Review Article - Interventional Cardiology (2013) Volume 5, Issue 5
Complications of chronic total occlusion percutaneous coronary intervention
- Corresponding Author:
- Tony J DeMartini
Prairie Heart Institute, St John’s Hospital
Springfield, IL, USA
E-mail: ctoskrat@comcast.net
Abstract
Chronic total occlusions (CTOs) are typically characterized by thrombolysis in myocardial infarction (TIMI) grade 0 (i.e., complete) or TIMI grade 1 flow in a coronary artery for a minimum of 3 months.
Keywords
chronic total occlusion, complications, percutaneous coronary intervention
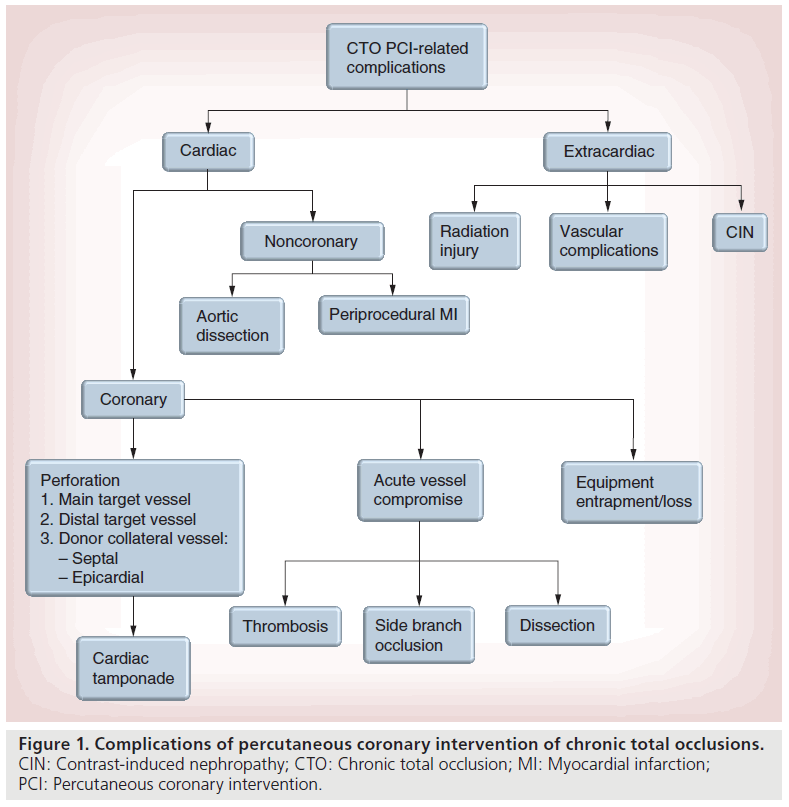
Chronic total occlusions (CTOs) are typically characterized by thrombolysis in myocardial infarction (TIMI) grade 0 (i.e., complete) or TIMI grade 1 flow in a coronary artery for a minimum of 3 months [1,2]. However, TIMI grade 2 or 3 flow to the distal vessel can occur in CTOs with large bridging collaterals. The true prevalence of CTOs remains unknown, as most patients are asymptomatic. However, CTOs are common and observed in up to 25% of patients undergoing angiography [3]. Percutaneous coronary intervention (PCI) of CTOs is most commonly performed in patients with refractory stable or progressive angina, despite optimal medical therapy. Revascularization of CTOs are challenging, namely owing to the marked anatomic and lesion variability that respresent the hallmark of CTOs and the variety of techniques and devices required for recanalization. The rates of successful recanalization of CTOs continue to improve and are 80% in contemporary practice at centers with higher CTO PCI volumes [4,5]. Advances in techniques and devices to treat CTOs have also reduced the rate of complications, where the rate of a major complications (death, emergent coronary artery bypass graft and stroke) is as low as 0.5% [5]. Regardless, operators should be aware of the complications that are more common in performing CTO PCI and include both cardiac (coronary and noncoronary) and extracardiac complications (Figure 1). In this review, the authors discuss the complications of CTO revascularization and strategies for prevention and treatment.
Cardiac complications: coronary
▪ Coronary perforation
For most operators, coronary perforation is a dreaded complication of CTO PCI, given the potential to cause a hemorrhagic pericardial effusion and/or cardiac tamponade requiring emergent pericardiocentesis or cardiac surgery. The estimated incidence of coronary perforation is 2.9% (95% CI: 2.3–3.6), but observed rates are as high as 11.9% in the literature [5]. By comparison, the incidence of coronary perforation in non-CTO PCI is 0.2% [6]. Cardiac tamponade occurs in approximately 10% of patients with a coronary perforation (pooled overall incidence rate: 0.3%; 95% CI: 0.2–0.5) [5]. Thus, the majority of coronary perforations are self-limited and can be managed without progression to tamponade. Coronary perforations are classified according to the Ellis Criteria (Table 1) [7]. Rates of coronary perforation and tamponade are higher in unsuccessful PCI attempts compared with successful recanalization of CTOs (perforation: 10.7 vs 4.7%; p < 0.0001; tamponade: 1.7 vs 0%; p < 0.0001) [5]. The rate of coronary perforation is higher using a retrograde approach compared with an antegrade approach (4.7 vs 2.1%; p = 0.04), but rates of cardiac tamponade are similar between the two approaches [8]. Importantly, the mechanisms and subsequent management of coronary perforations depend on vessel location. Perforations are categorized according to three main coronary vessel locations: main target vessel (i.e., at or near the CTO), distal target vessel and donor collateral vessel.
| Perforation type | Description | |
|---|---|---|
| Type I | Extraluminal crater without myocardial blush, extravasation or evidence of dissection | |
| Type II | Myocardial or pericardial blush without extravasation | |
| Type III | Extravasation through a ≥1 mm perforation | |
| Type III: cavity spilling | Perforation and extravasation into an anatomic cavity chamber | |
| Adapted with permission from [7]. | ||
Table 1. Ellis Criteria of coronary perforations.
Main target-vessel coronary perforation can occur with either the antegrade or retrograde percutaneous approaches. Contrary to belief, guidewire perforations alone via wire escalation or dissection and re-entry techniques are typically self-limited and rarely lead to a hemorrhagic pericardial effusion and/or cardiac tamponade. However, if a balloon or device (e.g., stent) is advanced into the pericardial space after guidewire perforation, then the risk for hemorrhagic pericardial effusion and cardiac tamponade increases owing to manual expansion of the coronary perforation. Contrast extravasation may not be readily apparent until balloon inflation and/or stent deployment. It is also important to recognize that oversized balloons or stents can lead to main target-vessel coronary perforation.
The initial step in management of any main target-vessel coronary perforation is to position a balloon proximal to the area of contrast extravasation and occlude the perforation with balloon inflation. Prolonged balloon inflations may be required to achieve hemostasis. If bleeding persists despite balloon occlusion, then a covered stent (e.g., JOSTENT Graftmaster, Abbott Vascular, CA, USA; Symbiot stent, Boston Scientific Corp., MA, USA; Over and Under stent, IGTI Medical, Or Akiva, Israel) should be placed [9,10]. Type III (Table 1) coronary perforations (i.e., contrast- streaming or cavity-spilling) usually result in cardiac tamponade, and a covered stent should be implanted for this type of perforation [11]. The most efficient method to minimize bleeding in patients requiring a covered stent is to use a ping-pong catheter (i.e., dual catheter) technique [9,12]. With this technique, a second guide catheter is advanced near the coronary ostium next to the first guide catheter that is currently engaged with the balloon occluding the perforation [12]. While maintaining balloon occlusion, the first guide catheter is disengaged, then a second guide catheter is advanced to the coronary ostium and covered stent is positioned proximal to the occluding balloon [12]. Rapidly, the occluding balloon is deflated and withdrawn proximally, then a covered stent is advanced and deployed to fully cover the perforated site [12]. Prior to removing any equipment, adequate sealing of the coronary perforation should be verified [12].
Distal target-vessel perforation typically occurs after crossing the CTO using an antegrade approach. One reason dual injection is essential for CTO PCI is the ability to delineate the natural course of the target vessel and identify branches beyond the distal cap of the CTO [4]. After crossing a CTO either through wire escalation or dissection and re-entry, advancement of the guidewire distally can lead to coronary vessel perforation. This scenario occurs more often when the guidewire is advanced into a smaller branch of the distal target vessel. Importantly, exchanging the stiffer, polymer-jacketed and/or tapered guidewires for workhorse wires immediately after crossing the CTO lesion and re-entering the true lumen can minimize the risk for distal target-vessel perforation. Distal target-vessel perforation can be less angiographically apparent than main target-vessel perforation, thus it is critical that operators pay careful attention to the distal guidewire position during CTO PCI. Operators should also consider guidewire trapping for distal wire protection during PCI of CTOs [9].
The initial step in managing a distal targetvessel perforation is to use balloon occlusion proximal to the coronary perforation. In addition, a microcatheter can be advanced into the distal target vessel and aspirated via suction to collapse the vessel [9,13]. The technique may enhance hemostasis and prevent the need for embolization. However, if bleeding persists, embolization is typically required using coils, vascular plugs, thrombus, subcutaneous fat or fibrin glue [9,14–17].
Unique to retrograde CTO PCI is the risk for donor collateral-vessel perforation. However, progression to cardiac tamponade following a donor collateral-vessel perforation depends on the location of the collateral vessel (i.e., septal vs epicardial). Collateral-vessel perforation normally occurs owing to advancing the guidewire and/or devices when attempting to reach the distal cap of the CTO. To facilitate passage to the target vessel, some operators may dilate the collateral vessels, which can also lead to coronary vessel perforation.
Septal collateral-vessel perforation carries a unique set of downstream consequences. However, cardiac tamponade rarely occurs [9,18]. Guidewire perforation of a septal collateral commonly results in bleeding into interventricular septum (i.e., septal wall hematoma) and not the pericardial space and is typically a benign event. It is also possible to perforate a septal collateral coronary vessel into any cardiac chamber, including the coronary sinus; however, this also rarely leads to any adverse clinical consequence [9,19]. Septal wall hematomas are easily identified on echocardiography (i.e., echo-free space) and usually asymptomatic, but can result in chest discomfort and rarely heart block depending on its size and location [9,20,21]. Rarely, a septal wall hematoma can progress to a septal wall rupture requiring percutaneous or surgical treatment [22]. Left ventricular outflow tract obstruction has also been observed in unique situations of septal wall hematomas and may require surgical intervention.
Epicardial collateral perforation carries a higher risk of hemorrhagic pericardial effusion and cardiac tamponade compared with septal collaterals [9]. Bleeding from an epicardial collateral perforation can be difficult to control due to the limited options available for management. Thus, only experienced retrograde CTO operators should attempt recanalization through an epicardial collateral vessel. During CTO PCI, dilation of an epicardial collateral vessel is strongly discouraged and should be avoided [4]. The key aspect in epicardial collateral perforation is to recognize that epicardial collaterals will have access to blood flow from both the antegrade and retrograde sources. If an epicardial collateral-vessel perforation is noticed, one initial measure is to balloon occlude either the perforated epicardial collateral or its donor vessel. Then, the perforation should be approached both antegrade and retrograde, with an attempt to achieve hemostasis using microcatheters with suction to collapse the perforated vessel and/or embolization (e.g., coils) [9]. Unfortunately, this approach presupposes that the CTO has been recanalized and the perforated vessel can be approached from both sides. If bleeding continues despite these measures, cardiac surgery may be required.
In general, operators should be aware of the natural history of coronary perforation in CTO PCI. Complications resulting from a perforation may not manifest for hours to days after PCI. Thus, the threshold for prompt evaluation of any cardiac or atypical symptoms is essential following CTO PCI. Anticoagulation with unfractionated heparin may be considered over bivalirudin, given the ability to reverse the anticoagulant effects of heparin with protamine in the setting of a coronary perforation [4,9]. Routine use of glycoprotein IIb/IIIa inhibitors is also not advised during CTO PCI and should be avoided. Wire and other microperforations that are not identifiable on angiography may manifest with the administration of glycoprotein IIb/IIIa inhibitors. It is critical that all catheterization centers performing CTO PCI have emergency equipment immediately available to treat a perforated coronary vessel, which includes a pericardiocentesis kit, varying sizes of covered stents, embolization equipment and a 2D echocardiography machine. Intravenous fluids and or vasoactive agents should be administered rapidly for hypotension and suspected coronary perforation. In this setting, the patient should also undergo immediate evaluation for pericardial effusion and meticulous review of the coronary angiogram. The decision to perform emergent pericardiocentesis should be dictated based on the patient’s hemodynamics. In certain patients, a hemorrhagic effusion may be focal (e.g., prior coronary artery bypass graft) and can potentially self-tamponade the perforation. Unfortunately, postcoronary artery bypass graft patients may also develop focal tamponade of the right or left atrium that is not amenable to pericardiocentesis and require surgical intervention. The steps described previously to ensure hemostasis are essential to the management of coronary perforation. Finally, cardiac surgery should be notified of any patient with a coronary perforation requiring percutaneous intervention.
▪ Stent/guidewire/device entrapment or loss
An additional coronary complication in CTO PCI is the entrapment or loss of any equipment required for CTO recanalization including stents, guidewires and other devices. The incidence of equipment entrapment or loss is unknown but considered a very rare complication [9,23,24]. The risk for entrapment or device loss increases in CTO PCI owing to the lesion complexity, extent of calcification, vessel tortuosity and techniques required for recanalization. During a retrograde approach, balloon and/or stent delivery can lead to entrapment or stent embolization [23]. In addition, any attempt to cross the CTO poses a risk for equipment entrapment and potential for embolization down the distal target vessel. This complication can lead to vessel injury and/or acute donorvessel closure when attempting to mitigate a solution. Retrieval should be pursued in all cases of equipment loss. Any equipment that is embolized and not retrievable should be crushed against the coronary vessel wall via a series of balloon inflations and stent deployment [9,23,24]. Intravascular imaging with intravascular ultrasound or optical coherence tomography should be used to ensure that the crushed equipment is not exposed anywhere in the coronary vessel [9,24]. In addition, guidewire fracture may also occur during CTO PCI. In this instance, retrieval should be attempted, but rarely leads to any clinical sequelae.
▪ Donor-vessel injury: dissection & acute closure
During retrograde CTO PCI, nontarget donorvessel injury (e.g., dissection or thrombosis) with or without acute closure can occur as attempts are made to advance guidewires and/or devices to the distal cap of the CTO. The aggressive guide catheters that are required to support retrograde CTO PCI can dissect the proximal donor vessel. This risk may be higher as equipment is withdrawn, which can suddenly cause ‘deep seating’ of the guide catheter [9]. The incidence of coronary dissection during retrograde PCI ranges from 0.5 to 10% and is significantly higher in unsuccessful versus successful attempts (10 vs 3.1%) [25–28]. The downstream consequences of coronary dissection largely rest on the amount of myocardium supplied and the severity of the dissection. Management (i.e., stenting vs conservative management) of any nontarget-vessel dissection will be dictated based on its location and downstream clinical consequences. It is also important to note that acute vessel closure of the donor vessel may occur without dissection (e.g., thrombosis). In CTO PCI, vessel thrombosis is rare but the risk may be higher compared with non-CTO PCI, due to the prolonged coronary guidewire intubation times required for recanalization [9]. Collateral vessels are particularly susceptible to dissection and thrombosis during retrograde attempts. Prevention of donor-vessel injury is essential during retrograde CTO PCI. It is important to pay careful attention to guide catheter position, especially during guidewire or device withdrawal. Guide catheters without side holes should be used to avoid masking any pressure dampening that may occur with donor-vessel injury [9]. Importantly, contrast should not be injected in the setting of pressure dampening, which can increase the risk for coronary dissection. With any CTO PCI, activated clotting times should be closely monitored and maintained above 350 s to minimize risk for thrombosis. Donor-vessel thrombosis can be a catastrophic event with compromise of multiple myocardial territories. Prevention and rapid identification are the keys to successful management of this potential complication.
▪ Target-vessel injury: side-branch occlusion & target-vessel dissection/thrombosis
In any attempt to recanalize a CTO, there is a risk of target-vessel injury both proximal and distal to the CTO lesion. Side branches at or near the CTO are common and observed in 16–79% of CTO lesions [25,26,29]. In one study, side-branch compromise occurred in 22% of patients, and the rate of side-branch occlusion was significantly higher in successful CTO procedures compared with unsuccessful attempts (4.4 vs 0.88%; p = 0.008) [25]. Side-branch occlusion is particularly common during dissection and re-entry techniques, but also may occur during stent deployment after successfully crossing the CTO. Given the risk for periprocedural myocardial infarction (MI) with side-branch compromise, it is critical that attempts are made to reduce its occurrence. Dual injection of the target and contralateral donor vessels is mandatory in all CTO PCIs to allow for visualization of any side branches associated with the CTO lesion [4]. With any dissection and re-entry technique, the subintimal dissection length should be minimized by re-entering the true lumen as soon as possible after successfully crossing the lesion. Distal target-vessel dissection can arise from long subintimal dissection planes, where wire position is unknown or proximal re-entry was unsuccessful. Dissection and reentry techniques can also result in a subintimal hematoma, which can expand and lead to side-branch or distal vessel compromise. The retrograde approach should be considered in patients with appropriate collateral vessels and large side branches located at or near the distal cap of the CTO. The subintimal tracking and re-entry technique establishes early proximal subintimal wire position, then a dissection plane is advanced into the distal artery [30]. Reentry into the true lumen is attempted at a distal entry site, typically at or near a target-vessel side branch [30]. Thus, there is a risk for permanent side-branch occlusion and distal targetvessel injury with the subintimal tracking and re-entry technique. Importantly, specialized techniques (e.g., limited antegrade subintimal tracking [LAST] technique) and crossing/reentry devices (CrossBoss™ catheter and Stingray ™ balloon/guidewire system; Boston Scientific, MN, USA) should be utilized to facilitate successful crossing and lessen the risk for side-branch occlusion. With the crossing and re-entry device system, over a third of CTOs can be crossed via the true lumen using the CrossBoss catheter without a subintimal dissection [31]. If subintimal tracking occurs with the CrossBoss catheter, then a controlled dissection plane is only extended to the angiographic true lumen and re-entry is attempted into the main target vessel using a re-entry device (i.e., Stingray balloon/guidewire system) [31]. Intravascular imaging with intravascular ultrasound and optical coherence tomography can minimize target-vessel complications by enhanced subintimal wire tracking [32]. Another simple strategy to avoid inadvertently expanding the antegrade subintimal dissection with contrast injection is to disconnect the control syringe.
Target-vessel thrombosis is a rare and avoidable complication of CTO PCI that is typically due to catheter injection of thrombus. As dissection planes are created, long periods of time may occur when antegrade injections are avoided and thrombus may form within the guide catheter. Prior to contrast injections through the antegrade guide, complete clearing of the guide should be done to avoid thrombus injection.
Other cardiac complications
▪ Aortic dissection
As mentioned previously, stiff and/or aggressively shaped guide catheters are often required to support CTO PCI, which can increase the risk of aortic injury. The incidence of aortic dissection in CTO PCI is low (<1%) [25]. Historically, iatrogenic aortic dissection is associated with high mortality rates (~35%) and MI (15%) [33]. However, the clinical presentation of iatrogenic aortic dissection can be insidious. Patients will not present with the ‘classical’ symptoms associated with spontaneous aortic dissection [33]. Instead, ‘atypical’ symptoms or the absence (25%) of chest discomfort is common, and hemodynamic compromise occurs in approximately one-quarter of patients [33]. It is important to consider aortic dissection in any patient with sudden hemodynamic collapse and/or ischemia during CTO PCI. Meticulous attention to guide catheter position can minimize the risk of aortic dissection. Operators should never inject contrast into any guide catheter with a dampened pressure waveform [9]. If an aortic dissection occurs and is located at or within the vicinity of the coronary ostium, operators should consider immediately stenting the coronary vessel at the aorto-ostium to minimize the risk that the dissection plane will compromise the coronary vasculature [9,34]. All iatrogenic aortic dissections should be imaged with transesophageal echocardiography or computed tomography to determine subsequent management (i.e., conservative vs emergent endovascular or surgical repair). Close monitoring, serial noninvasive imaging and early consultation with cardiac surgery is critical for the management of iatrogenic aortic dissection. Aortic insufficiency is another potential complication of aortic dissection and, depending on its severity, may require surgery or close monitoring with serial transthoracic echocardiography.
▪ Periprocedural MI
In CTO PCI, periprocedural MI can occur from any of the coronary complications discussed previously and is one of the most common procedural complications [5]. Approximately 2.5% (95% CI: 1.9–3.0) of patients undergoing CTO PCI will experience an MI; however, the majority of MIs are not ST-elevation MIs (0.2%; 95% CI: 0.1–0.3) [5]. The rate of periprocedural MI varies widely between studies (0–19.4%), largely owing to practice variability in routinely measuring cardiac biomarkers following PCI [5]. The rates of periprocedural MI are similar between antegrade and retrograde approaches, as well as in successful versus unsuccessful PCI attempts [5]. In addition, acute stent thrombosis is a rare procedural complication of CTO PCI (0.3%; 95% CI: 0.1–0.5) and cause of in-hospital MI [5]. The long-term clinical consequences of periprocedural MI in CTO patients is less understood. Despite a reduction in overall mortality, recanalization of CTOs is not associated with a lower long-term risk of MI [35]. As part of secondary prevention, all patients should receive dual antiplatelet therapy for a minimum of 12 months following CTO PCI [36].
Extracardiac complications
▪ Contrast-induced nephropathy
Following PCI, contrast-induced nephropathy (CIN) is a major cause of morbidity and mortality and occurs in approximately 10–15% of patients [5,37–39]. In CTO PCI, rates of CIN range from 2.4 to 18.1% with a pooled estimate of 3.8% (95% CI: 2.4–5.3) [5]. However, CIN is only reported in 20% of studies published on CTO PCI complications, thus the estimate is likely underestimated [5]. One concern with CTO PCI is the risk for CIN with the administration of higher volumes of contrast compared with non-CTO PCI. However, limited evidence exists to support this notion. Regardless, renal function should be assessed in all patients undergoing CTO PCI by measuring the estimated glomerular filtration rate [40]. In addition, the risk for CIN can be further assessed using a risk prediction rule [39,101]. Prevention of CIN is important in all patients undergoing PCI; however, the best preventative treatment strategy remains controversial. Despite initial enthusiasm, studies on the efficacy of N-acetylcysteine or sodium bicarbonate for preventing CIN are inconclusive [41,42]. Recently, the POSEIDON trial was presented and demonstrated that hydration using left ventricular end-diastolic pressure-guided hydration reduced the rate of CIN by 59% compared with standard hydration (absolute risk difference: 9.5%; number needed to treat of 11 to prevent one case of CIN) [43]. The median volume of hydration in POSEIDON was significantly higher in the left ventricular end-diastolic pressure-guided group compared with standard therapy (1711 vs 807 ml; p < 0.001), which suggests that higher volumes of hydration with normal saline can reduce the rates of CIN [43].
As experience with CTO PCI grows, the need for higher volumes of contrast should decrease. Repeated contrast injections, especially through the antegrade guide, become unnecessary and frequently are deleterious. Retrograde procedures typically require less contrast. Therefore, in a patient with antegrade and retrograde options for CTO PCI and renal insufficiency, the retrograde approach may be preferred.
▪ Radiation injury
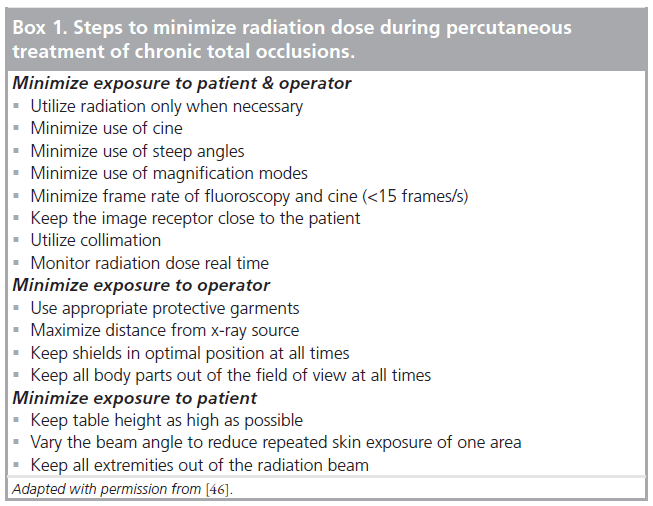
CTO PCI requires longer fluoroscopy times compared with non-CTO PCI [44]. In a recent meta-analysis, radiation injury was reported in three cases out of 2857 patients, but is infrequently reported in studies of CTO PCI [5]. The risk of radiation injury is dose-dependent, and there is a wide variability in the radiation dose ranges with different operators and institutions [44,45]. In all cases of CTO PCI, careful attention to the fluoroscopy time and radiation dose is necessary. Any patient with an exposure dose of >5 Gy can cause radiation skin injury, while doses of >10 Gy may cause significant injury [4]. Patients who do receive more than 5 Gy should be evaluated in 2–4 weeks for radiation skin injury and followed for a minimum of 1 year. As shown in Box 1, all operators should take steps to minimize radiation exposure during CTO PCI [46]. The combination of a change from 15 to 7.5 frames/s and change in field size from 7 to 10 inches can significantly reduce radiation exposure.
▪ Vascular complications
Vascular complications occur in an estimated 0.6% (95% CI: 0.3–0.9) of patients undergoing CTO PCI [5]. As the safety of PCI has evolved, rates of groin complications have surpassed ischemic complications [47]. Radial access is associated with a lower risk of bleeding and adverse cardiac events compared with femoral access [48]. However, radial access can prolong procedural times, increase radiation doses and provide less guide support during CTO PCI. It is important that operators evaluate prior iliofemoral angiograms to select the safest and most efficacious approach for vascular access. Bifemoral, femoral–radial, or biradial access can be utilized for dual injection during CTO PCI, and should be individualized for each patient based on operator experience. For femoral access cases, operators should use fluoroscopy and/or ultrasound to facilitate access. Use of a micropuncture needle with direct visualization under fluoroscopy can optimize needle placement in relation to the femoral head. All patients should undergo femoral angiography after sheath placement, preferably with a smaller sheath that can be upsized if optimal access site entry is confirmed.
Conclusion
CTO PCI is technically challenging for interventional cardiologists. The percutaneous strategies used to treat CTOs continue to evolve and the success rates for successful recanalization are improving. As CTO PCI gains adoption, it is crucial that CTO operators are aware of the unique set of complications associated with CTO revascularization. Prevention and management of CTO PCI complications is critical. Knowledge of the equipment and options available for management of CTO PCI-related complications will provide operators with the necessary skills required for safe percutaneous treatment of CTOs.
Future perspective
Significant innovations in equipment and device technology will continue to emerge for PCI of CTOs. Each step forward in the techniques and devices used for the percutaneous treatment of CTOs will improve the rate of successful recanalization and minimize the risk for complications. With these advances, pivotal trials will be conducted to determine the efficacy of CTO PCI compared with optimal medical therapy.
Financial & competing interests disclosure
TJ DeMartini works as a consultant at Boston Scientific. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Chronic total occlusions (CTOs) are characterized by thrombolysis in myocardial infarction grade 0–1 flow for a minimum of 3 months and are observed in up to 25% of patients undergoing angiography.
▪ Percutaneous coronary intervention (PCI) of CTOs is commonly performed in patients with refractory angina who have failed medical therapy.
▪ Successful recanalization of CTOs is ≥80% in contemporary practice and the rate of major complications is approximately 0.5%.
▪ CTO PCI has a unique set of cardiac and extracardiac complications.
▪ Coronary perforation occurs in approximately 2.9% of patients and rarely leads to cardiac tamponade:
– Main target-vessel perforations are treated with balloon occlusion ± covered stents;
– Distal target-vessel perforation can be treated using microcatheters and embolization;
– Septal collateral-vessel perforation typically does not result in cardiac tamponade;
– Epicardial collateral-vessel perforation has a high risk of cardiac tamponade and ideally should be treated with antegrade and retrograde embolization.
▪ Equipment entrapment or loss is rare but requires retrieval and/or crushing of equipment against the coronary vessel wall.
▪ Coronary dissection and side-branch occlusion are common complications that can be prevented by careful monitoring of guide catheters/wires/devices.
▪ With any dissection and re-entry technique, subintimal tracking should be minimized to reduce the risk of side-branch occlusion and/or distal target-vessel injury.
▪ Periprocedural myocardial infarctions are common but ST-elevation myocardial infarctions are rare.
▪ All patients undergoing CTO PCI should be assessed for their risk of contrast-induced nephropathy and receive aggressive hydration as tolerated.
▪ The risk for radiation injury is higher with CTO PCI, and patients should be followed closely for up to 1 year following any radiation dose >5 Gy.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Stone GW, Kandzari DE, Mehran R et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part I. Circulation 112(15), 2364–2372 (2005).
- Shah PB. Management of coronary chronic total occlusion. Circulation 123(16), 1780–1784 (2011).
- Christofferson RD, Lehmann KG, Martin GV, Every N, Caldwell JH, Kapadia SR. Effect of chronic total coronary occlusion on treatment strategy. Am. J. Cardiol. 95(9), 1088–1091 (2005).
- Brilakis ES, Grantham JA, Rinfret S et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc. Interv. 5(4), 367–379 (2012).
- Patel VG, Brayton KM, Tamayo A et al. Angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. JACC Cardiovasc. Interv. 6(2), 128–136 (2013).
- Javaid A, Buch AN, Satler LF et al. Management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am. J. Cardiol. 98(7), 911–914 (2006).
- Ellis SG, Ajluni S, Arnold AZ et al. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation 90(6), 2725–2730 (1994).
- Galassi AR, Tomasello SD, Reifart N et al. In-hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: insights from the ERCTO (European Registry of Chronic Total Occlusion) registry. EuroIntervention 7(4), 472–479 (2011).
- Brilakis ES, Karmpaliotis D, Patel V, Banerjee S. Complications of chronic total occlusion angioplasty. Interv. Cardiol. Clin. 1, 373–389 (2012).
- Romaguera R, Waksman R. Covered stents for coronary perforations: is there enough evidence? Catheter. Cardiovasc. Interv. 78(2), 246–253 (2011).
- Al-Mukhaini M, Panduranga P, Sulaiman K, Riyami AA, Deeb M, Riyami MB. Coronary perforation and covered stents: an update and review. Heart Views 12(2), 63–70 (2011).
- Ben-Gal Y, Weisz G, Collins MB et al. Dual catheter technique for the treatment of severe coronary artery perforations. Catheter.Cardiovasc. Interv. 75(5), 708–712 (2010).
- Yasuoka Y, Sasaki T. Successful collapse vessel treatment with a syringe for thrombus-aspiration after the guidewire-induced coronary artery perforation. Cardiovasc. Revasc. Med. 11(4), e261–e263(2010).
- Ponnuthurai FA, Ormerod OJ, Forfar C. Microcoil embolization of distal coronary artery perforation without reversal of anticoagulation: a simple, effective approach. J. Invasive Cardiol. 19(8), e222–e225 (2007).
- Oda H, Oda M, Makiyama Y et al. Guidewire-induced coronary artery perforation treated with transcatheter delivery of subcutaneous tissue. Catheter. Cardiovasc. Interv. 66(3), 369–374 (2005).
- Storger H, Ruef J. Closure of guide wire-induced coronary artery perforation with a two-component fibrin glue. Catheter. Cardiovasc. Interv. 70(2), 237–240 (2007).
- Fischell TA, Korban EH, Lauer MA. Successful treatment of distal coronary guidewire-induced perforation with balloon catheter delivery of intracoronary thrombin. Catheter. Cardiovasc. Interv. 58(3), 370–374(2003).
- Matsumi J, Adachi K, Saito S. A unique complication of the retrograde approach in angioplasty for chronic total occlusion of the coronary artery. Catheter. Cardiovasc. Interv. 72(3), 371–378 (2008).
- Sachdeva R, Hughes B, Uretsky BF. Retrograde approach to a totally occluded right coronary artery via a septal perforator artery: the tale of a long and winding wire. J. Invasive Cardiol. 22(4), E65–E66 (2010).
- Lin TH, Wu DK, Su HM et al. Septum hematoma: a complication of retrograde wiring in chronic total occlusion.Int. J. Cardiol. 113(2), e64–e66 (2006).
- Fairley SL, Donnelly PM, Hanratty CG, Walsh SJ. Interventricular septal hematoma and ventricular septal defect after retrograde intervention for a chronic total occlusion of a left anterior descending coronary artery. Circulation 122(20), e518–e521 (2010).
- Joyal D, Thompson CA, Grantham JA, Buller CE, Rinfret S. The retrograde technique for recanalization of chronic total occlusions: a step-by-step approach. JACC Cardiovasc. Interv. 5(1), 1–11 (2012).
- Utsunomiya M, Kobayashi T, Nakamura S. Case of dislodged stent lost in septal channel during stent delivery in complex chronic total occlusion of right coronary artery. J. Invasive Cardiol. 21(11), E229–E233 (2009).
- Sianos G, Papafaklis MI. Septal wire entrapment during recanalisation of a chronic total occlusion with the retrograde approach. Hellenic J. Cardiol. 52(1), 79–83 (2011).
- Rathore S, Matsuo H, Terashima M et al. Procedural and in-hospital outcomes after percutaneous coronary intervention for chronic total occlusions of coronary arteries 2002 to 2008: impact of novel guidewire techniques. JACC Cardiovasc. Interv. 2(6), 489–497 (2009).
- Ma JY, Qian JY, Ge L et al. Retrograde approach for the recanalization of coronary chronic total occlusion: collateral selection and collateral related complication.Chin. Med. J. 126(6), 1086–1091 (2013).
- Muramatsu T, Tsukahara R, Ito Y et al. Changing strategies of the retrograde approach for chronic total occlusion during the past 7 years. Catheter. Cardiovasc. Interv. 81(4), E178–E185 (2013).
- Karmpaliotis D, Michael TT, Brilakis ES et al. Retrograde coronary chronic totalocclusion revascularization: procedural and in-hospital outcomes from a multicenter registry in the United States. JACC Cardiovasc. Interv. 5(12), 1273–1279 (2012).
- Morino Y, Kimura T, Hayashi Y et al. In-hospital outcomes of contemporary percutaneous coronary intervention in patients with chronic total occlusion insights from the J-CTO Registry. JACC Cardiovasc. Interv. 3(2), 143–151 (2010).
- Colombo A, Mikhail GW, Michev I et al. Treating chronic total occlusions using subintimal tracking and reentry: the STAR technique. Catheter. Cardiovasc. Interv. 64(4), 407–411 (2005).
- Whitlow PL, Burke MN, Lombardi WL et al. Use of a novel crossing and re-entry system in coronary chronic total occlusions that have failed standard crossing techniques: results of the FAST-CTOs (Facilitated Antegrade Steering Technique in Chronic Total Occlusions) trial. JACC Cardiovasc. Interv. 5(4), 393–401 (2012).
- Tsujita K, Maehara A, Mintz GS et al. Intravascular ultrasound comparison of the retrograde versus antegrade approach to percutaneous intervention for chronic total coronary occlusions. JACC Cardiovasc. Interv. 2(9), 846–854 (2009).
- Januzzi JL, Sabatine MS, Eagle KA et al. Iatrogenic aortic dissection. Am. J. Cardiol. 89(5), 623–626 (2002).
- Abdou SM, Wu CJ. Treatment of aortocoronary dissection complicating anomalous origin right coronary artery and chronic total intervention with intravascular ultrasound guided stenting. Catheter. Cardiovasc. Interv. 78(6), 914–919 (2011).
- Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and meta-analysis. Am. Heart J. 160(1), 179–187 (2010).
- Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. J. Am. Coll. Cardiol. 58(24), e44–e122 (2011).
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. Am. Coll. Cardiol. 56(23), 1897–1907(2010).
- Bartholomew BA, Harjai KJ, Dukkipati S et al. Impact of nephropathy afterpercutaneous coronary intervention and a method for risk stratification. Am. J. Cardiol. 93(12), 1515–1519 (2004).
- Mehran R, Aymong ED, Nikolsky E et al. simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J. Am. Coll. Cardiol. 44(7), 1393–1399 (2004).
- McCullough PA, Adam A, Becker CR et al. Risk prediction of contrast-induced nephropathy. Am. J. Cardiol. 98(6A), 27K–36K (2006).
- Brar SS, Hiremath S, Dangas G, Mehran R, Brar SK, Leon MB. Sodium bicarbonate for the prevention of contrast induced-acute kidney injury: a systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 4(10), 1584–1592 (2009).
- Stenstrom DA, Muldoon LL, Armijo-Medina H et al. N-acetylcysteine use to prevent contrast medium-induced nephropathy: premature Phase III trials. J. Vasc. Interv. Radiol. 19(3), 309–318 (2008).
- Brar SK. POSEIDON (Prevention of Contrast Renal Injury with Different Hydration Strategies): a prospective, randomized trial of sliding-scale hydration for prevention of contrast nephropathy. Presented at: Transcatheter Cardiovascular Therapeutics.
- Grantham JA, Marso SP, Spertus J, House J, Holmes DR Jr, Rutherford BD. Chronic total occlusion angioplasty in the United States.JACC Cardiovasc. Interv. 2(6), 479–486(2009).
- Suzuki S, Furui S, Isshiki T et al. Factors affecting the patient’s skin dose during percutaneous coronary intervention for chronic total occlusion. Circ. J. 71(2), 229–233 (2007).
- Chambers CE, Fetterly KA, Holzer R et al. Radiation safety program for the cardiac catheterization laboratory. Catheter. Cardiovasc. Interv. 77(4), 546–556 (2011).
- Berry C, Kelly J, Cobbe SM, Eteiba H. Comparison of femoral bleeding complications after coronary angiography versus percutaneous coronary intervention. Am. J. Cardiol. 94(3), 361–363 (2004).
- Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am. Heart J. 157(1), 132–140 (2009).
- The Zunis Foundation. A risk score to predict contrast-induced nephropathy (2009). www.zunis.org/Contrast-Induced%20 Nephropathy%20Calculator2.htm
▪▪ Landmark article written by chronic total occlusion (CTO) experts that details the contemporary clinical management of CTO percutaneous coronary intervention (PCI) and provides an algorithm strategy for CTO treatment.
▪▪ Excellent recent meta-analysis on the success rates and complication rates associated with CTO PCI.
▪ Excellent review on the complications of CTO PCI and strategies for prevention and management.
▪ Important article that provides a detailed explanation of the dual-catheter technique for treatment of coronary preforation and clinical evidence for its efficacy.
▪ Unique observational study that compares the intravascular ultrasound findings from the retrograde and antegrade CTO PCI approaches.
▪ Describes a commonly used risk prediction rule for estimating the risk of contrast-induced nephropathy.
▪ Excellent review on radiation safety guidelines for the modern cardiac catheterization laboratory.
▪ Website