Case Report - Interventional Cardiology (2022)
Congenital heart disease patients with fever suspected of COVID-19: A case report
- Corresponding Author:
- Ren-Qiang Yang
Department of Cardiology,
Institute of Cardiovascular Disease,
the Second Affiliated Hospital of Nanchang University,
Nanchang,
Jiangxi,
China,
E-mail: yangrenqiangcn@163.com
Received date: 11-Apr-2022, Manuscript No. FMIC-22-60206; Editor assigned: 13-Apr-2022, PreQC No. FMIC-22-60206 (PQ); Reviewed date: 02-May-2022, QC No. FMIC-22-60206; Revised date: 09-May-2022, Manuscript No. FMIC-22-60206 (R); Published date: 16-May-2022, DOI: 10.37532/1755- 5310.2022.14(S9).210
Abstract
Background: Fever, dry cough and fatigue are the most common symptoms of the Coronavirus Disease-2019 (COVID-19). During the COVID-19 pandemic in China, we treated a patient with fever and finally diagnosed congenital heart disease.
Case presentation: An 18-year-old lady came to the fever clinic with a complaint about the symptoms of fever, dry cough and dyspnea for 15 days. She had a travel history of epidemic area two weeks ago. She had a low fever and dry cough accompanied with chest tightness and fatigue. Eventually she diagnosed ventricular septal defect complicated by infective endocarditis. Two months after surgery, the patient returned to normal social life and physical activity.
Conclusion: Early surgical treatment is an effective strategy for ventricular septal defect patients complicated with IE, which can improve the early survival rate of patients.
Keywords
The coronavirus disease-2019 • Fever • Congenital heart disease
Abbreviations
COVID-19: Coronavirus Disease-2019; WBC: White Blood Cells; HB: Hemoglobin; PLT: Platelets; CRP: C-Reactive Protein; VSD: Ventricular Septal Defect; PDA: Patent Ductus Arteriosus; IE: Infective Endocarditis
Introduction
The coronavirus disease-2019 (COVID-19) is a newly emerging acute infectious disease in the world. The epidemic is widespread in the country. Fever, dry cough and fatigue are the most common symptoms of COVID-19. In the early stage of lung imaging, multiple small plaques and interstitial changes were frequently observed, and the outer lung area was obvious. We treated a suspected COVID-19 patient with recurrent fever and eventually diagnosed congenital heart disease. We treated her surgically and got a good prognosis. The report is as follows.
Case Presentation
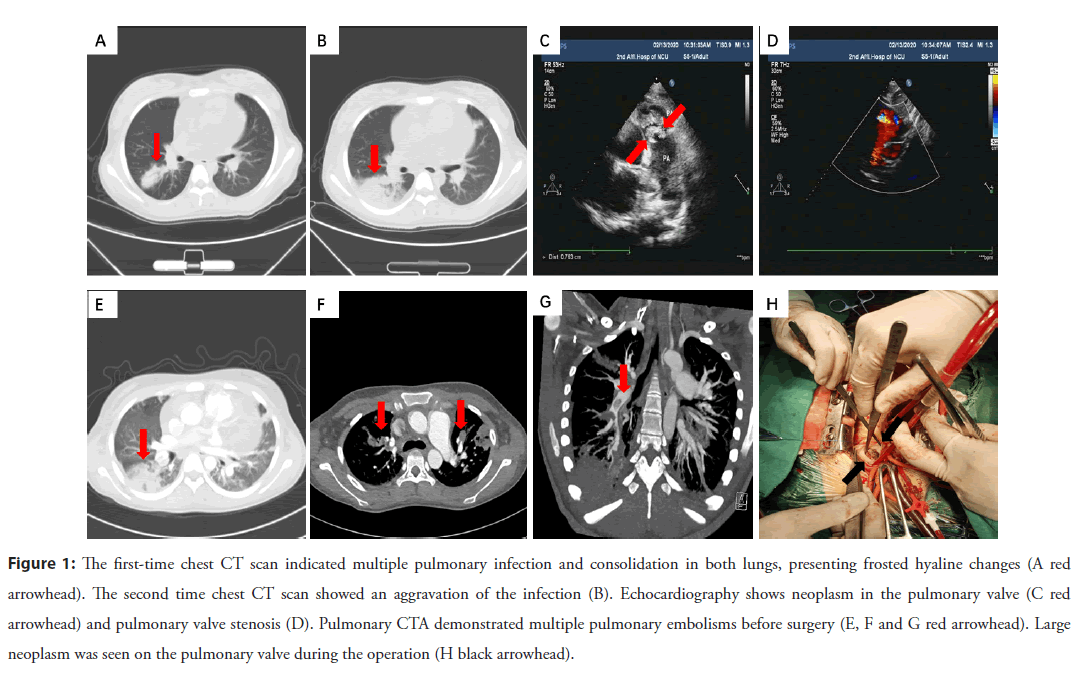
An 18-year-old lady came to the fever clinic with a complaint about the symptoms of fever, dry cough and dyspnea for 15 days. She had a travel history of epidemic area two weeks ago and had a tooth extracted four months ago. She had a low fever and dry cough accompanied with chest tightness and fatigue. There were no other concomitant symptoms. Physical examination showed her body temperature was 37.5℃, coarse respiratory and moist rales were found in her both lungs. The pulmonary valve auscultation area could be heard a loud systolic wind-like murmur of grade 4 up to 6. Biochemical examination indicated that the White Blood Cells (WBC), Hemoglobin (HB) and Platelets (PLT) were 15.36 × 10^9/L, 69 g/L and 66 × 10^9/L, respectively. The percentage of lymphocytes was 8%, and C-Reactive Protein (CRP) was 185.66 mg/L. Lung CT scan displayed multiple infections and consolidation occurred in both lungs, presenting frosted hyaline changes, peripheral ground-glass opacities, and pericardial effusion (Figure 1A). Primary cardiac ultrasound revealed the enlarged right atrium and ventricle. There seemed to be a slightly hyperechoic mass on the right ventricular outflow tract and pulmonary valve. Ultrasonic cardiogram indicated pulmonary valve stenosis and pulmonary artery enlargement with moderate pulmonary hypertension. Admission was considered as a suspected case of COVID-19 and was treated in isolation ward. Some kinds of antibiotic and antivirus therapy were prescribed. The second lung CT scan displayed an aggravation of the infection several days later (Figure 1B). The patient’s condition continued to deteriorate with fever, dyspnea, and hypoxemia. CRP increased progressively HB decreased to 57 g/ L, and PLT decreased to 7 × 109/L.
Figure 1: The first-time chest CT scan indicated multiple pulmonary infection and consolidation in both lungs, presenting frosted hyaline changes (A red arrowhead). The second time chest CT scan showed an aggravation of the infection (B). Echocardiography shows neoplasm in the pulmonary valve (C red arrowhead) and pulmonary valve stenosis (D). Pulmonary CTA demonstrated multiple pulmonary embolisms before surgery (E, F and G red arrowhead). Large neoplasm was seen on the pulmonary valve during the operation (H black arrowhead).
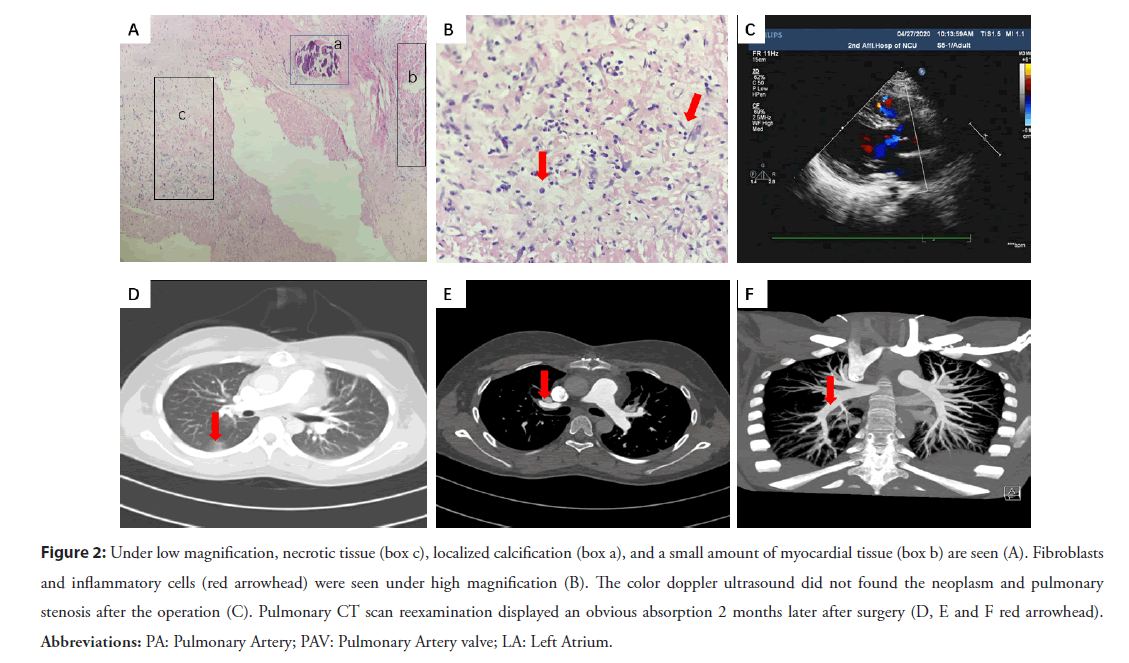
While multiple pharyngeal swabs of the patient were negative for COVID-19. The second ultrasonic cardiogram revealed enlarged right atria and ventricle, pulmonary stenosis and multiple neoplasm (Figures 1C and 1D). Blood culture of this patient collected a kind of streptococcal infection. Pulmonary artery CTA showed multi-location pulmonary embolisms and pulmonary infarction (Figures 1E-1G). The diagnosis was amended with congenital heart disease, pulmonary valve stenosis, infective endocarditis, sepsis, and pulmonary infection. High doses of penicillin, levofloxacin, vancomycin was prescribed according to sensitivity. Platelet suspension was infused to decrease the possibility of spontaneous hemorrhage. The patient still had fever, chest tightness, and dyspnea symptoms with HB 65 g/L, PLT 28× 109/L. So, surgical operation was decided to perform thoracotomy for pulmonary valvuloplasty and resection of neoplasm. A large neoplasm was seen on the pulmonary valve, which caused to the severe pulmonary valve stenosis (Figure 1H). An intra-cristae ventricular septal defect with a size of 1.5 × 1.8 cm2 was found by cardiac exploration. Due to being blocked by the thick muscle bundles nearby, the ventricular septal defect could not be detected by conventional echocardiography. Pathological examination showed the neoplasm filled with denatured and necrotic tissue, cellulose, lymphocytes and plasma cells, focal calcification, a small amount of myocardial and endocardial tissue (Figures 2A and 2B). The patient was re-amended with congenital Ventricular Septal Defect (VSD), infective endocarditis, secondary pulmonary valve stenosis. She received tissue patch repair for VSD, right ventricular outflow tract dredging, and tricuspid valvuloplasty. Two months later, the patient had no any special discomfort, and no valvular neoplasm was found by echocardiography (Figure 2C). Pulmonary artery CT scan did not show obvious embolism, and the inflammation of lung was obviously absorbed (Figures 2D- 2F). The patient backed to normal social life and physical activity.
Figure 2: Under low magnification, necrotic tissue (box c), localized calcification (box a), and a small amount of myocardial tissue (box b) are seen (A). Fibroblasts and inflammatory cells (red arrowhead) were seen under high magnification (B). The color doppler ultrasound did not found the neoplasm and pulmonary stenosis after the operation (C). Pulmonary CT scan reexamination displayed an obvious absorption 2 months later after surgery (D, E and F red arrowhead). Abbreviations: PA: Pulmonary Artery; PAV: Pulmonary Artery valve; LA: Left Atrium.
Results and Discussion
During the COVID-19 pandemic in China, the patient characterized by fever, cough, and dyspnea was initially diagnosed as COVID-19 suspected case comorbidity with congenital pulmonary stenosis. She was amended with congenital ventricular septal defect with the right heart infective endocarditis finally. Due to the low pressure of right heart system, the neoplasm of infective endocarditis was always large, crisp, and easy to fall off to form multiple pulmonary embolisms. That would lead to bacterial pulmonary small artery embolism and pulmonary infection [1]. Similar symptoms and lung image change in CT scan with COVID-19 caused to the initially diagnosis in this patient. Following up the patient’s medical history, she had a history of tooth extraction 4 months ago the onset of the disease, which may be the inducing factor causing to the Infective Endocarditis (IE).
Infective endocarditis with high morbidity and mortality is one of the most serious and destructive complication of valvular heart disease [2]. Congenital heart disease is a common cause of infective endocarditis [3], and studies have shown that the incidence can reach 10% to 14%, which is more common in VSD, congenital bicuspid aortic valve, Patent Ductus Arteriosus (PDA), and tetralogy of Fallot. Furthermore, VSD is the most common intracardiac malformation resulting in IE [4]. From this case, it was suggested that a diagnosis of pulmonary stenosis with IE should be more cautious in evaluation of VSD.
Endocarditis of the right heart system mainly involved the tricuspid valve and pulmonary valve, which were relatively rare in clinic. They were mainly seen in injecting drug abuser, patients with intracardiac implantation and congenital heart disease [5]. Compared with endocarditis in left heart system, right heart system of endocarditis not only showed infection symptoms, such as fever of unknown origin, anemia, bacteremia and so on, but also showed complications caused by pulmonary infection and right heart system function damage [6]. In this case, pulmonary valve stenosis and the formation of neoplasm were initially diagnosed, and it was finally amended with congenital ventricular septal defect complicated with infective endocarditis.
Early surgical treatment is an effective strategy for ventricular septal defect patients complicated with IE, which can improve the early survival rate of patients. Some studies have also shown that surgery should be actively considered for patients with congestive failure, severe valvular dysfunction, perivalvular abscess, recurrent systemic embolism, large neoplasm and persistent sepsis [7,8]. Although the preoperative general condition of this patient was extremely poor with HB 65 g/L, PLT 28 × 109/L, we decided to take the risk of surgical treatment and finally achieved a good result, considering that the pulmonary valve neoplasm of the patient was too large and she had multiple pulmonary embolisms.
Conclusion
This case gave a positive feedback. Ventricular septal defects can lead to infective endocarditis of the right heart system, which can lead to pulmonary embolism. Early treatment of the disease requires aggressive anti-infective medications. If medication does not work, surgery is also a good option.
References
- Darabant S, Oberton SB, Roldan LP, et al. Ventricular septal defect from aortic regurgitation jet lesion in aortic valve infective endocarditis. J Heart Valve Dis. 25(2): 150-152 (2016).
[Google Scholar] [PubMed]
- Abramczuk E, Stępińska J, Hryniewiecki T. Twenty-Year Experience in the Diagnosis and Treatment of Infective Endocarditis. PloS one. 10(7): e0134021 (2015).
[Cross Ref] [Google Scholar] [PubMed]
- Ma L, Ge Y, Ma H, B. et al. Infective endocarditis at a tertiary-care hospital in China. J Cardiothorac Surg. 15(1): 135 (2020).
[Cross Ref] [Google Scholar] [PubMed]
- Lee PT, Uy FM, Foo JS, et al. Increased incidence of infective endocarditis in patients with ventricular septal defect. Congenit Heart Dis. 13(6): 1005-1011 (2018).
[Cross Ref] [Google Scholar] [PubMed]
- Delahaye F, De Gevigney G. Infective endocarditis and specific situations: Right heart, valve prosthesis, cardiac implantable electronic device. Presse Med. 48(5): 549-555 (2019).
[Cross Ref] [Google Scholar] [PubMed]
- Sattwika PD, Hartopo AB, Anggrahini DW, et al. Right-sided infective endocarditis in patients with uncorrected ventricular septal defect and patent ductus arteriosus: Two case reports. Clin Case Rep. 6(11): 2168-2173 (2018).
[Cross Ref] [Google Scholar] [PubMed]
- Jia YX, Li Y, Meng X, et al. Clinical analysis of 161 cases of surgical treatment of infective endocarditis. Surg Infect (Larchmt). 20(8): 637-642 (2019).
[Cross Ref] [Google Scholar] [PubMed]
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs, Pettersson GB, Coselli JS, et al. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J Thorac Cardiovasc Surg. 153(6): 1241-1258.e29 (2017).
[Cross Ref] [Google Scholar] [PubMed]