Case Report - Interventional Cardiology (2017) Volume 9, Issue 3
Complete diagnostic work-up and prognosis prediction for cardiac sarcoidosis with atrioventricular block: Importance of cardiac magnetic resonance image (MRI) and positron emission tomography (PET)
- Corresponding Author:
- Ki Hong Lee
Chonnam National University Hospital
Gwangju, South Korea
Tel: +82622206572
Fax: +82622233105
E-mail: drgood2@naver.com
Submitted: 25 March 2017; Accepted: 18 April 2017; Published online: 24 April 2017
Abstract
A 56-year old woman complained of chest discomfort and dyspnea on exertion for one month. She had been diagnosed as mediastinal lymph node sarcoidosis through endobronchial ultrasonography guided transbronchial lymph node aspiration. Electrocardiogram at presentation demonstrated sequential first and second degree 2:1 atrioventricular block. Further imaging study with gadolinium enhanced cardiac magnetic resonance image and 18F-fluoro-2-deoxyglucose positron emission tomography confirmed the diagnosis of sarcoidosis involving heart. Intravenous steroid therapy without pacemaker implantation improved conduction abnormality to normal sinus rhythm. Follow-up 18F-FDG-PET demonstrated full improvement of cardiac and extracardiac lesion with maintenance oral steroid therapy for 5-month.
Keywords
Sarcoidosis; Atrioventricular block; Pacemaker artificial
Introduction
Sarcoidosis is a systematic granulomatous disease which can involve any organ of the body and the etiology has not been known yet [1]. The most commonly involved organs are lungs and lymphatic system [2]. An involvement of the heart is occurred in 20-30% of the patients [3], which constitutes a major cause of death of sarcoidosis [4]. Cardiac sarcoidosis is manifested as arrhythmia, cardiomyopathy, or pericarditis. Arrhythmia includes atrioventricular (AV) block, bundle branch block, supraventricular tachycardia, or sudden cardiac death (SCD) [5]. AV block usually presents complete AV block [6], therefore almost of all patients with AV block needs permanent pacemaker implantation [7]. In patients with aborted SCD, implantable cardioverter-defibrillator (ICD) implantation should be considered [7]. We report a woman diagnosed as sarcoidosis involving heart through complete diagnostic work-up. Endobronchial ultrasonography guided transbronchial lymph node aspiration (EBUS-TBNA) confirmed histologic diagnosis. Furthermore, imaging study with cardiac magnetic resonance image (MRI) and 18F-fluoro-2-deoxyglucose positron emission tomography (18F-FDG-PET) confirmed clinical diagnosis of cardiac and extracardiac sarcoidosis. The patient accompanied first and second degree AV block, which promoted new onset dyspnea, and were improved by systemic steroid therapy without need for pacemaker implantation. Follow-up 18F-FDG-PET confirmed improvement of cardiac sarcoidosis.
Case Description
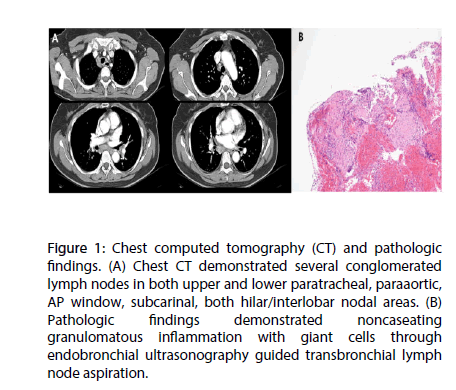
A 56-year-old female who complained of chest discomfort and dyspnea on exertion of New York Heart Association (NYHA) class III sustained for 1 month. She had no history of hypertension, diabetes, pulmonary tuberculosis or hepatitis. Multiple lymphadenopathies were identified at peribronchial, para-aortic, subcarinal, hilar, interlobar areas in chest CT 6 months ago for health care evaluation (Figure 1A). EBUS-TBNA confirmed histologic diagnosis of lymph node sarcoidosis by identifying non-caseating granulomatous inflammatory lesion including giant cells (Figure 1B).
Figure 1: Chest computed tomography (CT) and pathologic findings. (A) Chest CT demonstrated several conglomerated lymph nodes in both upper and lower paratracheal, paraaortic, AP window, subcarinal, both hilar/interlobar nodal areas. (B) Pathologic findings demonstrated noncaseating granulomatous inflammation with giant cells through endobronchial ultrasonography guided transbronchial lymph node aspiration.
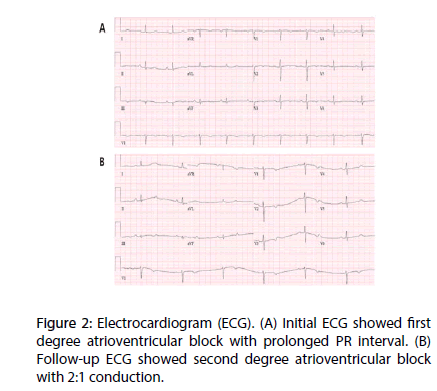
However, she did not have any treatment, because of absence of any clinical symptoms. After 5 months observation, she complained of progressive aggravation of chest discomfort and dyspnea on exertion. Electrocardiogram demonstrated new-onset first degree and second degree AV block with 2:1 conduction (Figure 2).
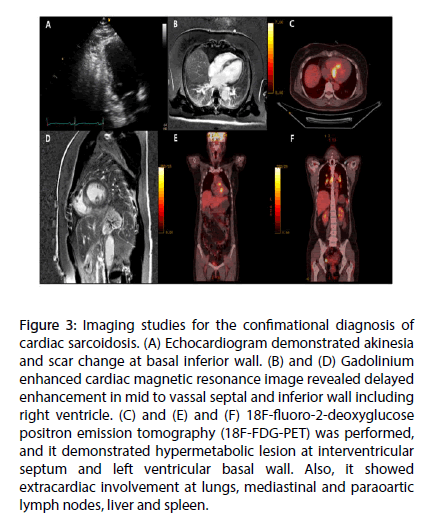
Echocardiogram revealed normal left ventricular systolic function with ejection fraction 65%, and scar change accompanied with wall motion abnormality limited at basal inferior wall, which suggested cardiac infiltrative disease (Figure 3A).
Figure 3: Imaging studies for the confimational diagnosis of cardiac sarcoidosis. (A) Echocardiogram demonstrated akinesia and scar change at basal inferior wall. (B) and (D) Gadolinium enhanced cardiac magnetic resonance image revealed delayed enhancement in mid to vassal septal and inferior wall including right ventricle. (C) and (E) and (F) 18F-fluoro-2-deoxyglucose positron emission tomography (18F-FDG-PET) was performed, and it demonstrated hypermetabolic lesion at interventricular septum and left ventricular basal wall. Also, it showed extracardiac involvement at lungs, mediastinal and paraoartic lymph nodes, liver and spleen.
Further imaging studies were performed to confirm cardiac involvement of sarcoidosis. Cardiac MRI demonstrated late gadolinium enhancement at basal to mid portion of interventricular septum and inferior myocardium including right ventricle with perfusion defect at same areas (Figures 3B and D). For further conformational diagnosis, 18F-FDG-PET was performed, and it showed hypermetabolic lesion at interventricular septum and inferior myocardium of both ventricles (maximum standardized uptake value [SUVmax]=8.0 g/mL) (Figures 3C and E). Also, it revealed extracardiac involvement at lungs, mediastinal and paraoartic lymph nodes (SUVmax=9.3 g/mL), liver (SUVmax=6.2 g/mL) and spleen (SUVmax=3.7 g/mL) (Figure 3F). Above findings suggested sarcoidosis involving both cardiac and extracardiac organs.
According to both Japanese Ministry of Health and Welfare criteria and Heart Rhythm Society criteria, all of the evidences were sufficient to make the diagnosis of cardiac sarcoidosis. Initial treatment was decided to perform systemic steroid therapy delaying pacemaker implantation. Three days after intravenous prednisolone 60 mg/day, 2:1 AV block was restored to normal sinus rhythm with 1:1 conduction and normal PR interval, and she had marked improvement of chest discomfort and dyspnea. Intravenous steroid therapy was tapered off oral steroid therapy was maintained for 5 months after that. Follow-up 18F-FDG-PET demonstrated complete improvement of cardiac and extracardiac lesions after oral steroid therapy for 5- months. The patient has been followed up without any symptoms so far.
Discussion
Sarcoidosis is a systemic inflammatory disease which forms granuloma in any organ of the body. Although varies depending on each report, cardiac sarcoidosis may be presented in considerable number of patients with sarcoidosis. Furthermore, because clinical presentation of cardiac sarcoidosis varies from no subjective symptom to sudden cardiac death, many clinicians are hard to decide to whether to treat it or to choose treatment modality. So, many are applying the general guideline for device-based therapy of cardiac rhythm abnormalities with making a decision to insert a device or not.
In the present case, the patient developed second degree AV block because of sarcoidosis infiltrating heart. According to the current guideline for devicebased therapy, she should be implanted pacemaker for advanced AV block. Some experts advocate ICD implantation in patients presenting advanced conduction disturbance in cardiac sarcoidosis8. However, we decided to initiate immunosuppressive therapy using systemic steroid administration rather than applying any device to the patient, because the nature of sarcoidosis is a systemic inflammation. After induction and maintenance of systemic steroid therapy, the conduction abnormality converted to normal sinus rhythm and the patient has been followed up without recurrence so far.
Additionally, further imaging studies were performed to confirm a diagnosis of cardiac sarcoidosis with cardiac MRI and 18F-FDG PET. Recent studies suggested that cardiac MRI and 18F-FDG PET are very useful for diagnosing cardiac sarcoidosis and even reported that it is possible to predict the responsibility and to monitor the response to immunosuppressive therapy depending on the findings of these imaging studies6. In this case, we reassured the diagnostic value of cardiac MRI and 18F-FDG PET in the patient who had shown new onset conduction abnormality and had been already diagnosed of extracardiac sarcoidosis.
Systemic steroid therapy was very effective in the present case, which was consistent with recent studies. So, in the case of cardiac sarcoidosis with advanced AV block, cardiac device implantation might be delayed or even unnecessary, if early diagnosis and immunosuppressive therapy is initiated.
Conclusion
In conclusion, clinicians should make early diagnosis through proper imaging studies and might delay device-based therapy while using a systemic immunosuppressive agent, if cardiac sarcoidosis is suspected to make conduction abnormalities.
Executive Summary
A 56-year old woman complained of chest discomfort and dyspnea on exertion for one month. She had been diagnosed as mediastinal lymph node sarcoidosis through endobronchial ultrasonography guided transbronchial lymph node aspiration.
Electrocardiogram at presentation demonstrated sequential first and second degree 2:1 atrioventricular block. Further imaging study with gadolinium enhanced cardiac magnetic resonance image and 18F-fluoro-2-deoxyglucose positron emission tomography confirmed the diagnosis of sarcoidosis involving heart.
Intravenous steroid therapy without pacemaker implantation improved conduction abnormality to normal sinus rhythm. Followup 18F-FDG-PET demonstrated full improvement of cardiac and extracardiac lesion with maintenance oral steroid therapy for 5- month.
References
- Sekhri V, Sanal S, Delorenzo LJ, Aronow WS, Maguire GP. Cardiac sarcoidosis: a comprehensive review. Arch. Med. Sci. 7: 546-554 (2011).
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N. Engl. J. Med. 357: 2153-2165 (2007).
- Houston BA, Mukherjee M. Cardiac sarcoidosis: clinical manifestations, imaging characteristics, and therapeutic approach. Clin. Med. Insights. Cardiol. 8: 31-37 (2014).
- Dubrey SW, Falk RH. Diagnosis and management of cardiac sarcoidosis. Prog. Cardiovasc. Dis. 52: 336-346 (2010).
- Aggarwal NR, Snipelisky D, Young PM, Gersh BJ, Cooper LT, Chareonthaitawee P. Advances in imaging for diagnosis and management of cardiac sarcoidosis. Eur. Heart. J. Cardiovasc. Imaging. 16: 949-958 (2015).
- Schatka I, Bengel FM. Advanced imaging of cardiac sarcoidosis. J. Nucl. Med. 55: 99-106 (2014).
- Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. Heart. Rhythm. 5: e1-e62 (2008).
- Youssef G, Beanlands RS, Birnie DH, Nery PB. Cardiac sarcoidosis: applications of imaging in diagnosis and directing treatment. Heart. 97: 2078-2087 (2011).