Review Article - Interventional Cardiology (2009) Volume 1, Issue 2
Complications of transcatheter atrial septal defect closure
- Corresponding Author:
- Daniel De Wolf
Department of Pediatric Cardiology
Ghent University Hospital UZ Gent
De Pintelaan 185, 9000 Gent, Belgium
Tel: +32 9332 2421
Fax: +32 9332 3856
E-mail: daniel.dewolf@ugent.be
Abstract
Keywords
atrial septal defect, closure, complications, device, patent foramen ovale
Surgical closure of atrial septal defect (ASD) has been the treatment of choice for decades, with excellent results, negligible mortality and limited morbidity. Percutaneous closure of ASD has been reported since the 1970s [1], became widely used in the 1990s in many centers, and replaced surgical ASD closure for anatomically suitable ASD. Since the first attempts, several new devices have been introduced with a continuous increase in indications (size, rims and complexity) and better results. Percutaneous closure of patent foramen ovale (PFO) in stroke patients was introduced in the 1990s and is now widely practiced [2].
Defects amenable for closure
Two types of atrial defects are amenable to percutaneous closure. The secundum ASD (ASD 2) is a congenital defect in the fossa ovalis of the atrial septum. Typically the defect is located in the middle of the atrial septum with rims surrounding the defect. Not infrequently, the anterior rim between the defect and the aorta is missing. In certain cases, the posterior, posteroinferior or coronary sinus rims are missing, limiting the chances of successful percutaneous closure. Sinus venosus ASD and primum ASD are not suitable for percutaneous closure. The ASD 2 is responsible for a variable left-to-right shunt, depending on its size. Importantly, leftto- right atrial shunting can be symptomatic at a young age if the ASD is large, but in most cases diagnosis is made on the occasion of a cardiac evaluation for an asymptomatic cardiac murmur. Some children are symptomatic at a young age and need closure of their ASD. In a series of pediatric patients treated either surgically or by catheter intervention for isolated ASDs, 2.2% were infants with pulmonary hypertension [3]. The postoperative course can be complicated, but the ultimate outcomes were good, with persistent normalization of pulmonary artery pressures [3]. In another series of small children that were treated with percutaneous closure of ASD, the majority of the smallest children had associated cardiac (21%) or noncardiac lesions (33%). Infants with a weight below 10 kg presented with 70% associated lesions [4]. If undetected during childhood, most patients will present with symptoms beyond the age of 40 years with progressive exercise intolerance and rhythm disturbances as their most frequent symptoms. Large ASDs can eventually lead to pulmonary hypertension and even Eisenmenger syndrome if left untreated.
A PFO occurs in up to 25% of adults and is a remnant of the fetal circulation [2]. During fetal life, the two overlaying structures of the atrial septum, the fibrous septum primum and the muscular septum secundum, leave a central opening, the foramen ovale. During fetal life, the septum primum serves as a one-way ‘valve’, allowing right-to-left shunting of placental blood towards the left atrium. After birth, left atrial pressure rises and right atrial pressure drops, resulting in a functional closure of the foramen ovale by apposing the septum primum against the septum secundum. In the majority of people (75%) those two layers fuse permanently, sealing the foramen. In 25% of cases however, fusion is not complete and the foramen ovale remains patent. The size of this PFO can differ considerably. In most people, this PFO will not produce any symptoms, but in some, paradoxical emboli will pass the foramen and be responsible for stroke. In approximately 2% of patients, a PFO is associated with an aneurysm of the interatrial septum, characterized by septal tissue in the area of the foramen ovale that is very redundant and very thin and membrane-like [2]. The association of a PFO and an aneurismal interatrial septum has been shown to increase the risk for recurrences in patients with cryptogenic stroke [5,6].
Indications
The commonly accepted indications for closure are hemodynamically significant ASD with left-to-right shunt and right ventricular volume overload. Patients presenting with a stroke and a PFO are considered to present a high risk of paradoxical embolism. PFO closure is thought to prevent recurrences in young patients with a PFO in the absence of another cause of the stroke being found (cryptogenic stroke). Other more miscellaneous indications for closure of ASD or PFO are the platypnea–orthodexia syndrome, characterized by dyspnea and desaturation in an upright position due to right-to-left shunting through a PFO, decompression illness in divers and migraine. The real value of the technique for these indications has yet to be proven.
Devices
Several types of devices are used for percutaneous closure of ASD and/or PFO. One type of device features two parallel disks made of a nitinol mesh, which makes them very flexible and easily retrievable, allowing for accurate and safe closure of most ASD 2s and PFOs with sufficient rims, not exceeding 40 mm of stretched diameter (Amplatzer®, AGA Medical Corporation, MA, USA, and Figulla®, Occlutech, Germany).
The second type of device consists of two parallel umbrellas fixed by a connection that is able to angulate to a certain degree. Each umbrella consists of a skeleton of different fabric covered by tissue (Cardioseal ®/StarFlex®, NMT Medical, MA, USA, and PFO-Star®/Intrasept/Cardiasept, Cardia, MN, USA). The third is a variation of the second, consisting of a double umbrella, covered by a biodegradable layer (Biosta r®, NMT Medical, MA, USA). Several other types of devices are on the market: coil-like (Helex®, Gore, NJ, USA), adjustable (Premere™, St Jude Medical, MN, USA), and others.
Results
The results of percutaneous closure are excellent and although analysis of the overall success rate is complicated by biases such as indications, size of ASD, ASD versus foramen and selection (attempts to close, intention-to-treat and completed procedures), complete closure after 6 months to 1 year can be expected in the vast majority of patients. Percutaneous closure is now the treatment of choice in many centers for closure of foramen ovale and ASD 2 if the anatomy is suitable.
Complications
Although successful, the technique is not complication- free. Complications can occur during the procedure, during immediate follow-up and in the long term.
▪ Overall complication rate
The overall complication rate with the actual devices should be low in experienced centers and periprocedural complications should be below 1% for closure of a PFO [2]. The reported complication rate in the literature approaches 8% in the largest series [7–13]. Comparison of complication rates is difficult since literature reports deal with different devices, indications, age groups, length of follow-up, focus and definition of complications. The severity of complications is categorized as mild, moderate and severe or as minor and major in most series. But even if the minor/major classification of complications is adopted, the definition of major complications differs: for example, life-threatening, needing surgery or needing treatment. In most recent reports the overall complication rate reaches 8%. Globally, major complications are reported in 1–3% and minor complications in up to 5%. The complication rate tends to vary considerably with the length of follow-up: in a report of ASD closure in adults the complication rate during the procedure was 1%, peaked to 5% in the month following the procedure and lowered to 2.8% after 1 month [12].
▪ Complication rate compared with surgical closure
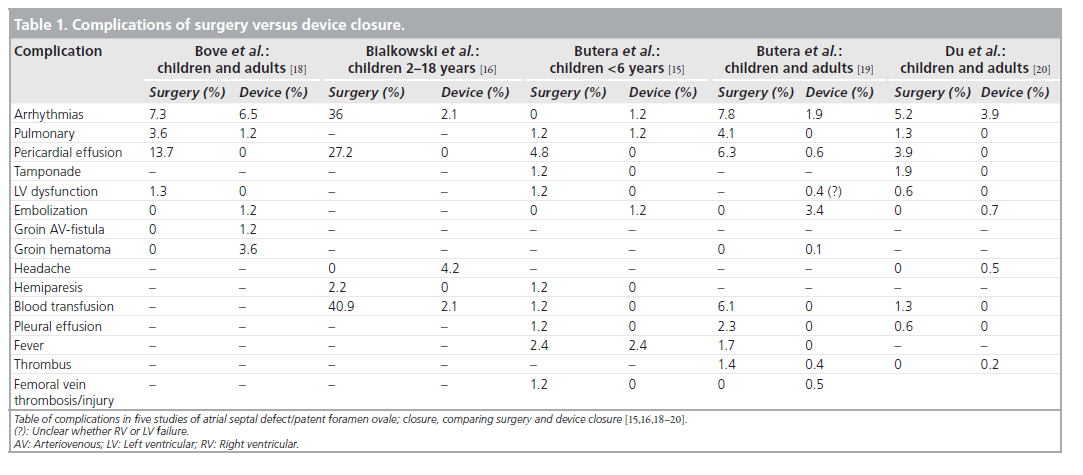
Although the reported success rate is excellent in carefully selected patients, surgery is still necessary in some patients with very large defects, deficient rims and other cardiac lesions that necessitate concomitant surgery (i.e., important valvar dysfunction) or after failure or complications of the percutaneous technique [9,10,14–16]. For this reason, comparison of complications of the surgical and percutaneous closure techniques is always biased by patient selection (Table 1). Nevertheless, some reports compared results and complications between surgery and percutaneous approaches in adults and children [14–20]. An analysis of the US FDA Manufacturer and User Facility Device Experience Database for adverse events involving Amplatzer septal occluder devices in comparison with the Society of Thoracic Surgery Congenital Cardiac Surgery Database, found a similar overall mortality of 0.1% for the two techniques [14]. The need for a rescue operation after a device closure attempt was twice as likely as the need for reoperation after surgery. The mortality for surgical management of a device adverse effect was 20-fold higher than for primary elective surgical ASD closure [14]. Major and minor complications seem more frequent in the surgical group, whether surgery is conventional or less invasive. The overall complication rate for surgery varies between 12 and 68%, depending on the criteria, compared with 4 and 15% for the interventional techniques [14–16,18–20]. Complications delaying hospital discharge account for 12% in the surgical group and 4% in the interventional group [14,17]. Mild and moderate or minor complications are found in 23–63% of surgical patients with emphasis on pericardial effusions, bleeding requiring blood transfusions and innocent arrhythmias. In the percutaneous group, this class of complications accounted for 6–8% of cases, including headache, arrhythmias and groin hematoma [14–16,18–20]. Major complications were found in up to 10% of the surgical group, including pericardial tamponade, hemiplegia and left ventricular (LV) dysfunction, compared with 1% in the percutaneous group, which mostly included embolizations and groin bleeding. In the surgical group, the typical complications consisted of pericardial effusion and respiratory problems and device embolization was the typical complication in the interventional group [14–16,18].
Table 1. Complications of surgery versus device closure.
▪ Risk factors
Although again not specifically studied in most series, some risk factors can be identified when reviewing the available literature. Age, defect morphology, center experience and device type influence results and complication rate.
Age of patients at time of closure
In a review of the data from the 2001–2005 Nationwide Inpatient Sample in the USA, evaluating the adverse effects of primary ASD/PFO closure in 2555 adult patients, patient age was one of the predictors of adverse effects [8]. Adverse effects occurred in 6.6% of patients between 20 and 39 years of age, 7.2% of patients between 40 and 59 years and 10.6% in those older than 60 years. Although increasing age was of course associated with a greater number of comorbidities, this did not entirely account for the relationship between age and adverse effects [8].
When comparing children and adults, preexisting atrial arrhythmias are almost never an issue in children, but do raise questions in adults [4,9,17,21]. Since a considerable percentage (40%) of these arrhythmias persist despite closure, the ASD/PFO closure device can preclude later transcatheter ablation of the arrhythmia substrate, if located in the left atrium (pulmonary veins). However, it has been shown recently that despite the presence of a PFO/ASD device, transseptal passage is not impossible and could even be facilitated by using the caudal rim of the device as a safe landmark for the puncture [22]. New persisting arrhythmias are also very rare in children, but not infrequent in adults and the incidence seems to be influenced by increasing age [4–10,21,23].
Although the complication rate does not differ significantly between adults and children, relatively more children are referred for surgery after transesophageal echocardiography (TEE) defect evaluation during the procedure [4,9,21]. Age and size of the children does not seem to increase the complication rate [4,9,10].
Morphology of the defect
Closing a PFO or an ASD is technically somewhat different and periprocedural success and complications will differ depending on the defect’s morphology [9,24]. In experienced hands, a simple PFO should not pose a problem to close. Careful examination of the size of the PFO, the length of the tunnel and the firmness of the atrial septum can be evaluated by pre- or peri-procedural TEE or intracardiac echocardiography (ICE). According to the findings, a suitable device can be chosen. Embolization is only a problem due to technical failures (premature release or inadequate fixing). On rare occasions, the PFO is small with a long fixed tunnel and is impossible to cross with catheter or wire. Some advocate the use of a central transseptal puncture and insertion of a device that will cover the PFO concomitantly. This author and others think that the chances that such a small, rigid PFO will be the substrate for a significant right-to-left shunt and a paradoxical embolus are so limited that a transseptal procedure is probably not warranted and the PFO should be left untouched.
It can be impossible to cover a large atrial aneurysm completely with one closure device. This could be a risk factor for early thrombotic events [25–27]. In my personal experience, any device tends to stabilize the aneurismal atrial septum over time and recurrences are not more frequent in these patients if the foramen is closed completely. However, for some devices the presence of an atrial aneurysm could prolong the time interval for complete closure and hence the time that anticoagulation or antiplatelet treatment should be continued [27].
Closure of an ASD can be more difficult if the ASD is large, if part of the rim is missing or if the ASD extends towards the inferior septum. Small ASDs can be closed with the ease and safety of PFO closure, while larger ASDs harbor significant risk of device embolization. However, any single ASD 2 with a stretched diameter of 35 mm or less and with posterior and/or inferior rims of more than 5 mm can be closed safely with adequate material and delivery techniques. Between 36 and 40 mm stretched diameter the risk of malposition and embolization becomes important, but if carefully selected, still feasible with reasonable safety [28]. In my personal opinion and experience, evaluation of the exact morphology of the ASD (by 2D and/or 3D echocardiography), adequate sizing and careful monitoring of deployment and position with intraprocedural TEE or ICE are the cornerstones of a correct and safe percutaneous closure procedure. A deficient anterosuperior rim is not very rare (17% of cases) [9] and by no means a contraindication for device closure but can make correct device positioning more cumbersome. Moreover, oversizing of the device should be avoided in those cases, in order to try and avoid erosion of the aortic wall by the device. A deficient posteroinferior rim is less frequent (4.2%) [9] and not always easily identified before starting the procedure. The odds for malposition and embolization are too important in these cases, and patients with this type of ASD should be referred for surgery. Complex ASDs (large ASDs with a deficient rim or a multifenestrated atrial septum) are more difficult to close (86% success) and procedure‑related complications are higher (12.5%) [29].
Operator or center experience
A review of the data from the 2001–2005 Nationwide Inpatient Sample in the USA, evaluating the adverse effects of primary ASD/PFO closure in 2555 adult patients, showed that hospital procedure volume was correlated with in-hospital adverse events. Higher hospital annual procedure volume was associated with a shorter length of stay and a lower risk of minor and major adverse events [8]. The overall rate of adverse events as well as the rate of major adverse events was higher in those hospitals performing less than ten procedures per year (below the recommendations of the American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions 2007 update of the Clinical Competence Statement on Cardiac Interventional Procedures) [8]. Nevertheless, many low-volume hospitals provided high-quality and safe care [8].
Type of device used
Device choice is limited by ASD or PFO morphology. For most types of PFO, the choice of the device does not alter the rate of success, provided devices with a fixed disc connector (distance) are avoided if a long fixed tunnel is present. In cases of ASD 2, size will be the limiting factor for most devices. The Amplatzer atrial septal occluder and the Figulla ASD occluder are more suitable for ASDs with a diameter of 20 mm or more, and are the only devices suitable for ASDs larger than 30 mm.
Differences in outcome between several devices for closure of ASD and/or PFO have been reported.
Patent foramen ovale
A comparison between the Amplatzer PFO occluder and the PFO-Star in 100 consecutive patients with a PFO showed more procedural complications, more residual shunts after 6 months and more recurrent thromboembolic events in the PFO-Star group [7].
In a large comparative study of 795 patients, the results of three contemporary devices for PFO closure were compared: PFO-Star, Intrasept and the Amplatzer PFO occluder [30]. The periprocedural complication rate of 1.8% was comparable for the three devices. Residual shunting immediately following the procedure was higher in patients treated with the PFO‑Star, but after a mean follow-up period of 26 months, no difference in residual shunting was apparent. The annual incidence of recurrent thromboembolic events was 1.4%, which was not affected by the presence of a residual shunt or the device used. New-onset atrial fibrillation following PFO closure was the only predictor of recurrent thromboembolic events and was more common in patients treated with the Amplatzer PFO occluder (10%). In another large study of PFO closure with different devices in 1349 patients, the use of the Sta rFlex device predicted atrial fibrillation [23]. This was confirmed by another report comparing the results of the Amplatzer PFO occluder, the Helex device and the Cardioseal –Sta rFlex device in 660 patients [31]. The Helex device showed more periprocedural complications. Device thrombus formation (3.6%) and atrial fibrillation (5%) were more common in the Cardioseal –Sta rFlex occluders. Anzai et al. looked at thrombus formation and found that thrombus formation occurred more frequently on the Cardioseal device (22%) than on the Amplatzer device (0%) [32]. However, no patient developed a systemic thromboembolic episode during follow-up in this study. In 1000 consecutive patients with ASD and PFO, different devices were tested for thrombus formation: the incidence was between 5 and 7% for the PFO‑Star, Cardioseal and Sta rFlex compared with 0 and 1% for Helex and Amplatzer closure devices [25]. Postprocedural atrial fibrillation and persistent atrial septal aneurysm were the only predictors for thrombus formation.
Atrial septal defect
Three generations of ASD closure devices have been compared in a single-center study in patients of 2 years or older. The Clamshell (Bard, GA, USA), Cardioseal and Sta rflex devices were compared. Each device modification resulted in improved closure rates (79, 93 and 98%, respectively). Severe complications were more frequent in the Clamshell (the oldest device) compared with the two others [21]. Another study compared Cardioseal /Sta rflex, Amplatzer and ASDOS (Osypka, Germany) devices. The large Cardioseal /Sta rflex devices showed a higher rate of embolization [33]. In any series, the embolization rate of the devices studied is unacceptably high for closure of large ASDs compared with the Amplatzer septal occluder.
▪ Specific complications Groin hematoma & femoral arteriovenous fistula
Groin hematoma and femoral arteriovenous fistula are not infrequent and are probably under-reported [7,8,10,11,18,34]. They result from inadvertent arterial puncture and/or the introduction of relatively large venous sheaths and an anticoagulation/antiplatelet protocol in the immediate postprocedural period. The postcatheterization groin should be treated with meticulous care.
Device embolization
Device embolization is not much of an issue in the PFO group and is mostly a consequence of technical mistakes during manipulation of the device. In the ASD group, correct sizing of the defect and hence device choice is not always easy and can cause the device to be in an unstable position with subsequent embolization. This can be avoided in many cases (but not always) by testing the device’s stability before release (‘wiggle’). Moreover, deficiency of the posterior rims, especially the posteroinferior rim, renders device stability very unlikely with device embolization within 12–24 h postimplantation. In most reports, device embolization occurs in less than 1% of procedures [4,9,10,12,15,33,35–37]. Most devices can be retrieved by catheter techniques, but for the larger device in particular, one must be careful not to damage cardiac structures (valves and atrial wall) and surgical retrieval can be a safer alternative [35].
Erosion/perforation of the atrial wall with pericardial effusion or tamponade
This potentially lethal complication is rare, but can occur at any moment postimplantation and with any device. The incidence of this complication is estimated to be approximately 0.1%. The majority occurs within 3 days after the procedure, but a delay of several weeks is possible. The erosion can result in perforation of atrial and aortic wall, creating a fistula, but can also affect the atrial free wall, resulting in hemopericardium and eventually tamponade and death [7,8,10–12,16,18,33,37–45]. Most patients will complain of chest pain and this specific complaint should always be taken seriously and checked using echocardiography. If a pericardial effusion has been confirmed, a cardiac CT can be ordered to differentiate between a (rare) inflammatory pericardial effusion and hemopericardium. Oversizing of the device is thought to be one of the causes and should be avoided, especially in the presence of deficient rims [45].
Aortic incompetence
One report found an incidence of new or worsening aortic incompetence of approximately 10% after closure of PFO or ASD with the Amplatzer septal occluder or the Cardia PFO/ASD occluder [46]. The incidence seems to increase with the length of follow-up and is thought to result from traction on the noncoronary cusp due to endothelialization and tissue overgrowth. The aortic incompetence does not appear to be hemodynamically significant to date. No other series in the literature mentions this complication. Recently, another study addressed the impact of percutaneous closure of PFO on valve insufficiencies, evaluating the regurgitation fraction of the semilunar and atrioventricular (AV) valves by cardiac magnetic resonance imaging. No significant changes in regurgitation fraction of the four valves were found at 12 months of follow-up [47].
Coronary compression
A rare case of coronary insufficiency producing exercise angor has been described after closure of a PFO in a patient with an abnormal course of the circumflex coronary artery originating from the right coronary sinus. The abnormal coronary artery was compressed by the device [48].
New-onset migraine
Although it is known that pre-existing migraine with aura tends to improve in some patients after PFO closure [49], some patients suffer from newonset migraine after closure of PFO or ASD. Up to 10% of patients develop new-onset migraine in the first month after percutaneous closure of PFO and ASD [50]. Younger patients and those with previous migraine seem to be at risk. And although spontaneous recovery has been described [51], migraine seems to persist in the majority of patients (69%) [50]. Symptoms often respond to the combination of aspirin and clopidogrel.
Arrhythmias
Atrial arrhythmias, supraventricular arrhythmias and transient AV block have been described after ASD and PFO closure. Atrial fibrillation is of particular concern since the occurrence or persistence of atrial fibrillation after the closure of ASD/PFO seems to be associated with an increased risk of device thrombosis and recurrence of thromboembolic events [25,30]. Atrial fibrillation does not occur in children, but the risk in adults increases with age [10,23]. In fact, atrial fibrillation is part of the natural history of older patients with ASD. Some devices seem to increase the risk for postprocedural atrial fibrillation, but the data are contradictory [23,25,30,31,52,53]. A series of 1349 patients was examined for the incidence and risk factors associated with the development of atrial fibrillation after PFO closure [23]. Over a mean follow-up period of more than 3 years, 3.9% of patients developed new-onset atrial fibrillation. Of these, 62.3% developed atrial fibrillation within 4 weeks and 15% within 6 months following PFO closure. These account for 3% of the total population studied and probably represent the patients in which the deployment of the device itself influenced the onset of the arrhythmia. Chronic atrial fibrillation was documented in only half of the affected patients. In those with persisting atrial fibrillation, aggressive treatment seems warranted, since persistent atrial fibrillation is associated with thrombus formation and recurrent thromboembolic events. If necessary, cardioversion can be performed safely (under anticoagulation).
Transient AV block is reported in up to 6%, varying from first to third degree [19,54–56]. Most patients recover normal AV conduction within 6 months [54]. On rare occasions, temporary or permanent pacing is necessary [56]. A study addressing the association of AV block and closure of ASDs with the Amplatzer septal occluder found a larger shunt and device size to be the only determinant factors for AV block [54]. The variable timing of the occurrence of AV block postdevice implantation (1 day to 1 week) and the predominantly transient nature do not facilitate treatment options [54]. Close follow-up without medication or administration of steroids have been reported [54,55]. Early device removal is probably not necessary.
Thrombosis & recurrent thromboembolic events
Recurrent thromboembolic events are an important issue after percutaneous closure of a PFO, because closure is aimed at prevention of recurrences after a stroke with paradoxical embolus. A systematic review of nonrandomized studies of transcatheter closure or medical therapy for PFO reported a 1‑year rate of recurrent thromboembolism of 0–4.9% with catheter intervention and 3.8–12% with medical therapy [57]. Prospective, randomized trials are still ongoing and the results are not yet available. The mechanism of the recurrences is not clear: thrombosis on the device has been reported, but without an association with clinical thromboembolic events [25,27,31,34]. As it is never certain in cryptogenic stroke that the PFO is part of the pathogenesis of the stroke, recurrent stroke is much more likely to be due to the original cause, which has not been diagnosed. Atrial fibrillation and increasing age have been identified as predictors of recurrent thromboembolic events [30]. Residual shunting was also presumed to be a risk factor [58], but this was not confirmed in other studies [7,27]. It has been postulated that the left atrial disk would be the substrate of the thromboembolic events. The missing association between device thrombi and thromboembolic events questions this hypothesis. For that matter, the development of new biodegradable devices such as the Biosta r [53], designed to diminish the amount of synthetic material in the heart, has not yet provided any evidence that the rate of complications or recurrent thromboembolic events decreases.
Left ventricular dysfunction
In elderly patients in particular, a restrictive physiology of the left ventricle can lead to LV dysfunction and pulmonary edema after ASD closure [15,18–20,59–61]. In elderly patients, left atrial pressures can be measured during temporary balloon occlusion of the ASD. In patients with normal mean left atrial pressure during occlusion, definitive device closure of the ASD can be performed during the same session. Patients with LV restriction due to increased mean atrial pressures (>10 mmHg) can be preconditioned with intravenous inotropes and diuretics for 2 to 3 days [60]. If during a second session the mean left atrial pressures are significantly decreased with balloon-occluded ASD, permanent device closure can be performed [60]. If mean left atrial pressures remain elevated, the ASD can be closed with a fenestrated device, providing significant reduction of left-to-right shunting and avoiding excessive volume overload of the restrictive left ventricle [61].
Conclusion
Device closure for ASD and PFO has become an established treatment in many centers. In most, device closure has replaced surgery as the treatment of choice for the treatment of ASD 2 in children and adults. The success rates are comparable to surgery for those ASDs that are anatomically suitable and the complication rate is lower than the complication rate of surgical closure. In stroke patients with presumed paradoxical embolus through a PFO, percutaneous closure seems feasible with a reasonable complication rate. Randomized trials are ongoing to establish the real advantages compared with medical therapy. Even though the complication rate seems reasonably low and acceptable, major complications of device closure are dangerous and potentially lethal, and patients and physicians should be aware of these possible risks.
Complications can be prevented in many cases and adequately treated in others, if meticulous defect visualization, device choice, technique, monitoring and follow-up are adopted.
The results of the ongoing randomized trials are expected to assess the real risk–efficiency ratio of percutaneous closure of PFO versus medical therapy in patients with thromboembolic events and/or migraine.
Future perspective
The results of ongoing trials will hopefully establish the real advantages of percutaneous closure of PFO in patients with cryptogenic stroke. We need to evaluate the morphologic and clinical features identifying those patients that benefit from PFO closure with the lowest recurrence of thromboembolic events. New designs from PFO and ASD devices will decrease trauma to cardiac structures, lowering the risk of perforation and arrhythmia. New designs do and will decrease the embolization rate in cases of large ASDs and ASDs with deficient rims. If the problem of a higher rate of residual shunting can be solved, the pursuit of biodegradable devices will continue, although the advantages of these devices are currently hypothetical.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Defects & percutaneous closure
▪ Morphologically suitable atrial septal defects (ASDs) and patent foramen ovales (PFOs) can be closed using percutaneous techniques with very good results.
▪ Different types of devices are available, but only one type is suitable for large ASDs.
▪ Results are excellent and percutaneous closure is now the treatment of choice for anatomically suitable ASDs and PFOs.
Complications overall
▪ Minor complications are reported in up to 5% of patients, and major complications in 1–3%.
Complication rate compared with surgical closure
▪ The overall complication rate for surgery is higher.
▪ Typical complications in the surgical group consist of pericardial effusion and respiratory problems, with device embolization being the typical complication in the interventional group.
Risk factors
▪ Increasing age is associated with more complications.
▪ Large ASDs are more difficult to close and absence of a posteroinferior rim is a major risk factor for device embolization.
▪ Better training and larger hospital procedure volume are associated with a lower risk of adverse events.
▪ Some complications appear to be device-dependent.
Specific complications
▪ Some complications are potentially lethal (perforation/erosion of the atrial wall).
▪ New-onset migraine is not uncommon after ASD closure.
▪ Arrhythmias are more frequent with increasing age.
▪ Recurrent thromboembolic events do occur. Atrial fibrillation and increasing age are risk factors for recurrence, probably reflecting the uncertain role of PFO in cryptogenic stroke.
▪ Left ventricular dysfunction should be identified prospectively before closure of an ASD in the elderly.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- King T, Thompson S, Steiner C, Mills N: Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 235, 2506–2509 (1976).
- Wahl A, Meier B: Patent foramen ovale and ventricular septal defect closure. Heart 95, 70–82 (2009).
- Goetschmann S, Dibernardo S, Steinmann H, Pavlovic M, Sekarski N, Pfammatter JP: Frequency of severe pulmonary hypertension complicating ‘isolated’ atrial septal defect in infancy. Am. J. Cardiol. 102, 340–342 (2008).
- Cardenas L, Panzer J, Boshoff D, Malekzadeh-Milani S, Ovaert C: Transcatheter closure of secundum atrial defect in small children. Catheter Cardiovasc. Interv. 69, 447–452 (2007).
- Overell J, Bone I, Lees K: Interatrial septal abnormalities and stroke: a meta-analysis of case–control studies. Neurology 55, 1172–1179 (2000).
- Mas JL, Arquizan C, Lamy C et al.: Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N. Engl. J. Med. 345, 1740–1746 (2001).
- Schwerzmann M, Windecker S, Wahl A et al.: Percutaneous closure of patent foramen ovale: impact of device design on safety and efficacy. Heart 90, 186–190 (2004).
- Opotowsky A, Landzberg M, Kimmel S, Webb G: Percutaneous closure of patent foramen ovale and atrial septal defects in adults: the impact of clinical variables and hospital procedure volume on in-hospital adverse events. Am. Heart J. 157, 867–874 (2009).
- Butera G, Romagnoli E, Carminati M et al.: Treatment of isolated secundum atrial septal defects: impact of age and defect morphology in 1013 consecutive patients. Am. Heart J. 156, 706–712 (2008).
- Wilson N, Smith J, Prommete B, O’Donnell C, Gentles T, Ruygrok P: Transcatheter closure of secundum atrial septal defects with the amplatzer septal occluder in adults and children – follow-up closure rates, degree of mitral regurgitation and evolution of arrhythmias. Heart Lung Circ. 17, 318–324 (2008).
- Egred M, Andron M, Albouaini K, Alahmar A, Grainger R, Morrison WL: Percutaneous closure of patent foramen ovale and atrial septal defect: procedure outcome and medium-term follow-up. J. Interv. Cardiol. 20, 395–401 (2007).
- Majunke N, Bialkowski J, Wilson N et al.: Closure of atrial septal defect with the Amplatzer septal occluder in adults. Am. J. Cardiol. 103, 550–554 (2009).
- O’Gara PT, Messe SR, Tuzcu EM, Catha G, Ring JC: Percutaneous device closure of patent foramen ovale for secondary stroke prevention: a call for completion of randomized clinical trials. A science advisory from the American Heart Association/American Stroke Association and the American College of Cardiology Foundation. J. Am. Coll. Cardiol. 53, 2014–2018 (2009).
- Di Bardino DJ, McElhinney DB, Kaza AK, Mayer JE: Analysis of the US Food and Drug Administration Manufacturer and User Facility Device Experience database for adverse events involving Amplatzer septal occluder devices and comparison with the Society of Thoracic Surgery Congenital Cardiac Surgery Database. J. Thorac. Cardiovasc. Surg. 137, 1334–1341 (2009).
- Butera G, Lucente M, Rosti L et al.: A comparison between the early and mid-term results of surgical as opposed to percutaneous closure of defects in the oval fossa in children aged less than 6 years. Cardiol. Young 17, 35–41 (2007).
- Bialkowski J, Karwot B, Szkutnik M, Banaszak P, Kusa J, Skalski J: Closure of atrial septal defects in children: surgery versus Amplatzer device implantation. Tex. Heart Inst. J. 31, 220–223 (2004).
- Formigari R, Di Donato RM, Mazzera E et al.: Minimally invasive or interventional repair of atrial septal defects in children: experience in 171 cases and comparison with conventional strategies. J. Am. Coll. Cardiol. 37, 1707–1712 (2001).
- Bove T, Francois K, De Groote K, Suys B, De Wolf D, Van Nooten G: Closure of atrial septal defects: is there still a place for surgery? Acta Chir. Belg. 105, 497–503 (2005).
- Butera G, Carminati M, Chessa M et al.: Percutaneous versus surgical closure of secundum atrial septal defect: comparison of early results and complications. Am. Heart J. 151, 228–234 (2006).
- Du Z, Hijazi Z, Kleinman C, Silverman N, Larntz K: Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults. J. Am. Coll. Cardiol. 39, 1836–1844 (2002).
- Nugent AW, Britt A, Gauvreau K, Piercey GE, Lock JE, Jenkins KJ: Device closure rates of simple atrial septal defects optimized by the Sta rFlex device. J. Am. Coll. Cardiol. 48, 538–544 (2006).
- Zaker-Shahrak R, Fuhrer J, Meier B: Transseptal puncture for catheter ablation of atrial fibrillation after device closure of patent foramen ovale. Catheter Cardiovasc. Interv. 71, 551–552 (2008).
- Staubach S, Steinberg DH, Zimmermann W et al.: New onset atrial fibrillation after patent foramen ovale closure. Catheter Cardiovasc Interv J. S1522–S1726X (2009).
- Harper RW, Mottram PM, McGaw DJ: Closure of secundum atrial septal defects with the Amplatzer septal occluder device: techniques and problems. Catheter Cardiovasc. Interv. 57, 508–524 (2002).
- Krumsdorf U, Ostermayer S, Billinger K et al.: Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J. Am. Coll. Cardiol. 43, 302–309 (2004).
- Krumsdorf U, Keppeler P, Horvath K, Zadan E, Schrader R, Sievert H: Catheter closure of atrial septal defects and patent foramen ovale in patients with an atrial septal aneurysm using different devices. J. Interv. Cardiol. 14, 49–55 (2001).
- von Bardeleben RS, Richter C, Otto J et al.: Long term follow up after percutaneous closure of PFO in 357 patients with paradoxical embolism: difference in occlusion systems and influence of atrial septum aneurysm. Int. J. Cardiol. 134, 33–41 (2009).
- Lopez K, Dalvi B, Balzer D et al.: Transcatheter closure of large secundum atrial septal defects using the 40 mm Amplatzer septal occluder: results of an international registry. Catheter Cardiovasc. Interv. 66, 580–584 (2005).
- Santoro G, Bigazzi MC, Lacono C et al.: Transcatheter closure of complex atrial septal defects: feasibility and mid-term results. J. Cardiovasc. Med. (Hagerstown) 7, 176–181 (2006).
- Spies C, Reissmann U, Timmermanns I, Schrader R: Comparison of contemporary devices used for transcatheter patent foramen ovale closure. J. Invasive Cardiol. 20, 442–447 (2008).
- Taaffe M, Fischer E, Baranowski A et al.: Comparison of three patent foramen ovale closure devices in a randomized trial (Amplatzer versus CardioSeal –STARFlex versus Helex occluder). Am. J. Cardiol. 101, 1353–1358 (2008).
- Anzai H, Child J, Natterson B et al.: Incidence of thrombus formation on the CardioSeal and the Amplatzer interatrial closure devices. Am. J. Cardiol. 93, 426–431 (2004).
- Post MC, Suttorp MJ, Jaarsma W, Plokker HW: Comparison of outcome and complications using different types of devices for percutaneous closure of a secundum atrial septal defect in adults: a single-center experience. Catheter Cardiovasc. Interv. 67, 438–443 (2006).
- Huang TC, Hsieh KS, Lin CC, Lee CL: Clinical results of percutaneous closure of large secundum atrial septal defects in children using the Amplatzer septal occluder. Heart Vessels 23, 187–192 (2008).
- Costache V, Chavanon O, Thony F, Blin D: Aortic arch embolization of an Amplatzer occluder after an atrial septal defect closure: hybrid operative approach without circulatory arrest. Eur. J. Cardiothorac. Surg. 28, 340–342 (2005).
- Ponnuthurai FA, van Gaal WJ, Burchell A, Mitchell A, Wilson N, Ormerod O: Single centre experience with GORE–HELEX septal occluder for closure of PFO. Heart Lung Circ. 18, 140–142 (2009).
- Spies C, Timmermanns I, Schrader R: Transcatheter closure of secundum atrial septal defects in adults with the Amplatzer septal occluder: intermediate and long-term results. Clin. Res. Cardiol. 96, 340–346 (2007).
- Wilson WM, Goh TH, Oqueli E et al.: Aorto-left atrial fistula post-percutaneous device ASD closure. Heart Lung Circ. (2009) (Epub ahead of print).
- Brown S, Gewillig M: Perforation of the aortic sinus after closure of atrial septal defects with the Atriasept occluder. Catheter Cardiovasc. Interv. 74(2), 298–301 (2008).
- Rickers C, Hamm C, Stern H et al.: Percutaneous closure of secundum atrial septal defect with a new self centering device (‘angel wings’). Heart 80, 517–521 (1998).
- De Ridder S, Suttorp MJ, Ernst SM et al.: Percutaneous transcatheter closure of atrial septal defects: initial single-centre experience and follow-up results. Initial experience with three-dimensional echocardiography. Acta Cardiol. 60, 171–178 (2005).
- Aggoun Y, Gallet B, Acar P et al.: Perforation of the aorta after percutaneous closure of an atrial septal defect with an Amplatz prosthesis, presenting with acute severe hemolysis. Arch. Mal. Coeur Vaiss 95, 479–482 (2002).
- Grayburn PA, Schwartz B, Anwar A, Hebeler RF: Migration of an Amplatzer septal occluder device for closure of atrial septal defect into the ascending aorta with formation of an aorta-to-right atrial fistula. Am. J. Cardiol. 96, 1607–1609 (2005).
- Divekar A, Gaamangwe T, Shaikh N, Raabe M, Ducas J: Cardiac perforation after device closure of atrial septal defects with the Amplatzer septal occluder. J. Am. Coll. Cardiol. 45, 1213–1218 (2005).
- Amin Z, Hijazi Z, Bass J, Cheatham J, Hellenbrand W, Kleinman C: Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc. Interv. 63, 496–502 (2004).
- Schoen SP, Boscheri A, Lange SA et al.: Incidence of aortic valve regurgitation and outcome after percutaneous closure of atrial septal defects and patent foramen ovale. Heart 94, 844–847 (2008).
- Wörhle J, Kochs M, Spiess J, Nusser T, Hombach V, Merkle N: Impact of percutaneous device implantation for closure of patent foramen ovale on valve insufficiencies. Circulation 119, 3002–3008 (2009).
- Casolo G, Gensini GF, Santoro G, Rega L: Anomalous origin of the circumflex artery and patent foramen ovale: a rare cause of myocardial ischaemia after percutaneous closure of the defect. Heart 89, E23 (2003).
- Mortelmans K, Post M, Thijs V, Herroelen L, Budts W: The influence of percutaneous atrial septal defect closure on the occurrence of migraine. Eur. Heart J. 26, 1533–1537 (2005).
- Rodes-Cabau J, Mineau S, Marrero A et al.: Incidence, timing, and predictive factors of new-onset migraine headache attack after transcatheter closure of atrial septal defect or patent foramen ovale. Am. J. Cardiol. 101, 688–692 (2008).
- Fernandez-Mayoralas DM, Fernandez-Jaen A, Munoz-Jareno N, Gutierrez-Larraya F, Calleja-Perez B, San Antonio Arce V: Migraine symptoms related to the percutaneous closure of an ostium secundum atrial septal defect: report of four paediatric cases and review of the literature. Cephalalgia 27, 550–556 (2007).
- Herrmann HC, Silvestry FE, Glaser R et al.: Percutaneous patent foramen ovale and atrial septal defect closure in adults: results and device comparison in 100 consecutive implants at a single center. Catheter Cardiovasc. Interv. 64, 197–203 (2005).
- Mullen MJ, Hildick-Smith D, De Giovanni JV et al.: BioSTAR Evaluation STudy (BEST): a prospective, multicenter, Phase I clinical trial to evaluate the feasibility, efficacy, and safety of the BioSTAR bioabsorbable septal repair implant for the closure of atrial-level shunts. Circulation 114, 1962–1967 (2006).
- Suda K, Raboisson M, Piette E, Dahdah N, Miro J: Reversible atrioventricular block associated with closure of atrial septal defects using the Amplatzer device. J. Am. Coll. Cardiol. 43, 1677–1682 (2004).
- Celiker A, Ozkutlu S, Karakurt C, Karagoz T: Cardiac dysrhythmias after closure of ASD with Amplatzer device. Turk. J. Pediatr. 47, 323–326 (2005).
- Nehgme R, Huddleston A, Cheatham J: Progression to late complete atrioventricular block following Amplatzer device closure of atrial septal defect in a child. Pediatr. Cardiol. 30, 367–370 (2009).
- Khairy P, O’Donnell C, Landzberg M: Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann. Intern. Med. 139, 753–760 (2003).
- Windecker S, Wahl A, Chatterjee T et al.: Percutaneous closure of patent foramen ovale in patients with paradoxical embolism: long-term risk of recurrent thromboembolic events. Circulation 101, 893–898 (2000).
- Ewert P, Berger F, Nagdyman N et al.: Masked left ventricular restriction in elderly patients with atrial septal defects: a contraindication for closure? Catheter Cardiovasc. Interv. 52, 177–180 (2001).
- Schubert S, Peters B, Abdul-Khaliq H, Nagdyman N, Lange P, Ewert P: Left ventricular conditioning in the elderly patient to prevent congestive heart failure after transcatheter closure of atrial septal defect. Catheter Cardiovasc. Interv. 64, 333–337 (2005).
- Peters B, Ewert P, Schubert S et al.: Self-fabricated fenestrated Amplatzer occluders for transcatheter closure of atrial septal defect in patients with left ventricular restriction: midterm results. Clin. Res. Cardiol. 95, 88–92 (2006).
▪▪ Well-documented database of adverse events, using international codes. Convincing conclusions on the influence of patient age and hospital procedure volumes on results and complications.
▪ Large series evaluating the influence of age and absence of rims on device implantation success.
▪ Well-written report focusing on specific features of atrial septal defect closure: right ventricular function and pulmonary hypertension.
▪▪ Despite some assumptions, the largest database of complications to compare surgery and device closure.
▪ Most complete focus on new-onset atrial fibrillation after patent foramen ovale closure. Identification of advanced age as a risk factor.
▪ Original report of a complication that was not studied, nor reported before. Although the hemodynamic consequences seem limited in the short term, this potentially important complication should be documented on a long-term basis.