higher resistance to Anti-TB drugs. Although there is no significant difference in number and size of cavities, actually the cavity wall thickness was thicker in XDR- than MDR-TB group. Although it was not proven pathologically or biologically, we finally concluded that the cavity wall thickness correlated with the drug resistance.
Our study has several limitations. First, as our study was retrospectively, this study contains a selection bias. Not all patients with XDR- and MDR-TB underwent CT. CT scans tended to be performed in patients with more aggressive symptoms and clinical manifestations of tuberculosis. So the patients that had severe symptoms or clinical manifestations tended to be included in this study. Second, we suggested that the thicker wall would expect the higher probability of resistance to the anti-TB drugs, it was not pathologically proven.
Conclusion
In conclusion, after 2:1 propensity score matching, the cavity wall thickness and size of cavity were significantly different between MDR-TB and XDR-TB groups.
References
- Organization WH. Extensively drug-resistant tuberculosis (XDR-TB): Recommendations for prevention and control. Wkly. Epidemiol. Rec. 81, 430-432 (2006).
- Gandhi NR, Moll A, Sturm AW et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 368, 1575-1580 (2006).
- Chung MJ, Lee KS, Koh WJ et al. Drug-sensitive tuberculosis, multidrug-resistant tuberculosis and non-tuberculous mycobacterial pulmonary disease in non-AIDS adults: comparisons of thin-section CT findings. Eur. Radiol. 16, 1934-1941 (2006).
- Control CfD Prevention. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs-worldwide, 2000-2004. MMWR. Morb. Mortal. Wkly. Rep. 55, 301 (2006).
- Raviglione MC, Smith IM. XDR tuberculosis-implications for global public health. N. Engl. J. Med. 356, 656-659 (2007).
- Kim HR, Hwang SS, Kim HJ et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin. Infect. Dis. 45, 1290-1295 (2007).
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate. Behav. Res. 46, 399-424 (2011).
- Kim HC, Goo JM, Lee HJ et al. Multidrug-resistant tuberculosis versus drug-sensitive tuberculosis in human immunodeficiency virus-negative patients: computed tomography features. J. Comput. Assist. Tomogr. 28, 366-371 (2004).
- Bai G, Park Y, Choi Y et al. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. Int. J. Tuberc. Lung. Dis. 11, 571-576 (2007).
- Park HS, Kim YI, Kim HY et al. Bronchial artery and systemic artery embolization in the management of primary lung cancer patients with hemoptysis. Cardiovasc. Intervent. Radiol. 30, 638-643 (2007).
- Cha J, Lee HY, Lee KS et al. Radiological findings of extensively drug-resistant pulmonary tuberculosis in non-AIDS adults: Comparisons with findings of multidrug-resistant and drug-sensitive tuberculosis. Korean. J. Radiol. 10, 207-216 (2009).
- Migliori GB, Besozzi G, Girardi E et al. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur. Respir. J. 30, 623-626 (2007).
- Keshavjee S, Gelmanova IY, Farmer PE et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: A retrospective cohort study. Lancet. 372, 1403-1409 (2008).
- Kim DH, Kim HJ, Park SK et al. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am. J. Respir. Crit. Care. Med. 178, 1075-1082 (2008).
- David HL. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl. Microbiol. 20, 810-814.
- Blanchard JS. Molecular mechanisms of drug resistance in Mycobacterium tuberculosis. Annu. Rev. Biochem. 65, 215-239 (1996).