Device Evaluations - Interventional Cardiology (2013) Volume 5, Issue 3
Conformable Gore TAG Thoracic Endoprosthesis for the treatment of thoracic aortic aneurysms
- Corresponding Author:
- G Chad Hughes
Division of Cardiovascular & Thoracic Surgery & Vascular Surgery
Duke University Medical Center, Durham, NC, USA
Tel: +1 919 668 0903
Fax: +1 919 613 5674
E-mail: gchad.hughes@duke.edu
Abstract
Keywords
aneurysm, aorta, endovascular, surgery
Although relatively uncommon, descending thoracic aortic aneurysms are lethal, with a natural history of progressive dilatation, rupture and death. The traditional treatment of these aneurysms involves open thoracotomy and replacement of the aneurysmal aorta with a prosthetic graft. Although successful and durable, this treatment is potentially associated with significant morbidity and mortality, especially given the advanced age and frequent comorbidities of patients harboring these aneurysms [1]. Rates of spinal cord ischemia (SCI) as high as 13% and operative mortality of 10% or more are reported [2], although superior results are found in select centers of excellence, including rates of SCI of 2% [3] and operative mortality of 5% [4]. Other potential major complications include respiratory failure, myocardial infarction, renal failure and stroke.
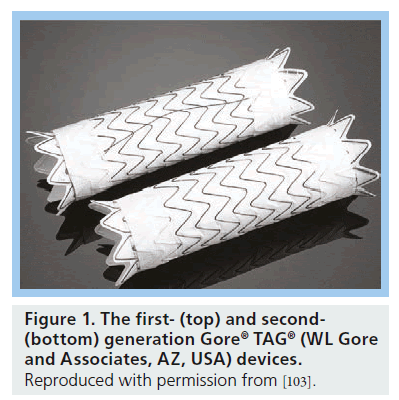
Given the notable mortality and complications associated with open-repair, endovascular approaches to the treatment of descending thoracic aortic aneurysms have been developed. This method is known as thoracic endovascular aortic repair (TEVAR). The procedure involves placement of a covered stent graft(s) through a peripheral access artery, usually in the groin, into the descending thoracic aorta over a guidewire under radiographic imaging guidance. The device is introduced in a collapsed state within a sheath or outer covering to reduce the entry profile. Once in position, the outer covering or sheath is retracted allowing the tubular stent graft to expand and seal against the wall of the aorta. This results in the exclusion of the aneurysmal portion of the aorta with the endograft(s) anchoring proximally and distally to more normal caliber aorta. This strategy reduces operative mortality and morbidity as it avoids open thoracotomy and clamping of the thoracic aorta. There are currently five US FDA-approved thoracic endografts, with the Gore® TAG® (WL Gore and Associates, AZ, USA) endograft the first to be introduced in 1997 as a means to repair descending thoracic aortic aneurysms via endovascular methods. After various modifications, including the removal of the longitudinal spines used for columnar support and reinforcement of the expanded polytetrafluoroethylene (ePTFE) material, the Gore TAG Thoracic Endoprosthesis was the first endovascular thoracic aortic stent graft approved for use by the FDA in the USA on 23 March 2005 (Figure 1).
Figure 1: The first- (top) and second- (bottom) generation Gore® TAG® (WL Gore and Associates, AZ, USA) devices. Reproduced with permission from [103].
The device was further modified in 2011 with the introduction of the third-generation Conformable TAG (C-TAG; WL Gore and Associates) device (Figure 2). Modifications were made in the new C-TAG with the goals of improving device conformability in the aortic arch and tortuous aortas, treating narrower diameter and tapered aortas, as well as resisting device compression.
Figure 2: Third-generation Gore® Conformable TAG® (WL Gore and Associates, AZ, USA) device. Reproduced with permission from [103].
Device specifications
As described in the instructions for use [101], the Gore C-TAG device is made of ePTFE, which is supported by a self-expanding Nitinol stent. At each end of the device is an ePTFE sealing cuff as well as two radiopaque gold bands to assist with visualization and placement during fluoroscopy. The prosthesis is available in diameters from 21 to 45 mm and 10, 15 and 20 cm lengths, with the longer lengths available only in larger diameter devices. The device can accommodate aortic neck diameters between 16 and 42 mm, and a minimum seal zone length of 20 mm in the proximal and distal neck. In addition, two tapered devices are available (Table 1).
The femoral or iliac arteries are used for device entry in the vast majority of cases, via introducer sheaths measuring 18–24 F depending on the largest diameter device utilized. Currently, the most commonly utilized sheath is the Gore® Dry- Seal Sheath (WL Gore and Associates), although we find the 65 cm hydrophilic Cook Flexor® sheath (Cook Medical, IN, USA) to be useful in very tortuous aortic anatomies, especially when proximal deployment in the aortic arch is required. The C-TAG device comes on a 100-cm delivery catheter, and is constrained in an ePTFE outer sleeve. This constraining sleeve is laced up with a cord that exits the base of the shaft of the delivery catheter. The graft is deployed by pulling this cord, which unlaces the covering sheath allowing the Nitinol stent to expand to its design diameter. Graft deployment is from the middle of the graft outwards towards both ends. This occurs rapidly, such that displacement during deployment is minimal. Hemodynamic manipulations, such as rapid ventricular pacing, temporary adenosine-induced asystole or pharmacologic lowering of blood pressure is unnecessary in the overwhelming majority of descending aneurysm cases. After deployment, the graft is typically balloon molded using the second-generation Gore Tri-Lobe Balloon Catheter (WL Gore and Associates) to ensure good apposition to the aortic wall and, thus, promote sealing of the proximal and distal landing zones, as well as any overlap zones in the case of multiple devices (Figure 3).
Figure 3: Gore® Tri-Lobe Balloon. Reproduced with permission from [103].
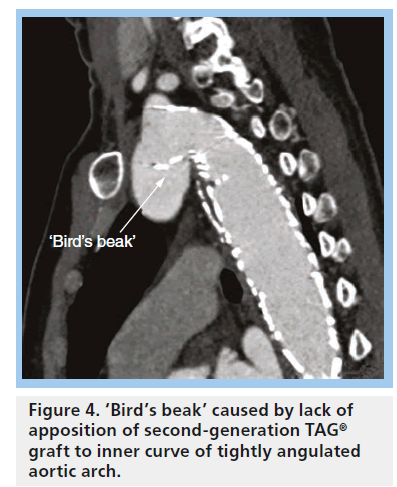
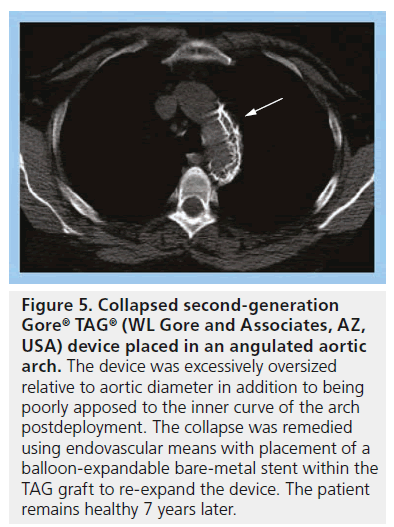
As mentioned above, the current third-generation TAG device is called the C-TAG graft. The new device is designed to better conform to the curvature of the aortic arch. With the normal stiffness of traditional stent grafts, including the Gore TAG device, there is often difficulty apposing the graft onto the aortic wall when the angulation of the aortic arch is tight (Figure 4). Lack of apposition can result in graft collapse, especially in the setting of excessive graft oversizing ( Figure 5), or type I endoleak.
Figure 5: Collapsed second-generation Gore® TAG® (WL Gore and Associates, AZ, USA) device placed in an angulated aortic arch. The device was excessively oversized relative to aortic diameter in addition to being poorly apposed to the inner curve of the arch postdeployment. The collapse was remedied using endovascular means with placement of a balloon-expandable bare-metal stent within the TAG graft to re-expand the device. The patient remains healthy 7 years later.
Numerous modifications have been made to C-TAG compared with the previous-generation TAG device to allow this improved performance. The flares at the ends of the older device have been removed, and the ends now consist of partially uncovered stents on the proximal end and a straight cut on the distal end. The new device also does not have a tendency to straighten. In addition, the device has an expanded oversizing window, allowing for more custom selection of outward radial force. The new device can be oversized between 6 and 33%. These features allow for a more tailored treatment of a variety of aortic pathologies, including dissection and trauma. The C-TAG was the first FDA-approved device for use in blunt aortic injury. The device is also available in tapered grafts, as well as smaller diameters to treat smaller aortas common in younger trauma patients. Overall, these changes may allow for a better seal between the graft and aorta, decreasing the rate of type I endoleaks.
In addition, these changes have allowed for the expanded use of TEVAR in patients with smaller and/or more tortuous aortas. Lastly, these changes may decrease the incidence of graft collapse.
Clinical data
Key clinical trials have led to the widespread use of the Gore device in treating descending thoracic aortic aneurysms. A Phase II, prospective, multicenter, pivotal trial of the original first-generation Gore TAG endoprosthesis was performed between 1999 and 2001 in 17 US centers and published in January 2005 [5]. The study followed 142 patients who were treated for descending thoracic aortic aneurysms. The trial enrolled generally lower-risk patients. For example, medical inclusion criteria included a life expectancy of greater than 2 years and assessment as a surgical candidate. Examples of exclusion criteria included myocardial infarction or stroke within 6 weeks of planned TEVAR, creatinine greater than 2 mg/dl and connective tissue disorder. All aneurysms were located between the left subclavian artery and celiac axis. Endograft coverage of the left subclavian artery (Ishimaru zone 2 [6]) was allowed as part of the trial, although more proximal deployment to cover the left common carotid or innominate arteries was not; likewise, coverage of the celiac axis or more distally was not permitted. The left subclavian artery was revascularized in 20% of patients, all of whom had planned coverage of the vessel. The device(s) were deployed using a transfemoral approach in the vast majority of patients, with a conduit being required in 15%. The trial results were generally positive with 98% of patients having the endograft(s) deployed in the intended location. Major adverse events within 30 days of surgery occurred in 32%, including stroke (4%), SCI (3%) and death (1.5%). There were no early aortic ruptures, although four patients suffered aneurysm-related deaths over the mean 24-month follow-up period. In addition, 2% of patients required re-intervention for endoleak during follow-up.
The initial studies were performed with the first-generation TAG device that contained longitudinal columnar spines (Figure 1), which were prone to fracture. FDA approval was not sought for the first-generation device; instead, a confirmatory study began in 2003 comparing the modified second-generation device to the pivotal study results [7]. This confirmatory study enrolled 51 patients at 11 US centers, comparing outcomes in patients undergoing open surgical repair versus TEVAR with the TAG device. The patients were similar in demographics, comorbidities and aortic morphology to the patients in the pivotal study. The American Society of Anesthesiologists score was similar between the surgical and TAG group, but the Society of Vascular Surgery risk score was higher in the TAG group. There was an 83% reduction in risk of major adverse events for the TAG-device group (12%) compared with the surgical-control group (70%) at 30‑day follow-up. The rates of vascular complications were similar between the groups. There were no 30‑day deaths or major device-related events. Overall, these results, along with those of the pivotal study, demonstrated the mid-term safety and efficacy of the device and led to its FDA approval for the treatment of aneurysms of the descending thoracic aorta in patients with appropriate anatomy (adequate iliac/femoral access, aortic inner diameter in the range of 23–37 mm, and ≥2 cm nonaneurysmal aorta proximal and distal to the aneurysm for device seal [102].
In February 2007, intermediate follow-up of the pivotal study was published [8]. The intermediate data from the study demonstrated decreased operative mortality (2.1 vs 11.7%; p = 0.004), SCI (3 vs 14%; p = 0.003), respiratory failure (4 vs 20%; p < 0.001) and renal insufficiency (1 vs 13%; p = 0.01) for the TAG patients compared with those treated with open surgical repair. Overall mortality was similar in both groups at 2 years after surgery, with three re-interventions in the endovascular group compared with none in the open-surgical group. Although the study had many limitations, including being nonrandomized and using nonconcurrently treated historicalcontrol open patients as part of the comparison group, the study did demonstrate superior short-term outcomes for TEVAR using the Gore TAG device compared with open surgical repair.
The 5‑year results of the Gore TAG pivotal trial were published in 2008 [9]. This long-term follow-up of those patients treated in the Phase II prospective multicenter trial comparing TEVAR with the Gore device to open surgical repair demonstrated maintained safety, efficacy and durability of the TAG device, results which remained numerically superior to open repair over the 5-year follow-up period. Aneurysm-related mortality during the 5-year follow-up period was 2.8% for the TEVAR patients versus 11.7% for open surgical repair (p = 0.008), although allcause mortality was equivalent between groups. Of note, this late aneurysm-related mortality difference was due entirely to the improved perioperative survival in the TAG group.
At 1 year following TAG deployment, 9% of patients had aneurysm enlargement, 48% had no change in aneurysm size and 43% had a decrease in aneurysm diameter. There were no late aortic ruptures in either group. Major adverse events at 5 years were lower in the TEVAR group (58 vs 79%; p = 0.001). Endoleaks occurred in 10.6% of TAG patients during the 5‑year followup, the majority of which were type Ia. The aneurysm reintervention rate was 2.1% in the opensurgical group and 3.6% in the TAG group. Late reinterventions in the TAG group were needed for spine fracture (n = 1), aortoesophageal fistula (n = 1) and endoleak (n = 3). Device-related issues included one case of migration and 20 fractures of the now obsolete longitudinal connecting bar on the first-generation device, one of which required reintervention as mentioned above. These data confirmed that the early/mid-term results with the Gore TAG device were durable over long-term follow-up.
In 2008, our group reported a single-institution series documenting ‘real-world’ results with the Gore TAG device 2 years after FDA approval [10]. A total of 83 TEVAR procedures were performed with the TAG device in the initial 2 years postapproval, 43 (52%) of which were performed for an on-label aneurysm indication. Primary technical success in the entire cohort of 83 patients was 98.8%. In-hospital/30-day rates of mortality and stroke were 3.6% each, and permanent paraparesis/ paraplegia occurred in 2.4% of patients. We have subsequently reported results with a larger series of on-label TEVAR use for the treatment of descending thoracic aneurysm in the postapproval era [11], which demonstrated 30 day/in-hospital rates of death, stroke and permanent paraplegia/ paresis of 5.1 (1.9% elective mortality), 2.5 and 1.3%, respectively, in a total of 79 patients, 85% of whom were treated using the second-generation TAG device. Overall actuarial survival was 73% at 55 months with an aorta-specific survival of 86%.
Newer studies have investigated the outcomes of the C-TAG device. The 1‑year results of the European C-TAG Registry were presented in 2009 [12]. The study involved 94 patients from five European centers. Indications for TEVAR included aneurysm (n = 50), acute symptomatic type B dissection (n = 22), intramural hematoma/penetrating atherosclerotic ulcer (n = 15) and traumatic transection (n = 7). Landing zones included zones 0, 1 or 2 in 57 patients. The procedure-related mortality rate was 11%. The rates of endoleak, paraplegia and stroke were 7, 3 and 12%, respectively. Retrograde type A dissection occurred in 1%. The primary technical success rate was 90.5%.
One of the main complications of TEVAR as with any device is endoleak, defined as the presence of persistent flow in the aneurysm sac despite endovascular stent graft placement (Table 2).
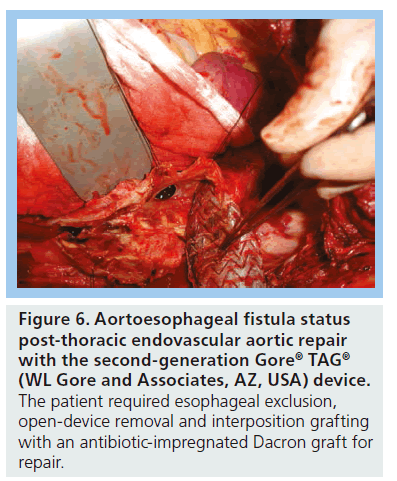
Certain device characteristics may predispose to this complication, such as low radial force and insufficient conformability, particularly with the earlier generation devices. According to cumulative data from Gore, 28.6% of patients have an endoleak at some point after device placement [103]. In patients in the initial 1999–2001 Gore TAG trial, 13.4% had a type 1 endoleak at some point postprocedure, 3% had type 2 and 3% had a type 3 endoleak. Other device-related complications have included migration, continued aneurysm enlargement in the absence of visible endoleak, retrograde type A dissection [13], infection, device collapse (Figure 5) and aortoesophageal fistula (Figure 6).
Figure 6: Aortoesophageal fistula status post-thoracic endovascular aortic repair with the second-generation Gore® TAG® (WL Gore and Associates, AZ, USA) device. The patient required esophageal exclusion, open-device removal and interposition grafting with an antibiotic-impregnated Dacron graft for repair.
Other FDA approved TEVAR devices currently on the market include the Cook Zenith® TX2® (Cook Medical), Bolton Relay® (Bolton Medical, Barcelona, Spain), Medtronic Talent® (Medtronic, MN, USA) and Medtronic Valiant® (Medtronic) endoprostheses. The Medtronic Talent device is made of self-expanding Nitinol rings covered by a polyester woven graft. The device is available in proximal and distal components, with the proximal devices available in diameters of 22–46 mm in 2 mm increments. These distal devices are tapered, with a 4 mm difference between the diameter of the proximal and distal portions. The 22–32 mm devices have a 22 F outer diameter; the 34–40 mm devices have a 24 F outer diameter; and the 42–46 mm devices have a 25 F outer diameter. The Valiant is the next-generation Medtronic device. It lacks the longitudinal connecting bar between the proximal and distal springs in the Talent device, and is more flexible at its proximal portion.
The Cook Zenith TX2 device is also a modular system with proximal and distal components made of woven polyester attached to self-expanding steel Cook-Z stents. The device is available in tapered and nontapered forms, and contains barbs at the proximal and distal ends of the device to assist in fixation to the aortic wall. The device is different from the Gore TAG and the Medtronic devices in that it requires a 25 mm neck (as opposed to a 20 mm neck in the other devices). The 28–34 mm devices are deployed through a 20 F delivery system, while the 36–42 mm devices are deployed through at 22 F delivery system.
The Bolton Relay device is composed of self-expanding Nitinol stents on a polyester vascular graft. The proximal portion of each stent graft can come with either a bare-metal stent or covered stent configuration. The device is available in 22–46 mm diameters in 2 mm increments, as well as straight and tapered configurations, and offers the longest stent graft on the market (up to 25 cm). The outer diameter of the delivery system ranges from 22 to 26 F.
All of these devices are approved for an aneurysm indication. The Medtronic Valiant and Gore C-TAG are also now approved for blunt traumatic aortic injury. Furthermore, both C-TAG and Valiant have completed trials for use in acute complicated type B dissection and are working towards FDA approval; a dissection trial is ongoing for the Cook Zenith dissection system. There have been no prospective studies comparing the various devices, although retrospective data from our own institution have not shown any meaningful differences in short- or long-term outcomes between devices [14,15]. One exception to this may prove to be the occurrence of retrograde type A dissection, which did appear lower with the Gore device in a single-center review from our institution [13], although more data in larger numbers of patients are needed to confirm or refute this finding.
Despite the decreased operative risk of TEVAR, appropriate patient selection remains to be critical. The decreased short-term morbidity and mortality after TEVAR has led to the expanded use of the procedure to different patient populations, including the elderly and those with significant comorbidities, who may not have been offered open repair in the past. In some patients with limited life expectancy, the application of TEVAR may represent clinical futility without meaningful survival benefit, similar to ‘cohort C’ in transcatheter aortic valve replacement [16]. Our group has identified risk factors for 1-year mortality after TEVAR, including age >75 years, aortic diameter >6.5 cm and American Society of Anesthesiologists class 4 [14]. In patients with these characteristics, one must carefully weigh the potential risks and benefits of the procedure and frankly discuss patient expectations and prognosis.
In addition, as described earlier, the outcomes after open repair of descending thoracic aortic aneurysms at select high-volume centers are excellent and match or exceed the outcomes after TEVAR, especially over the long term. Therefore, particularly in younger patients (age <60 years), many advocate for open repair in this population [17]. Open repair is also the standard treatment for patients with connective tissue disorders at most centers [18], given the poor outcomes reported for endovascular repair in this population [19].
Conclusion & future perspective
Thoracic endovascular aortic repair has been proven to be safe and effective for the treatment of descending thoracic aortic aneurysms both in the short and long term. Most of the literature focuses on stent grafts placed in the descending thoracic aorta given that, until recently, this was the only FDA-approved indication for use. However, advances are being rapidly made in branched- and fenestrated-endograft technology to allow treatment of aortic pathologies encompassing branch vessels in both the arch and thoracoabdominal aorta. In addition, improvements in device design have facilitated treatment of non-aneurysmal aortic pathologies, such as blunt traumatic aortic injury and dissection. In the future, these advances may allow total endovascular repair of ascending, arch and thoracoabdominal aortic aneurysms.
The future availability of smaller-profile devices may decrease vascular access-site-related complications. Furthermore, given the frequency with which the subclavian artery requires endograft coverage, several manufacturers plan to examine the safety and effectiveness of a thoracic endograft with a single side branch for the left subclavian artery in the near future. Finally, with regard to deployment, the controlled release system currently available on the Gore EXCLUDER® (WL Gore and Associates) endovascular abdominal aortic aneurysm repair graft will likely be added to the C-TAG graft in the near future as well. This system allows controlled expansion of the stent graft, as well as reconstraint and redeployment if needed, and should add incremental improvement for precise positioning, which is frequently required in the aortic arch.
Executive summary
Background
▪ Traditional open surgical repair of thoracic aortic aneurysms is effective; however, it is associated with significant potential morbidity and mortality.
▪ The development of thoracic aortic endografts in the past decade has allowed these aneurysms to be repaired via endovascular means, avoiding thoracotomy and its associated complications.
The Gore® Comfortable TAG® device
▪ The Gore® Comfortable TAG® (C-TAG; WL Gore and Associates, AZ, USA) Thoracic Endoprosthesis is US FDA approved for the treatment of isolated lesions, including thoracic aortic aneurysms and blunt traumatic aortic injury.
▪ This device is an endovascular stent graft made of expanded polytetrafluoroethylene supported by a self-expanding Nitinol stent.
▪ The device is typically deployed into the thoracic aorta through a sheath placed in a femoral or iliac artery.
▪ C-TAG is the third-generation device from WL Gore and Associates providing conformability for deployment in the aortic arch, as well as expanded sizing options.
Clinical efficacy
▪ The results of a multicenter, prospective, Phase II trial of the Gore TAG device were first published in 2005 demonstrating encouraging results for short-term morbidity and mortality.
▪ The 5‑year results of the trial were published in 2008, which demonstrated decreased aneurysm-related mortality and major adverse events in the Gore TAG group compared with open surgical repair.
▪ Recent multicenter, prospective, nonrandomized trials have demonstrated the safety and efficacy of the newer C-TAG device.
Other FDA-approved thoracic aortic endografts
▪ Bolton Relay® (Bolton Medical, Barcelona, Spain), Cook Zenith® TX2® (Cook Medical, IN, USA), Medtronic Talent® (Medtronic, MN, USA) and Medtronic Valiant® (Medtronic).
Conclusion
▪ The Gore TAG and C-TAG Thoracic Endoprostheses are FDA approved for the treatment of thoracic aortic aneurysms.
▪ The Gore TAG device has been well studied in numerous clinical trials and real-world clinical series, and has been shown to be safe and effective for the treatment of thoracic aortic aneurysms.
▪ Durability of the TAG device has been proven up to greater than 5 years of clinical follow-up.
▪ These characteristics have led to the expanded use of this device for the treatment of other aortic pathologies including aortic dissection and blunt traumatic injury.
▪ The newer, third-generation C-TAG provides improved conformability and sizing and has demonstrated encouraging results in multicenter trials.
Financial & competing interests disclosure
GC Hughes is a consultant and speaker for Medtronic and Vascutek Terumo. He has an unrestricted research grant from WL Gore and Associates, and is also a consultant and a speaker for them. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Conrad MF, Cambria RP. Contemporary management of descending thoracic and thoracoabdominal aortic aneurysms: endovascular versus open. Circulation 117(6), 841–852 (2008).
- Knepper J, Upchurch GR Jr. A review of clinical trials and registries in descending thoracic aortic aneurysms. Semin. Vasc. Surg. 23(3), 170–175 (2010).
- Estrera AL, Miller CC 3rd, Chen EP et al. Descending thoracic aortic aneurysm repair: 12‑year experience using distal aortic perfusion and cerebrospinal fluid drainage. Ann. Thorac. Surg. 80(4), 1290–1296 (2005).
- Fehrenbacher JW, Siderys H, Terry C, Kuhn J, Corvera JS. Early and late results of descending thoracic and thoracoabdominal aortic aneurysm open repair with deep hypothermia and circulatory arrest. J. Thorac. Cardiovasc. Surg. 140(Suppl. 6), S154–S160 (2010).
- Makaroun MS, Dillavou ED, Kee ST et al. Endovascular treatment of thoracic aortic aneurysms: results of the Phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J. Vasc. Surg. 41(1), 1–9 (2005).
- Mitchell RS, Ishimaru S, Ehrlich MP et al. First International Summit on thoracic aortic endografting: roundtable on thoracic aortic dissection as an indication for endografting. J. Endovasc. Ther. 9, 98–105 (2002).
- Cho JS, Haider S, Makaroun MS. Endovascular therapy of thoracic aneurysms: Gore TAG trial results. J. Semin. Vasc. Surg. 19, 18–24 (2006).
- Bavaria JE, Appoo JJ, Makaroun MS et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J. Thorac. Cardiovasc. Surg. 133(2), 269–277 (2007).
- Makaroun MS, Dillavou ED, Wheatley GH et al. Five-year results of endovascular treatment with the Gore TAG device compared with open repair of thoracic aortic aneurysms. J. Vasc. Surg. 47(5), 912–918 (2008).
- Hughes GC, Daneshmand MA, Swaminathan M et al. “Real world” thoracic endografting: results with the Gore TAG device 2 years after U.S. FDA approval. Ann. Thorac. Surg. 86(5), 1530–1537 (2008).
- Hughes GC, Lee SM, Daneshmand MA et al. Endovascular repair of descending thoracic aneurysms: results with ‘on-label’ application in the post Food and Drug Administration approval era. Ann. Thorac. Surg. 90(1), 83–89 (2010).
- Brunkwall J, Böckler D, Larzon T, Taylor P, Mangialardi N. Performance of the next generation conformable GORE TAG thoracic endoprosthesis in multiple etiologies. One year results of the European CTAG registry. Presented at: Leipzig Interventional Course 2009. Leipzig, Germany 14–17 January 2009.
- Williams JB, Andersen ND, Bhattacharya SD et al. Retrograde ascending aortic dissection as an early complication of thoracic endovascular aortic repair. J. Vasc. Surg. 55(5), 1255–1262 (2012).
- Shah AA, Craig DM, Williams JB et al. Risk factors for one year mortality after thoracic endovascular aortic repair. J. Thorac. Cardiovasc. Surg. 145(5), 1242–1247 (2013).
- Shah AA, Barfield ME, Andersen ND et al. Results of thoracic endovascular aortic repair six-years after U.S. FDA approval. Ann. Thorac. Surg. 94(5), 1394–1399 (2012).
- Miller DC. TAVI has a limited role in the treatment of AS. Presented at: The American Association of Thoracic Surgery 2012 Annual Meeting. San Francisco, CA, USA, 29 April 2012.
- Di Luozzo G, Geisbüsch S, Lin HM et al. Open repair of descending and thoracoabdominal aortic aneurysms and dissections in patients aged younger than 60 years: superior to endovascular repair? Ann. Thorac. Surg. 95(1), 12–19 (2013).
- Hughes GC. Aggressive aortic replacement for Loeys–Dietz syndrome. Tex. Heart Inst. J. 38(6), 663–666 (2011).
- Waterman AL, Feezor RJ, Lee WA et al. Endovascular treatment of acute and chronic aortic pathology in patients with Marfan syndrome. J. Vasc. Surg. 55(5), 1234–1240 (2012).
- Gore® TAG® Thoracic Endoprosthesis. Instructions for use. www.goremedical.com/tag/instructions
- Department of Health and Human Services. Gore® TAG® Thoracic Endoprosthesis. www.accessdata.fda.gov/cdrh_docs/pdf4/ p040043a.pdf
- Conformable Gore® TAG® Thoracic Endoprosthesis. www.goremedical.com/tag
■ Websites