Review Article - Interventional Cardiology (2012) Volume 4, Issue 4
Complications and safety of jugular and azygous angioplasty in CCSVI patients with multiple sclerosis
- Corresponding Author:
- Tristan R A Lane
Imperial College London, London, UK
Tel: +44 20 3311 7036
Fax: +44 20 3311 7362
E-mail: tristan.lane00@imperial.ac.uk
Abstract
Keywords
azygous, chronic cerebrospinal venous insufficiency, jugular, safety, venoplasty
Background
Chronic cerebrospinal venous insufficiency (CCSVI) has been described as ‘a syndrome characterized by stenoses of the internal jugular veins and azygous (AZY) venous system with opening of collaterals and insufficient drainage proved by increased mean transit time on cerebral MRI [1]. The presence of this phenomenon in multiple sclerosis (MS) patients was first reported in 2006 and has since been a polarizing topic among vascular surgeons, neurologists and patients [2]. Those in support of the CCSVI hypothesis have implicated its role in the pathogenesis of MS and suggested that it causes venous distension and iron deposition, initiating an inflammatory response [3]. Despite questions over whether the imaging findings are reproducible [4] or clinically significant [5], some have proceeded with venoplasty of stenotic internal jugular veins and AZYs and/or stent placement on the premise that treatment of CCSVI would be beneficial to MS patients [6].
Word of putative benefits in function and quality of life seen in relapsing–remitting patients from a pilot case series [7] rapidly spread to patients, media and the public, mounting a dramatic response worldwide with social network sites promoting CCSVI treatment [101]. However, the Cardiovascular and Interventional Radiology Society of Europe and the US FDA have warned against the procedure [8,102] until further data from clinical trials are available, leaving those seeking treatment to travel to independent ‘fee-per-procedure’ centers. A substantial number of procedures are thus conducted in overseas centers, without follow-up, as medical tourism [103]. This poses many problems of which the most pertinent are the complication rates, which remain largely unknown as there is no systematic documentation of complications or efficacy performed by independent observers. Local follow-up is variable and, as a result, complications can present at distant centers that have not been involved in the initial procedure [9]. This has led to the introduction of a US registry for endovascular procedures for CCSVI [104]. As a result, the FDA have now requested that patients submit post-venoplasty complications directly to them via MedWatch [102].
The aim of this review is to examine the safety profile of jugular and AZY angioplasty in patients with MS from the available literature.
Methods
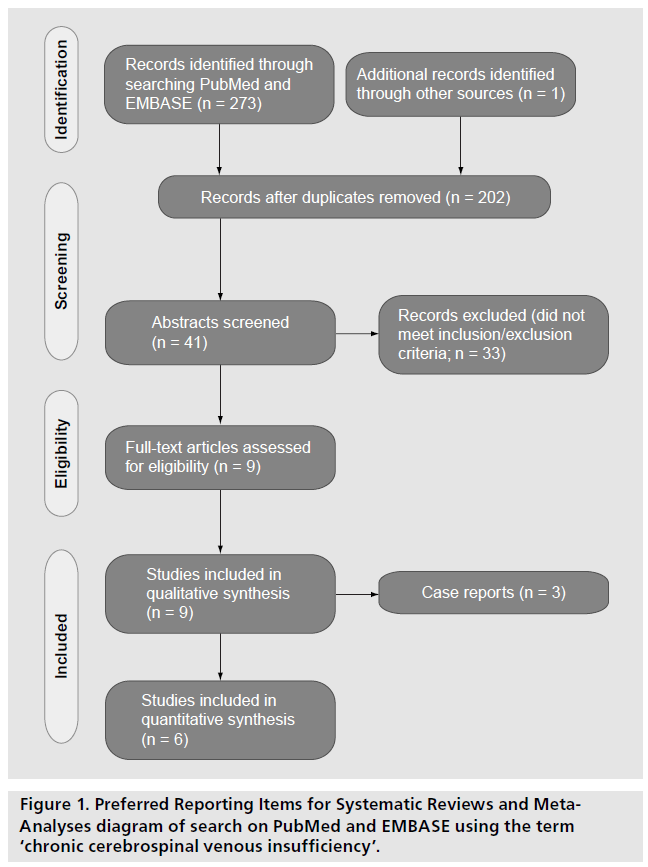
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines were used when conducting this systematic review (Figure 1) [10]. The PubMed and EMBASE databases were searched, from 1946 to date using the OVID portal, by three reviewers (L Varatharajan, T Lane and A Thapar) in March 2012. The keywords used were ‘chronic cerebrospinal venous insufficiency’. Inclusion criteria were interventional studies reporting complications following venoplasty of the internal jugular and AZY veins in MS patients. Exclusion criteria were duplicate publication, review articles, letters and abstracts. Case reports were considered qualitatively, as they did not report prevalence statistics. No limits, filters or language exclusions were applied. Authors of conference proceedings were contacted for data and, if they replied, these data were included.
After title and abstract screening, full-text articles were assessed independently for methodological quality by two reviewers. Authors of articles without clear technical details or follow-up protocols were contacted by email. If they did not respond they were excluded. Data on immediate (<24 h), early (<30 day) and late (>30 day) complications were extracted onto an Excel (Microsoft, USA) spreadsheet for analysis.
Results
▪ Search results
The search process is outlined in Figure 1. Nine relevant articles were identified for qualitative synthesis. Of these, three case reports were excluded from the quantitative analysis as they did not report prevalence statistics [9,11].
▪ Methodological quality
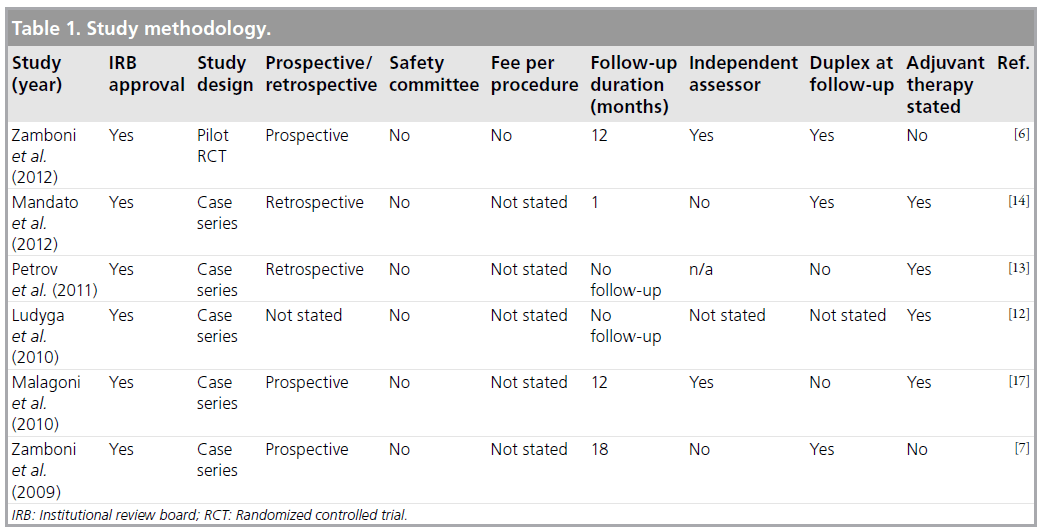
The six included studies were all institution review board-approved research studies, with five case series and one pilot randomized controlled trial. Three studies were prospective (Table 1). No safety committee was mentioned in any of the studies, nor were details regarding any fees received per procedure. Six studies followed up patients for at least 24 h, four studies for at least 1 month and three for at least 12 months. Two studies had an independent assessor for follow-up and four gave details of adjuvant pharmacotherapy used.
▪ Study demographics
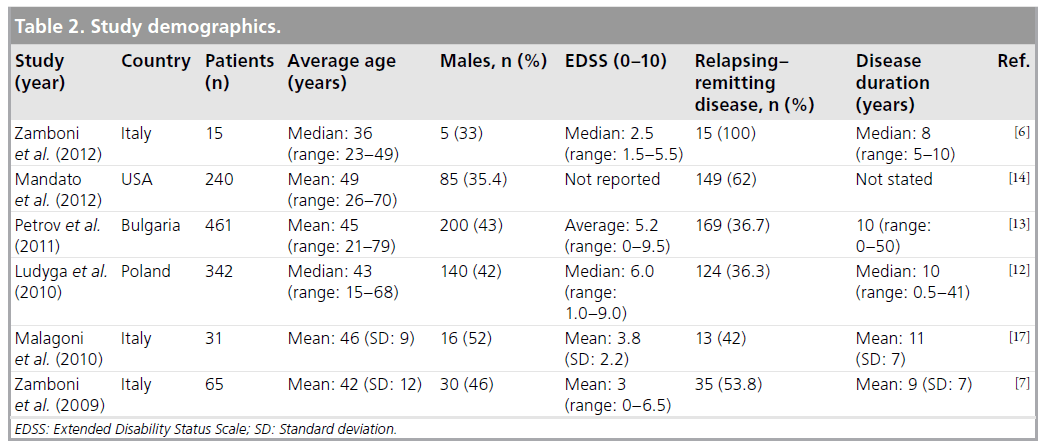
The study populations ranged from 15 to 461 patients with five European studies and one from the USA (Table 2). Average age ranged from 36 to 49 years, with the youngest patient reported as being 15 years of age and the eldest 79. Approximately 33–52% of patients were male, with average Expanded Disability Status Scale scores of 2.5–6.0. Two studies included severely disabled patients with Expanded Disability Status Scale scores of up to 9.5 (bed bound or unable to communicate or swallow) [12,13]. Relapsing–remitting disease was found in 36–100% of patients across the studies. The same two studies included a larger proportion of progressive patients (64%). Average time from diagnosis of MS was 8–11 years.
▪ Technical details
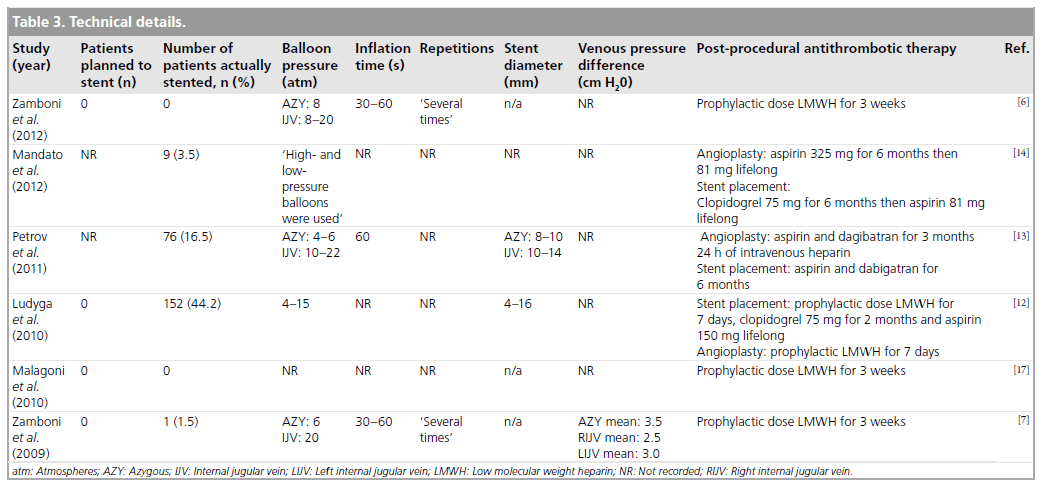
Perioperative care was described in four studies with all procedures being performed under local anesthetic with sedation (Table 3). All studies described the use of the standard percutaneous venographic technique via a femoral vein. Three of the studies referenced the technique used in the original Zamboni study [7]. Technical details were explicitly stated in four out of six studies. Angioplasty was the primary intention in all studies; however, stents were placed in 0–44% of cases. Four studies described the pressures used during balloon dilatation (4–15 atm) and three reported the inflation time (30–60 s). The number of balloon inflations was recorded in two studies only as ‘several times’. One study described a reduction in venous pressure postprocedure of 2.5–3.5 cm/H20 [7].
▪ Post-procedure antithrombotic therapy
All studies reviewed utilized postoperative antithrombotic therapy, varying from 1 week of prophylactic dose low molecular weight heparin to lifelong aspirin. Three studies utilized the same post-procedural regime of 3 weeks of prophylactic- dose low molecular weight heparin (Table 3).
▪ Immediate complications
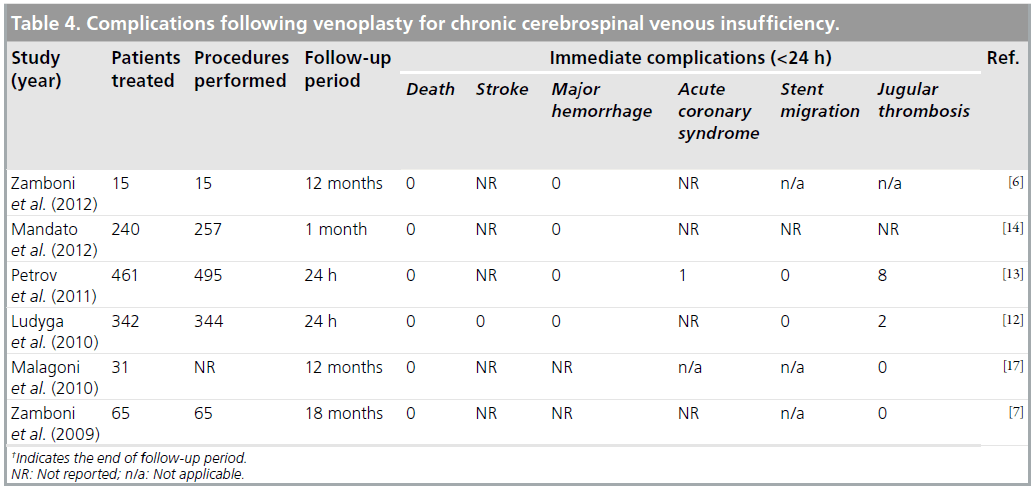
There was one case report of fatal intracranial hemorrhage and one stent migration to the right atrium, requiring thoracotomy [11]. Other complications are summarized in Table 4. The most frequently reported immediate complications were arrhythmia, groin hematoma and jugular thrombosis. Although most arrhythmias were benign atrial fibrillation, one patient developed a hemodynamically significant tachycardia requiring intensive care admission, another developed ventricular fibrillation secondary to coronary artery disease and a third developed ventricular tachycardia. Jugular thrombosis was reported in ten cases and, out of these, seven were in-stent thromboses. One group did not re-intervene in patients with in-stent thrombosis [12] but did not follow them further to establish outcome; however, others used thrombolysis and catheter aspiration to successfully recanalize eight out of eight patients [13].
▪ Early complications
There were three further jugular thromboses: two in-stent and one in a native vein (Table 4). The native jugular thrombosis was treated with stenting. Of the in-stent thromboses, one was successfully treated with angioplasty. Thrombectomy was attempted for the other patient with in-stent thrombosis; however, they developed a life-threatening tachycardia and required intubation and vasopressor support [14]. There were further case reports of symptomatic jugular thrombosis that were treated with thrombectomy, cranial nerve XI and XII palsy from oversized stent placement, extraperitoneal hematoma requiring blood transfusion, sigmoid sinus thrombosis requiring anticoagulation and asymptomatic trans-diaphragmatic AZY stent migration [9,15].
▪ Late complications
No late complications were reported by any group.
Discussion
The principal findings of this review are that studies reporting complications after venoplasty for CCSVI are poorly reported and prone to bias. It is unclear just how many patients travel to international centers to receive procedures or what the medium- and long-term safety outcomes are. This is a strong argument for national interventional registries of patients. Only after further study will we have a true appreciation of the safety profile of such procedures.
Regarding the reported complications, the most frequent immediate complications were arrhythmia, jugular thrombosis and puncture site hematoma. Most of these were innocuous; however, a handful resulted in cardioversion or defibrillation, anticoagulation or blood transfusion. However, there were rarer complications, often associated with the use of stents; these include: stent migration, intracranial hemorrhage secondary to anticoagulation for a stent and cranial nerve palsy from oversizing a stent. Practitioners and patients should be aware of these risks.
It is unclear how or by whom the complications from these procedures should be treated. First, there are no funding arrangements for reintervention. Second, there is little experience regarding long-term outcomes of jugular thrombosis or venous stent migration. Third, a proportion of thromboses will require anticoagulation to prevent cranial or caudal extension and the follow-up care this entails. It would be interesting for researchers to observe the neurological outcomes in patients returning with occluded jugular veins, in order to understand if there is a disease modifying effect.
The nearest comparable techniques are stenting procedures in the lower limb, which have demonstrated a substantial rate of stent thrombosis of 57–79% at 5 years [16]. Long-term management of these stents is likely to become a major issue in the future.
Although the studies reporting data in this review were all institutional review board approved, it was interesting that no safety committee was mentioned by any group. In some studies, the patients treated were as young as 15 years old [12], or included patients who were bed bound with difficulty feeding or communicating [13]. These individuals are classed as vulnerable individuals by research ethics boards and require special consideration.
This is the first analysis of the reported safety outcomes of venoplasty for CCSVI in the literature. It demonstrates that without registry data collection, complications may be underreported and may present to distant centers, without funding arrangements and without any evidence base on which to treat these complications. These findings are relevant to regulatory bodies, MS societies and healthcare providers, and will facilitate informed consent for patients. The limitations of this analysis are largely due to the paucity and variable quality of the source data, which did not permit meta-analysis.
Conclusion
The most commonly reported adverse events following venoplasty for CCSVI include arrhythmia, jugular thrombosis and puncture site bleeding; however, rare complications such as intracranial hemorrhage, cranial nerve palsy, stent migration and venous sinus thrombosis have been reported. The current data quality is the limiting factor; in particular, it is difficult to provide accurate complication rates owing to the limitations of the data presented above and the large number of cases performed outside of these studies. This issue can only be solved if centers submit data to a registry or perform the procedure within the context of a research trial. We note that in the USA, the first CCSVI endovascular registry has been formed, and, in Europe, a randomized controlled trial has commenced. Other institutions should follow suit and patients should look for data submission to a registry or trial as a safety mark. Intervention outside this setting is inappropriate and hard to justify. It is currently unclear who will fund and provide treatment for complications of CCSVI venoplasty and this will need to be considered carefully by healthcare providers.
Future perspective
To address safety concerns, registry data collection will capture large numbers of nontrial procedures, ref lecting real-world practice. European countries should follow suit and set up an international endovascular CCSVI registry, starting, perhaps, on a voluntary basis. The results of randomized controlled trials assessing the efficacy of the technique are still awaited.
Financial & competing interests disclosure
The research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
▪ Venoplasty for chronic cerebrospinal venous insufficiency is practiced at several international centers. The safety profile of the procedure is relatively unknown.
Methods
▪ A systematic review of the PubMed and EMBASE databases from 1947 to the present was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Results
▪ The quality of the studies obtained was generally poor, reporting limited follow-up with a high risk of bias.
▪ The most commonly reported complications were arrhythmia, groin hematoma and jugular thrombosis. There were case reports of stent migration, intracranial hemorrhage and cranial nerve palsy.
Implications
▪ Chronic cerebrospinal venous insufficiency venoplasty is still an experimental procedure and researchers should practice it within the context of a clinical trial or registry.
References
Papers of special note have been highlighted as:
▪ of interest
- Zamboni P, Galeotti R. The chronic cerebrospinal venous insufficiency syndrome. Phlebology 25(6), 269–279 (2010).
- Thapar A, Lane R, Nicholas R. Chronic cerebrospinal venous insufficiency: it is an entity and subset of multiple sclerosis. Against the motion. Presented at: 34th International Charing Cross Symposium. Greenhalgh RM (Ed.). BIBA Press, London, UK, 14–17 April 2012.
- Zamboni P. The big idea: iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis. J. R. Soc. Med. 99(11), 589–593 (2006).
- Thapar A, Lane TR, Nicholas R et al. Systematic review of sonographic CCSVI findings in multiple sclerosis. Phlebology 26(6), 254–256. (2011).
- Doepp F, Wurfel JT, Pfueller CF et al. Venous drainage in multiple sclerosis: a combined MRI and ultrasound study. Neurology 77(19), 1745–1751 (2011).
- Zamboni P, Galeotti R, Weinstock-Guttman B, Kennedy C, Salvi F, Zivadinov R. Venous angioplasty in patients with multiple sclerosis: results of a pilot study. Eur. J. Vasc. Endovasc. Surg. 43(1), 116–122 (2012).
- Zamboni P, Galeotti R, Menegatti E et al. A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency. J. Vasc. Surg. 50(6), 1348–1358. e1–e3 (2009).
- Reekers JA, Lee MJ, Belli AM, Barkhof F. Cardiovascular and Interventional Radiological Society of Europe commentary on the treatment of chronic cerebrospinal venous insufficiency. Cardiovasc. Intervent. Radiol. 34(1), 1–2 (2011).
- Thapar A, Lane TR, Pandey V et al. Internal jugular thrombosis post venoplasty for chronic cerebrospinal venous insufficiency. Phlebology 26(6), 254–256 (2011).
- Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology 22(1), 128; author reply 128 (2011).
- Experimental multiple sclerosis vascular shunting procedure halted at Stanford. Ann. Neurol. 67(1), A13–A15 (2010).
- Ludyga T, Kazibudzki M, Simka M et al. Endovascular treatment for chronic cerebrospinal venous insufficiency: is the procedure safe? Phlebology 25(6), 286–295 (2010).
- Petrov I, Grozdinski L, Kaninski G, Iliev N, Iloska M, Radev A. Safety profile of endovascular treatment for chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J. Endovasc. Ther. 18(3), 314–323 (2011).
- Mandato KD, Hegener PF, Siskin GP et al. Safety of endovascular treatment of chronic cerebrospinal venous insufficiency: a report of 240 patients with multiple sclerosis. J. Vasc. Interv. Radiol. 23(1), 55–59 (2012).
- Burton JM, Alikhani K, Goyal M et al. Complications in MS patients after CCSVI procedures abroad (Calgary, AB). Can. J. Neurol. Sci. 38(5), 741–746 (2011).
- 1Neglen P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J. Vasc. Surg. 46(5), 979–990 (2007).
- Malagoni AM, Galeotti R, Menegatti E et al. Is chronic fatigue the symptom of venous insufficiency associated with multiple sclerosis? A longitudinal pilot study. Int. Angiol. 29(2), 176–182 (2010).
- CCSVI online. www.ccsvi-online.com (Accessed 20 March 2012)
- US FDA Press Release. www.fda.gov/NewsEvents/Newsroom/ PressAnnouncements/ucm303538.htm (Accessed 29 May 2012)
- Safe Trip Medical. www.safemedtrip.com/ccsvi-liberationprocedure. html (Accessed 29 May 2012)
- Hubbard Multi-Center CCSVI Registry. www.hubbardfoundation.org/CCSVI_multicentered_ registry_introduction.html (Accessed 22 May 2011)
▪ Explains the premise of chronic cerebrospinal venous insufficiency (CCSVI).
▪ Explains the diagnostic problems associated with CCSVI.
▪ Explains the diagnostic problems associated with CCSVI.
▪ Explains the rationale for treatment of CCSVI.
▪ Explains the rationale for not treating CCSVI.
▪ Websites