Review Article - Imaging in Medicine (2012) Volume 4, Issue 2
Contemporary imaging of the pediatric chest
David Manson*The Hospital for Sick Children, Department of Diagnostic Imaging, University of Toronto, Ontario, Canada
- Corresponding Author:
- David Manson
The Hospital for Sick Children
Department of Diagnostic Imaging
University of Toronto, Ontario, Canada
E-mail: david.manson@sickkids.ca
Abstract
Keywords
chest ▪ digital radiography ▪ high-resolution CTn imaging ▪ lung ▪ MRI ▪ multidetector CT ▪ pediatric
Conventional radiography
For several decades, the conventional chest radiograph consisted of screen-film combinations of traditional radiographic film placed inside a light-proof cassette with an internal chemical lining, which emitted light on exposure to radiation. Contemporary computerized radiography has replaced the conventional film with a latent image, stored in the cassette’s chemical lining, which is read by a laser beam that transfers the latent analog image to a digital version. This is then processed by computer algorithms that produce a digital image, which can be manipulated digitally and disseminated through picture archive and computer storage systems. Newer systems are capable of acquiring the image through the use of a complex array of electrodes embedded in selenium, resulting in a more direct digital image [1]. At the current time, the utility of these direct digital imaging units is limited by their use in designated radiography rooms; however, portable units will probably be available soon.
These new systems are now widely used in both adult and pediatric imaging departments [2]. They have many advantages and some disadvantages for pediatric applications [3]. The most significant advantage is the ability to acquire, process and store images in a digital fashion.
The wider latitude of digital systems permits the manipulation of films previously considered to be nondiagnostic owing to hardware or technologist error, into useful diagnostic images. This has resulted in a plummeting rate of repeat examinations and a wide-scale drop in radiation exposure [3,4]. Most systems have algorithms that can detect the relative amount of incident radiation, thereby permitting more accurate measurements of the radiation dose to the child [5]. Furthermore, it is suspected that the dissemination and availability of the image to all clinical services involved in the care of the child has probably resulted in improved patient care [6]. In addition, there are some financial, as well as environmental, cost savings in that darkroom chemicals are no longer needed. However, these systems are more expensive to acquire, and significantly more expensive to maintain [4,7]. Teams of well-trained and technologically savvy personnel are now required to ensure the smooth functioning of most picture archives and computer storage systems. Fairly large computer storage and backup servers are also required. These factors must be weighed against increased overall physician efficiency and improved patient care.
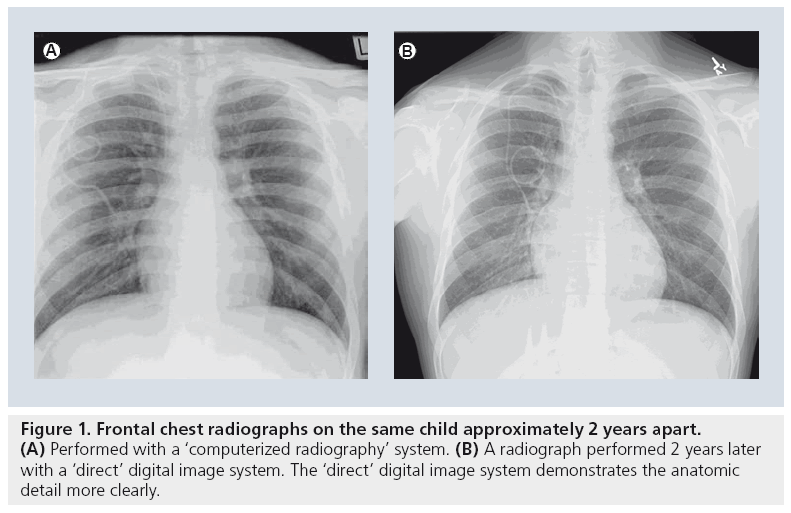
There are relatively few studies specifically evaluating digital plain film radiography in children. There is little question as to the subjective preference most radiologists have to the sharper and clearer images provided by either computerized or direct digital images (Figure 1) [8]. However, while contemporary radiographic practice has adopted digital imaging for pediatric pulmonary diseases as a generally beneficial imaging technique, this adoption has occurred with little critical analysis [9].
In the initial stages of the introduction of digital imaging to pediatric applications, the few studies comparing conventional images with digital images were performed in highly focused areas. Don et al. performed an animal- based study to demonstrate equivalent accuracy at pneumothorax detection between the two modalities, proposing that the animal model could simulate neonatal imaging [10]. Nakano et al. compared computerized radiography chest images and conventional film–screen images performed within 24 h of each other on 20 premature infants with hyaline membrane disease and found ‘comparable’ results [11]. Similarly, Kleinman et al. assessed the ability of digital radiography to detect rib fractures removed at autopsy from abused children and found that despite the lower spatial resolution of digital systems, the improved contrast resolution available with computerized systems permits equivalent performance of these systems to conventional high-detail film–screen systems [12].
While image quality is always a concern, recent pediatric imaging studies, in general, have concentrated on the crucial issue of radiation dose reduction. This issue has been given broad attention in both the medical and lay literature [13]. Although this issue has more significant repercussions when dealing with the relatively larger doses administered during pediatric CT examinations, the quest to reduce dose is being addressed throughout all imaging techniques. At least one study has reviewed the potential for radiation dose reduction with the use of digital imaging over conventional film–screen imaging, finding that, while more significant dose reductions are possible with abdominal, skull and pelvic imaging, modest reductions are possible in chest examinations in imaging centers that have used slower speed systems in the past [14]. While not producing tangible results, others have claimed a potential dose reduction of up to one-third with the use of some computerized radiography systems [15]. A recent pediatric digital radiology summit reviewed the significant obstacles that exist in evaluating radiation doses administered with digital imaging [16]. The measurement of the absorbed dose itself has not been standardized among vendors of the imaging equipment, organized educational programs to teach technologists how to use the equipment are lacking, and the tendency to increase radiation dose for each examination to provide a clearer (although not necessarily more diagnostic) image, the socalled ‘dose-creep’ factor [8], are all issues that were addressed as major hurdles to ensuring an optimal application of the ‘as low as reasonably achievable’ principle to digital plain film imaging.
Computer-assisted diagnosis, dual-energy imaging & digital tomosynthesis
Once an image has been acquired digitally, one of the most significant advantages is the ability to manipulate the data through various computerized programs and postprocessing algorithms. These systems include computer-assisted diagnosis, dual-energy imaging and digital tomosynthesis. Unfortunately for pediatric imagers, those who have developed most of these systems have targeted their applications to the adult population. As such, the detection and evaluation of parenchymal nodules in the quest to diagnose lung cancer and nodular metastases at their earliest stages has occupied many of those innovative physicists and imagers who are adept at digital image development. For example, computerassisted diagnosis is a research technology that uses complex computer algorithms that rely on a combination of computerized “…elaborate image processing, pattern recognition and artificial intelligence” [17]. The primary end point for the computer program is to accurately detect pulmonary nodules on the plain radiograph. While many studies have evaluated these systems in the adult, there is very little published concerning pediatric applications, mostly because nodule detection is not a primary diagnostic end point in most pediatric disease states. In adults, these systems generally are, at the current time, limited by high false-positive and false-negative rates [18]. In one of the few pediatric series, Helm et al. found a fairly poor overall detection rate of 38%, although this did improve to 80% if the nodules were greater than 4 mm, which compared positively with the radiologist overall sensitivity rate of 70–80% [19]. Similarly, the physics of dual-energy imaging system technologies seem to be primarily directed towards an end point most important to adult imagers. This imaging technique uses two different incident beam energies, exploiting the differential beam absorption of calcium in contrast to soft tissue. The result is the ability to individually and independently display calcific contents in contrast to soft tissue contents of the same image. The most attractive application is in the detection of calcification within a pulmonary nodule, thereby differentiating the nodule as a likely benign granuloma in contrast to a potential malignancy. In addition, this technique allows the imager to separate overlying structures of the thoracic cage from intraparenchymal structures [20,21]. The advantage is gained at a cost of a slightly higher radiation dose to the patient.
The imaging geometry inherent to digital tomosynthesis is similar to conventional tomography, whereby a computer-controlled moving x‑ray tube source is matched to a digital detector [17,22]. This results in the ability to blur anatomy at different depths while keeping the anatomy at a predetermined depth in focus. This application has great potential to detect pulmonary nodules in addition to parenchymal anatomy at different depths in the image. This has widespread repercussions in a variety of tissues, especially in imaging bone detail. This method of imaging only requires a slight increase in radiation dose to the child, and may reduce the overall dose if this technique is able to limit the use of CT in selected cases. However, digital tomosynthesis, dual-energy imaging and computer-assisted diagnosis remain in the translational research stage, without widespread clinical acceptance in most pediatric academic imaging centers.
■ Typical indications
▪ General purpose radiography
▪ General clinic and emergency room radiography
Ultrasound
At first thought, it would seem that the physics of the ultrasound beam’s inability to propagate through air would limit the utility of this technology in the pediatric chest. Nevertheless, as a relatively inexpensive, portable, real-time and radiation-free technology, it is very attractive to maximize its application to evaluating certain specific pathologies within the pediatric chest. Furthermore, most contemporary ultrasound units carry the ability to examine vascular flow characteristics through the use of Doppler technology. Improvements made to image quality, anatomic resolution and Doppler interrogation have resulted in fairly widespread and established uses of this technology in the evaluation of a number of pediatric intrathoracic disease states.
While CT and MRI excel in their relative abilities to provide excellent and sophisticated displays of normal and pathological anatomy, sonography can be an elegant imaging modality that can answer a host of clinical problems. Furthermore, most machines are portable, allowing bedside imaging for many of the most complicated and sick neonates and infants. It is performed in a real-time and multiplanar fashion, providing dynamic answers to questions of function (e.g., diaphragmatic motility). However, most attractive of all is the fact that it is radiation free, a key concern in the more radiation-susceptible child.
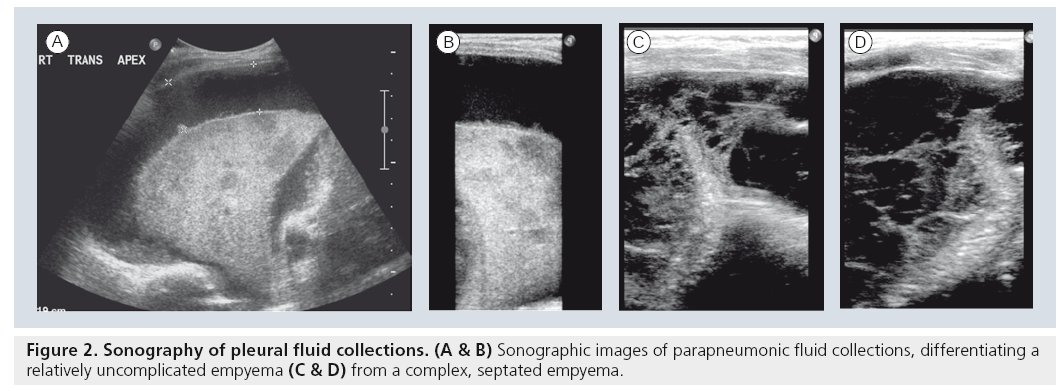
The application of sonography to imaging the noncardiac aspects of the chest initially began as an extension of abdominal sonography in the 1970s [23,24]. It was rapidly apparent that both mediastinal structures and pleural effusions at the lung bases could be easily examined by using various creative sonographic windows. This application has gained widespread acceptance and is now used as the initial imaging modality of choice in children with suspected pleural collections, or extensive thoracic opacification on standard chest radiograph [25,26]. Sonography easily differentiates simple pleural collections from complex collections, a finding that alone guides subsequent management in the case of infected empyemas and parapneumonic fluid collections (Figure 2). While the various therapeutic options, including draining by percutaneous image-guided thoracostomy tube placement versus video-assisted thoracoscopic drainage, and whether or not fibrinolytics are indicated, remain a matter of debate, the decision is now significantly based on the results of the initial sonographic examination [26–32]. Furthermore, the initial study in the setting of a completely opacified hemithorax can differentiate infectious consolidation from an underlying neoplasm as the causative source of the collection [23,25,26].
Sonography has been widely used in the initial investigation of suspected parenchymal congenital anomalies. The diagnosis of the classic systemic feeding vessel in lower thoracic lesions in children can characterize the lesion as either a sequestration or a ‘hybrid’ lesion, which in effect establishes the lesion as a congenital malformation. A somewhat complex controversy among radiological, surgical and pathological circles has arisen with the increase in the incidence of prenatally detected congenital lesions [33–35]. Since the debate involves the potential need for surgical resection of asymptomatic lesions, CT has assumed a central role in evaluating the characteristics of the lesion, in order to determine whether gross pathological changes of cystic adenomatoid malformation may be present. Most children with prenatally diagnosed congenital thoracic malformations will likely undergo postnatal CT evaluation, until such time as we better understand the natural history of these lesions [36–39].
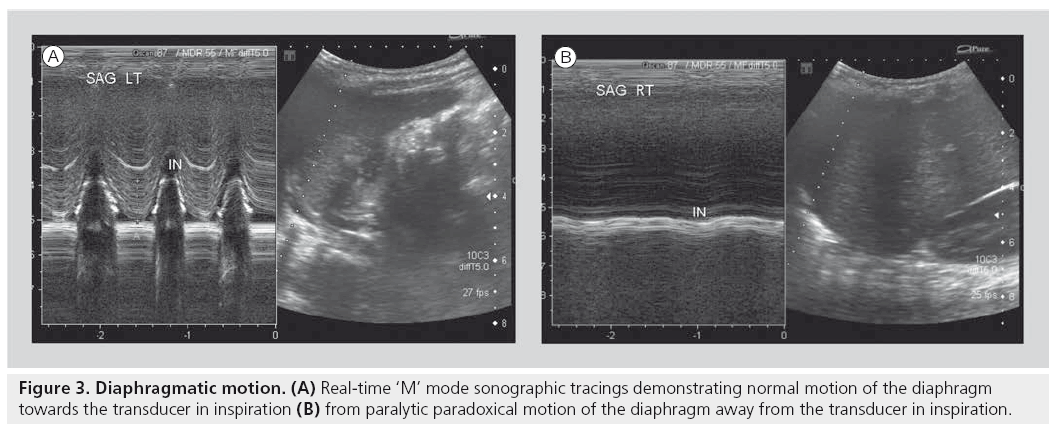
Another accepted and widely used application of sonography is in the evaluation of diaphragmatic activity and juxtadiaphragmatic structures [23,26,27,40–42]. Children with perinatal brachial plexus injuries may suffer phrenic nerve injury. However, in most tertiary settings, this application is most widely used for children who have undergone open thoracotomy for cardiac surgery and may demonstrate postoperative diaphragmatic elevation on plain film, or who may be inexplicably difficult to wean from ventilation. A bedside dynamic study can be performed, documenting as well as quantifying diaphragmatic activity by using ‘M’ mode Doppler technology. In this way, normal, paretic or paralytic diaphragmatic activity can be documented, the latter of which may require surgical placation (Figure 3). Similarly, this application can be used to differentiate the various severities of diaphragmatic eventrations. The use of sonography in children with congenital diaphragmatic hernia can be challenging. The actual documentation of the gap in a diaphragm can be quite difficult. However, the secondary changes of the presence of abdominal viscera in the chest are easily identifiable.
Evaluation of chest wall or palpable masses is uniquely suited to the sonographic examination, as these lesions usually do not have aerated lung situated between the lesion and the ultrasound transducer. However, chest wall lesions that demonstrate aggressive features on plain chest radiography (e.g., rib erosion and periosteal elevation) will probably require further imaging with CT or MRI in order to stage what may subsequently prove to be malignant. Nevertheless, a large number of chest wall lesions in children carry a fairly high pretest likelihood of being benign. Overall, up to 94% of chest wall masses in children are of benign etiology [43]. These include soft-mobile lesions, lesions with vascular discoloration and prenatally diagnosed cystic lesions. Vascular malformations, most commonly lymphatic malformations or hemangiomas, constitute the most common thoracic wall lesions in childhood [44].
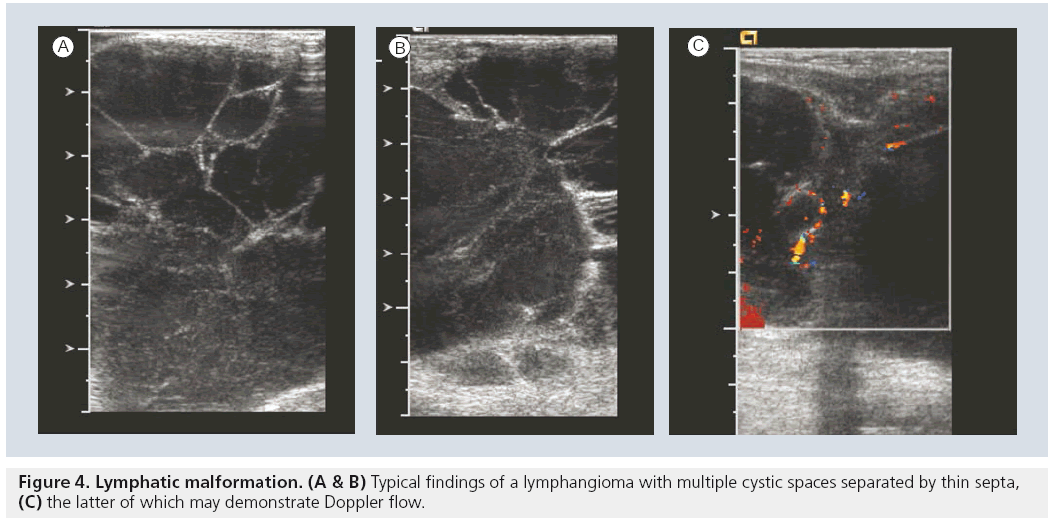
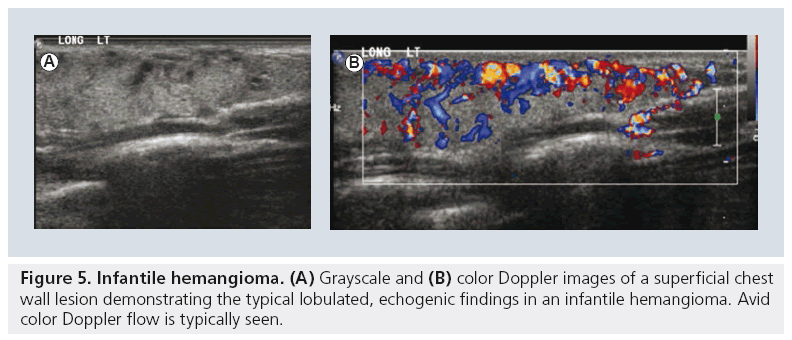
Lymphatic malformations are the most common cervico–thoracic masses of childhood, with 50% diagnosed at birth and 90% diagnosed before school age. In addition, the chest wall constitutes the site of 25% of all pediatric lymphatic malformations [45]. Sonography is an excellent initial mode of investigation for these lesions, which are easily identified by their cystic, fluid-filled nature and may only require further imaging if they are extensive in order to delineate their complete anatomic extent. Typically, they are avascular and devoid of internal flow on Doppler examination, although they may have thin internal septae that can contain Doppler flow (Figure 4). The more extensive lesions require MRI to fully evaluate the extent of the lesion and the potential involvement of regional structures. Also, infantile proliferative hemangiomas are found in approximately 18% of all infants. Most are small, superficial and require no treatment, although they may grow rapidly in the first year of life, gradually involute, and undergo fatty degeneration, with 50% gone by the age of 5 years [46]. At ultrasound examination, they are solid, lobulated and echogenic. At Doppler interrogation they are typically highly vascular (Figure 5) [26,47,48].
Similarly painless, asymptomatic palpable thoracic cage deformities are not uncommon throughout the general population, including children. These are most commonly due to rib or cartilaginous costochondral anatomic variants, which require no intervention. Sonography has been established as the initial investigation modality of choice owing to its lack of radiation, requiring no further imaging in most cases [49].
■ Typical indications
▪ Juxtaphrenic solid lesions
▪ Pleural collection analysis
▪ Opaque hemithorax
▪ Diaphragmatic motion
▪ Chest wall masses
CT
Contemporary CT technology has progressed rapidly in recent years. Fortunately, much of the progress has been directed to aspects of CT technology that make CT an even more attractive modality for pediatric applications. Improved multiple detector array technology coupled with more rapid scan times and rapid contrast power injectors have resulted in improved image resolution as well as a precipitous drop in the need for sedation [50]. Artifacts due to patient motion in smaller or uncooperative children, respiratory motion and cardiac activity can all be minimized with more rapid scan times. Sophisticated reformatting and reconstruction computer algorithms permit the ability to maximize the manipulation of the raw data into images that can provide even further diagnostic information. More recent attention to CT radiation concerns have resulted in published and even vendor supplied, dose modulation techniques. CT, for all practical purposes, remains the mainstay imaging technology for most pediatric indications that require comprehensive anatomic analysis of the lungs and cardiovascular structures at the same time.
HRCT
HRCT provides a relatively lower dose application of CT imaging, primarily designed to image the smaller structures of the lung parenchyma to a better extent. The actual technology is simple; thinner slice imaging reduces volume averaging, coupled with a highly edge-enhanced algorithm results in a higher conspicuity of smaller structures. As most indications are for children with diffuse parenchymal abnormalities, only representative samples of the lung need to be imaged, resulting in the ability to provide a diagnostic examination at a fraction of the usual chest CT dose [51,52].
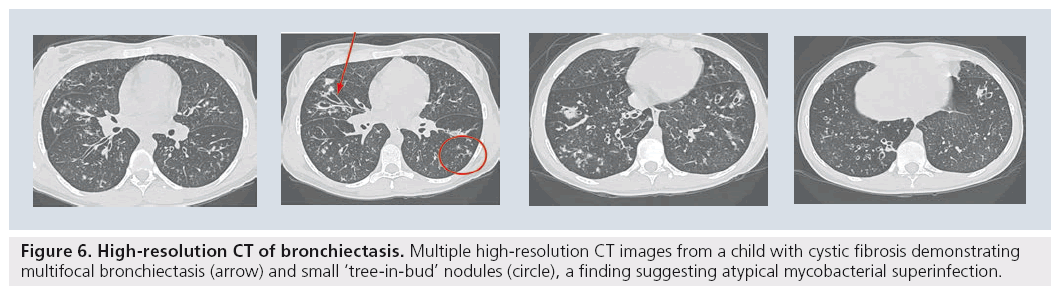
For the established indication of bronchiectasis, there has been widespread acceptance that HRCT is the gold standard to diagnose the extent and degree of bronchiectasis, be it in the child with cystic fibrosis [53,54], immune deficiency [55], ciliary dyskinesia or any of the various other causes of bronchiectasis in children (Figure 6) [56]. However the most significant difficulty with HRCT images in children is in understanding the pathologic processes that usually initiate the request for the CT. In recent years, there has been an overall acceptance that the usual ‘alphabet soup’ of established interstitial or diffuse lung diseases has limited applicability to pediatrics [57]. This has led to confusion regarding the indications and interpretations of imaging the child with diffuse lung disease [51]. Diagnostic accuracy of image interpretation has been negatively affected by widespread confusion over what diagnostic entities actually exist in children. Earlier studies have suggested that approximately 50% of cases could still remain without a definitive diagnosis, even after lung biopsy [58]. HRCT images that demonstrate characteristic changes that were similar to the adult carried favorable accuracy rates of 90–100%. These included a ‘normal’ study, bronchiolitis obliterans, alveolar proteinosis and pulmonary lymphangiectasia [59–61].
Figure 6: High-resolution CT of bronchiectasis. Multiple high-resolution CT images from a child with cystic fibrosis demonstrating multifocal bronchiectasis (arrow) and small ‘tree-in-bud’ nodules (circle), a finding suggesting atypical mycobacterial superinfection.
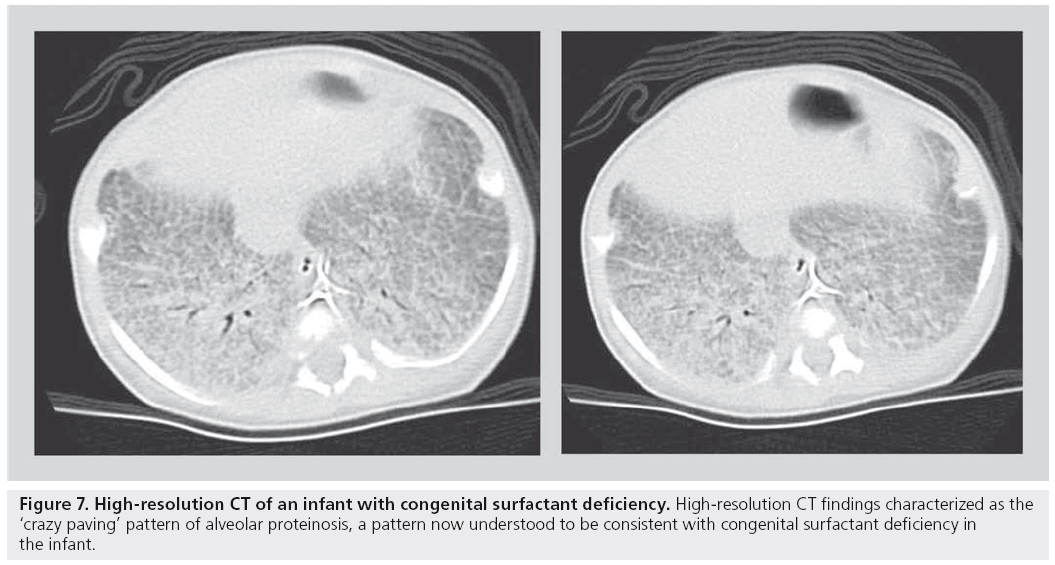
A more recent and somewhat revolutionary approach has been proposed by a number of pediatric pathologists, pulmonologists and respirologists, who evaluated a large number of biopsies, CT scans and clinical charts of children with idiopathic diffuse lung disease. This group, subsequently called the chILD consortium, published a summary of their findings in an attempt to organize pediatric diffuse lung disease into defined, and somewhat uniquely pediatric, entities [57,62]. Using this as a new template for diagnosis, the role of HRCT in accurately determining these new entities is yet to be established. For example, a recently described pathological entity in smaller children, neuroendocrine cell hyperplasia of infancy, is progressively being recognized on HRCT owing to its fairly characteristic central- and middle-lobe ground glass appearance [63]. Similarly, alveolar proteinosis has been described as a pathological entity seen in association with congenital surfactant deficiency states such as ABCA-3 deficiency. The HRCT finding of ‘crazy paving’ has been shown to be associated with alveolar proteinosis (Figure 7) [64,65]. Nevertheless, established indications for HRCT persist. While HRCT may not be able to determine a pathological diagnosis, useful clinical indications include the ability to determine the presence of disease in a child with a normal chest radiograph despite persistently unexplained diffuse symptoms/findings (e.g., wheezing, severe or atypical asthma and abnormal pulmonary function tests), to determine the extent/character of disease in a child with diffusely abnormal but nonspecific chest x‑ray findings, as a guide for appropriate site of biopsy, to help characterize a source of infection, as a modality to monitor response to administered therapies, in children who are at risk for bronchiolitis obliterans, such as bone marrow transplant recipients, and in children with systemic disorders known to predispose to parenchymal lung disease (e.g., mixed connective tissue disorder, sarcoidosis and histiocytosis).
■ Typical indications for HRCT
▪ Bronchiectasis
▪ Diffuse lung disease
▪ Diffuse small airways disease (e.g., bronchiolitis obliterans)
MDCT
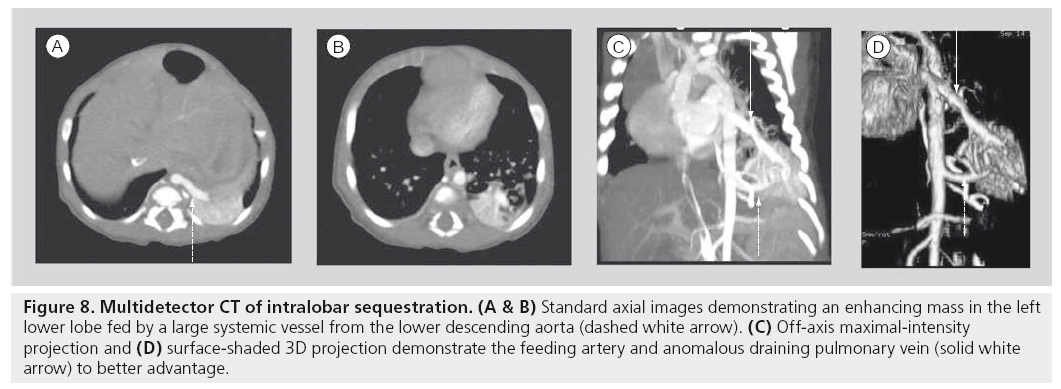
Multidetector helical scanning technology is now the generally accepted standard for combined imaging of both the pulmonary parenchyma and mediastinal structures. It is the single most useful imaging modality for complicated abnormalities that involve the cardiovascular structures of the mediastinum, airway and lung parenchyma (Figure 8) [66–72]. Various technological innovations in CT imaging, including the addition of multiple synchronous x‑ray detectors, slip-ring gantry capabilities and concomitant table motion, have revolutionized CT imaging. These innovations result in true, noninterpolated data acquisition from all three axes of the patient. Coupling these technologies to timed, intravenous contrast power injectors, as well as to dedicated computer reconstruction algorithms, has resulted in complex, sophisticated, multiplanar, reformational and 3D reconstruction capabilites. The added value of progressively shorter scan times is a critical issue to pediatric imaging as faster scan times can diminish artifacts from breathing and cardiac motion. More importantly, faster scan times require less time ‘on the table’, diminishing the need for sedation for smaller or uncooperative children. The precipitous drop in pediatric CT sedation rates with MDCT is a significant clinical advantage [50,73], especially to children with chest disorders that can compromise respiratory function.
Figure 8: Multidetector CT of intralobar sequestration. (A & B) Standard axial images demonstrating an enhancing mass in the left lower lobe fed by a large systemic vessel from the lower descending aorta (dashed white arrow). (C) Off-axis maximal-intensity projection and (D) surface-shaded 3D projection demonstrate the feeding artery and anomalous draining pulmonary vein (solid white arrow) to better advantage.
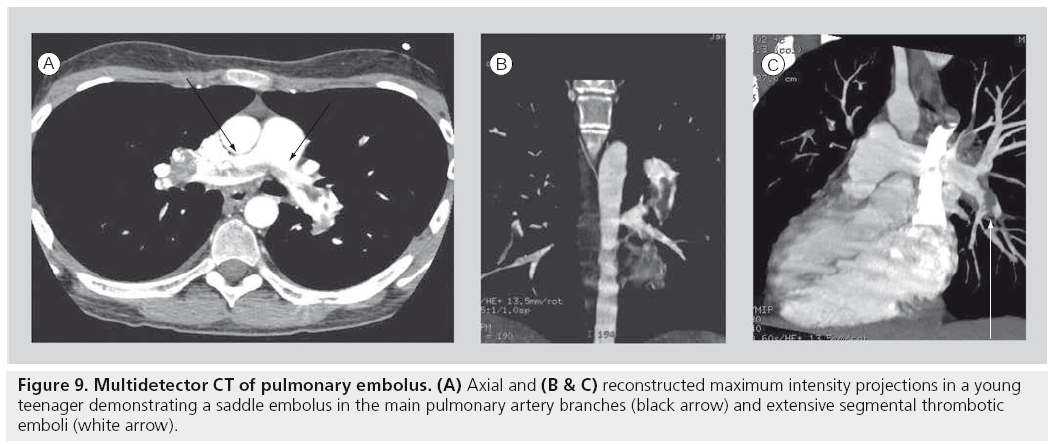
Many of the indications for adult CT pulmonary angiogram, including the evaluation for pulmonary embolism and post-thoracic traumatic indications, are very applicable in the pediatric setting (Figure 9) [74–76]. Specific pediatric indications for MDCT in children are numerous. Chest CT will remain the modality of choice for oncological staging, as it is the most sensitive modality to detect small parenchymal metastases. As noted previously, it will probably remain the most definitive study for the evaluation of the characteristics of congenital pulmonary anomalies until a more definitive approach to the requirement for surgical resection of these entities is established. It remains the mainstay modality of imaging the anatomy of pulmonary parenchymal disorders until MRI matures at imaging detailed pulmonary anatomy.
This general applicability unfortunately resulted in a somewhat uncontrolled increase in CT use until the hallmark articles by Brenner et al. These authors raised significant concerns over the rate of the use of CT examinations in children as well as concerns over the excessive radiation doses per examination that were unaltered for smaller children. They calculated a potential 500 cases of cancer per year over the lifetime of the children examined [13]. These calculations originated from estimations of the lifetime cancer risk to the survivors of Hiroshima who were exposed to lower radiation doses during the bombing. The subsequent reaction among the medical and lay communities has been impressive, including campaigns by the various pediatric imaging societies and the Alliance for Radiation Safety (the so called ‘as low as reasonably achievable’ principles and the Image Gently campaign), which have resulted in large-scale conferences and campaigns aimed at lowering administered CT doses and re-evaluating indications for pediatric CT [77]. Interestingly, as initial MDCT architecture expanded from four detectors to eight, 16 and up to 120 detectors, radiation doses increased concomitantly. However, recent studies are suggesting that the latest multidetector technology using an architecture of 320 detectors can reduce scan times to under 1 s and can provide dose reductions of up to 40% over previous MDCT architectures [78].
■ Typical indications for MDCT
▪ Mediastinal masses
▪ Mediastinal vascular malformations
▪ Congenital lung malformations
▪ Tumor staging for pulmonary metastases
▪ Pulmonary embolus
▪ Pulmonary rheumatologic disease
▪ Infection screening in immunocompromised host
▪ Complicated pulmonary infections
MRI
MRI is a modality that is continuously undergoing change, as a significant amount of research resources are applied to the various technical capabilities of the modality. The attraction of using MRI in pediatric disorders has increased with the acceptance that CT carries a potentially significant radiation risk, while MRI, when performed appropriately, appears to have little if any significant independent risk. Most of the potential risks from an MRI examination appear to be related to sedation and contrast use. The need for sedation will persist until established MRI protocols are sufficiently short, as they are for most MDCT examinations, to obviate the need for a sleeping patient. Ironically, the need for sedation in imaging chest disorders is frequently the most significant obstacle to optimal imaging. By necessity, many chest disorders compromise cardiorespiratory reserve, either through direct parenchymal disease or through compression of vital structures. It is in these children where the risk of performing an adequate MRI examination needs to be weighed against the potential radiation risk from the performance of a CT examination, the latter of which is a more dependabe diagnostic tool that is rapid enough that most children will not require sedation. While the longer overall scan time of the MRI examination continues to be a challenge, ongoing research into sequences that can be performed in progressively shorter scan times will probably eventually result in a gradual displacement of CT in favor of MRI for many clinical applications. Rapid, flow-sensitive and contrast-enhanced sequences are currently under investigation to examine regional pulmonary flow dynamics in the lung. Regional differences in perfusion can be detected earlier than using pulmonary function testing [79]. MRI investigation of pulmonary embolism is also a current area of investigation, although this application will probably take some time to reach the pediatric level, as MDCT for pulmonary embolism in children has only recently been somewhat validated [80].
The second major obstacle to anatomic pulmonary imaging in both the child and the adult is the difficulty inherent to pulmonary anatomy. Several factors limit the capability of MRI to provide fine anatomic detail. These include the low physical density of water molecules in the lung, the rapid degradation of signal from magnetic susceptibility of tissue interfaces, and the relatively limited anatomic resolution inherent to breathing motion during longer imaging times [81,82]. Therefore, it has been a very significant challenge to image smaller parenchymal processes, such as interstitial or diffuse lung diseases, small nodular processes (such as for tumor staging), and processes that diminish the parenchymal structures, such as emphysema or air trapping. While more rapid T2‑weighted breath-hold imaging techniques are being investigated, most investigators suggest sequences using half fourier acquisition singleshot turbo spin-echo or rapid gradient sequences in the axial and coronal planes. Furthermore, 3 T field strength imaging has excellent potential, as has been shown in recent imaging work in children with primary ciliary dyskinesia [83,84].
Other recent work in imaging pediatric lung anatomy has focused on disease processes that involve relatively larger areas of pulmonary airspace disease as can be seen in children with cystic fibrosis. As noted previously, there is a significant body of evidence developing that suggests that MRI can detect early zones of altered perfusion in regions of significant airway disease before pulmonary function testing can detect any abnormalities [79]. In addition, MRI can evaluate complicated lung infections with internal necrosis as seen in tuberculous disease [85] and can detect large airway bronchiectasis-associated changes in cystic fibrosis and primary ciliary dyskinesia demonstrating similar CT grading scores to HRCT [83,86].
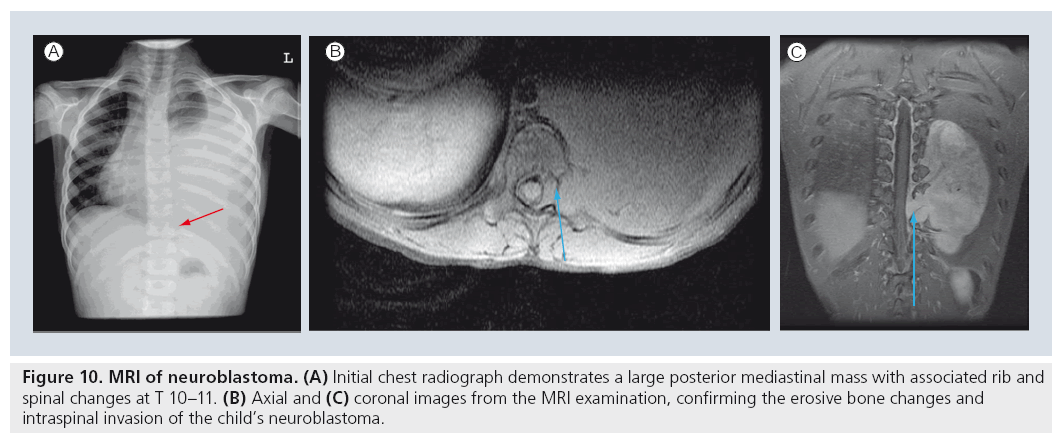
MRI has replaced the traditional CT examination for many selected applications. For example, the initial investigation of choice for a child with a posterior mediastinal mass is currently the MRI examination [72,87,88]. The MRI study will be able to image the internal characteristics of the mass, the extent of regional involvement including potential intraspinal extension (Figure 10) and the vascularity of the mass in lesions suspected to be in the pulmonary sequestration spectrum. Similarly, chest wall masses that are not identifiable by sonographic examination are best evaluated by MRI rather than by CT. The soft tissue contrast provided by MRI can evaluate bony and soft tissue masses to a significantly better extent than CT, differentiating soft tissue structures more effectively and differentiating cortical from intramedullary bone disease more optimally [72,89,90]. Although most aggressive lesions will require CT for ultimate staging, the current requirement for CT staging really only exists for the evaluation of pulmonary metastases. MRI still does not image small pulmonary nodules optimally, as the spatial resolution of MRI is exceeded by the capability of CT to image small structures. Currently accepted limitations suggest that parenchymal nodules of less than 5 mm are not reliably appreciated at MRI examination [82,90,91].
Figure 10: MRI of neuroblastoma. (A) Initial chest radiograph demonstrates a large posterior mediastinal mass with associated rib and spinal changes at T 10–11. (B) Axial and (C) coronal images from the MRI examination, confirming the erosive bone changes and intraspinal invasion of the child’s neuroblastoma.
MRI has, however, provided a new modality to perform functional pulmonary imaging. Pulmonary vascular flow studies have been standardized using MR angiographic techniques to capably image the major arterial and venous structures of the thorax in the adult. Also, ventilation maps have been generated using various inhaled agents, including oxygen, hyperpolarized helium and nebulized gadolinium [92]. These techniques carry the promise of radiation-free, sophisticated functional pulmonary imaging. Yet even those authors who are most experienced with these techniques admit the previously mentioned practical challenges still limit widespread acceptance of these procedures to replace CT for most pediatric anatomic applications [93–97].
■ Typical indications
▪ Chest wall masses
▪ Posterior mediastinal masses
▪ Cardiovascular imaging
Future perspective
Any attempt to look into the future with respect to innovative technologies is, admittedly, a bit of a guessing game. The rapidity of technological changes in our era is increasing faster than previously imaginable. However, if we use bench and translational research as a source of our future capabilities, it would seem that the technological innovations that are being devised in MRI are developing at a faster rate than any other single modality. Patient cooperation and anatomic resolution are today’s limiting factors. Faster imaging sequences will probably soon result in the same precipitous drop in sedation rates that occurred with the widespread application of MDCT. Anatomic resolution limitations will probably be quickly overcome. The issues of radiation dosage are likely to become obsolete with the transfer of most imaging to MRI. Functional MRI will soon replace nuclear medicine imaging of the lungs, resulting in a radiation-free future for complex cardiopulmonary imaging and function in children. Amazingly enough, this not only seems to be idyllic, but it seems to be inevitable.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Korner M, Weber CH, Wirth S, Pfeifer KJ, Reiser MF, Treitl M. Advances in digital radiography: physical principles and system overview. Radiographics 27(3), 675–686 (2007).
- Schaefer-Prokop CM, De Boo DW, Uffmann M, Prokop M. DR and CR: recent advances in technology. Eur. J. Radiol. 72(2), 194–201 (2009).
- Goodman TR, McHugh K. Advances in radiology. Arch. Dis. Chil. 77, 265–271 (1997).
- Kottamasu SR, Kuhns LR, Stringer DA. Pediatric musculoskeletal computed radiography. Pediatr. Radiol. 27, 563–575 (1997).
- Seibert JA, Morin RL. The standardized exposure index for digital radiography: an opportunity for optimization of radiation dose to the pediatric population. Pediatr. Radiol. 41(5), 573–581 (2011).
- Lissner J, Fink U. Digital imaging and picture archiving and communication systems. Curr. Opin. Radiol. 3(2), 267–274 (1991).
- Don S, Albertina MJ, Ammann DL, Evans RG, Siegel MJ. Soft copy computed radiography in neonatal and pediatric intensive care units: cost saving analysis. Radiology 197, 501–505 (1995).
- Willis CE. Optimizing digital radiography of children. Eur. J. Radiol. 72(2), 266–273 (2009).
- Shet N, Chu J, Siegel E. Continuing challenge in defining image equality. Pediatr. Radiol. 41, 582–587 (2011).
- Don S, Kruger RA, Winkler TA et al. Volume detection threshold: quantitative comparison of computed radiography and screen-film radiography in detection of pneumothoraces in an animal model that simulates the neonate. Radiology 194(3), 727–730 (1995).
- Nakano Y, Odagiri K. Use of computed radiography in respiratory distress syndrome in the neonatal nursery. Pediatr. Radiol. 19(3), 167–168 (1989).
- Kleinman PK, O’Connor B, Nimkin K et al. Detection of rib fractures in an abused infant using digital radiography: a laboratory study. Pediatr. Radiol. 32(12), 896–901 (2002).
- Brenner D, Elliston C, Halle BW. Estimated risks of radiation-induced fatal cancer from pediatric CT. Am. J. Roentgenol. 176(2), 288–296 (2001).
- Hutton AP, Doyle SM, Carty HM. Digital radiography in paediatrics: radiation dose considerations and magnitude of possible dose reduction. Br. J. Radiol. 71(842), 186–189 (1998).
- Tarver RD, Cohen M, Broderick NJ, Conces DJ Jr. Pediatric digital chest imaging. J. Thorac. Imaging 5(11), 31–35 (1990).
- Don S. Pediatric digital radiography summit overview. Pediatr. Radiol. 41(5), 567–572 (2011).
- McAdams HP, Samei E, Dobbins J 3rd, Tourassi GD, Ravin CE. Recent advances in chest radiography. Radiology 241(3), 663–683 (2006).
- Chen JJ, White CS. Use of CAD to evaluate lung cancer on chest radiography. J. Thorac. Imaging 23(2), 93–96 (2008).
- Helm E, Silva C, Roberts H et al. Computer aided detection for the identification of pulmonary nodules in pediatric oncology patients: initial experience. Pediatr. Radiol. 39, 685–693 (2009).
- Gilkeson RC, Sachs PB. Dual energy subtraction digital radiography: technical considerations, clinical applications, and imaging pitfalls. J. Thorac. Imaging 21(4), 303–313 (2006).
- Kuhlman JE, Collins J, Brooks GN, Yandow DR, Broderick LS. Dual-energy subtraction chest radiography: what to look for beyond calcified nodules. Radiographics 26(1), 79–92 (2006).
- Dobbins JT 3rd, McAdams HP, Godfrey DJ, Li CM. Digital tomosynthesis of the chest. J. Thorac. Imaging 23(2), 86–92 (2008).
- Haller J, Schneider M, Kassner EG¸ Freidman AP, Waldroup LD. Sonographic evaluation of the chest in infants and children. AJR Am. J. Roentgenol. 134, 1019–1027 (1980).
- Goldberg B. Superosternal ultrasonography. JAMA 215, 245–250 (1971).
- Ben Ami TE, O’Donovan JC, Yousafzadeh DK. Sonography of the chest in children. Radiol. Clin. North Am. 31(3), 517–531 (1993).
- Coley BD. Pediatric chest ultrasound. Radiol. Clin. North Am. 43(3), 405–418 (2005).
- Enriquez G, Serres X. Chest ultrasound. In: Pediatric Chest Imaging. Lucaya J, Strife J (Eds). Springer-Verlag, Berlin, Germany, 1–26 (2002).
- Sistrom CL, Wallace KK, Gay SB. Thoracic sonography for diagnosis and intervention. Curr. Probl. Diagn. Radiol. 26(1), 1–49 (1987).
- Hawkin JA, Scaife ES, Hillman ND, Feola GP. Current treatment of pediatric empyema. Semin. Thorac. Cardiovasc. Surg. 16(3), 196–200 (2004).
- Chen K, Liew Y, Wong H. Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J. Ultrasound Med. 19, 837–843 (2000).
- Kornecki A, Siran Y. Treatment of lobulated pleural effusions with intrapleural urokinase in children. J. Pediatr. Surg. 32, 1473–1475 (1997).
- Krishnan S, Amin N, Dozier AJ et al. Urokinase in the management of complicated parapneumonic effusions in children. Chest 112, 1579–1583 (1997).
- Pumberger W, Hörmann M, Deutinger J, Bernaschek G, Bistricky E, Horcher E. Longitudinal observation of antenatally detected congenital lung malformations (CLM): natural history, clinical outcome and long-term follow-up. Eur. J. Cardiothorac. Surg. 24(5), 703–711 (2003).
- Wallis C. Clinical outcomes of congenital lung abnormalities. Pediatr. Resp. Rev. 1(4), 328–335 (2000).
- Adzick NS. Management of fetal lung lesions. Clin. Perinatol. 36(2), 363–376 (2009).
- Shanmugam G, MacArthur K, Pollock JC. Congenital lung malformations – antenatal and postnatal evaluation and management. Eur. J. Cardiothorac. Surg. 27(1), 45–52 (2005).
- Davenport M, Warne SA, Cacciaguerra S, Patel S, Greenough A, Nicolaides K. Current outcome of antenally diagnosed cystic lung disease. J. Pediatr. Surg. 39(4), 549–556 (2004).
- Aziz D, Langer JC, Tuuha SE, Ryan G, Ein SH, Kim PC. Perinatally diagnosed asymptomatic congenital cystic adenomatoid malformation: to resect or not? J. Pediatr. Surg. 39(3), 329–334 (2004).
- Adzick NS. Management of fetal lung lesions. Clin. Perinatol. 36, 363–376 (2009).
- Liu P, Daneman A, Stringer DA. Real time sonography of mediastinal and juxtamediastinal masses in infants and children. Can. Assoc. Radiol. J. 39, 198–203 (1988).
- Baron R, Lee J, Mulsen G. Sonographic evidence of right juxtadiaphragmatic masses using transhepatic approach. J. Clin. Ultrasound 8, 156–159 (1980).
- Kangarloo A, Sukor R, Sample WF, Lipson M, Smith L. Ultrasonographic evaluation of juxtadiaphragmatic masses in children. Radiology 125, 785–787 (1977).
- Bissett GS 3rd. MR imaging of soft tissue masses in children. MRI Clin. N. Am. 4, 697–720 (1996).
- Eich G, Kellenberger C, Will U. Radiology of the chest wall. In: Pediatric Chest Imaging. Lucaya J, Strife J (Eds). Springer-Verlag, Berlin, Germany, 1–26 (2002).
- Glasson MJ, Taylor SF. Cervical, cervicomediastinal and intrathoracic lymphangioma. Prog. Ped. Surg. 27, 62–83 (1991).
- Garzon M. Hemangiomas: update on classification, clinical presentation and associated anomalies. Cutis 66(5), 325–328 (2000).
- Dubois J, Patriquin H, Garel L et al. Soft tissue hemangiomas in infants and children: diagnosis using color Doppler sonography. AJR Am. J. Roentgenol. 171, 247–252 (1998).
- Dubois J, Garel L, David M et al. Vascular soft tissue tumors in infancy: distinguishing features on Doppler sonography. AJR Am. J. Roentgenol. 178, 1541–1545 (2002).
- Donnelly L, Frish D. Abnormalities of the chest wall in pediatric patients. AJR Am. J. Roentgenol. 173, 1595–1601 (1999).
- Kaste SC, Young CW, Holmes TP, Baker DK. Effect of helical CT on the frequency of sedation in pediatric patients. AJR Am. J. Roentgenol. 168, 1001–1003 (1997).
- Kuhn JP, Brody AS. High resolution CT of pediatric lung disease. Radiol. Clin. North Am. 40(1), 89–110 (2002).
- Owens C. Radiography of diffuse interstitial pulmonary disease in children. Eur. Radiol. 14(S4), L2–L12 (2004).
- Brody AS, Molina PL, Klein JS, Rothman BS, Ramagopal M, Juritz DR. High resolution computed tomography of the chest in children with cystic fibrosis: support for use as an outcome surrogate. Pediatr. Radiol. 29(10), 731–735 (1999).
- Santamaria F, Grillo G, Guidi G et al. Cystic fibrosis: when should high resolution of congenital tomography of the chest be obtained? Pediatrics 101(5), 908–913 (1998).
- Manson D, Reid B, Dalal I, Roifman CM. Clinical utility of high resolution pulmonary congenital tomography in children with antibody deficiency disorders. Pediatr. Radiol. 27(10), 794–798 (1997).
- Kornreich L, Horev G, Zir N, Grunebaum M. Bronchiectasis in children: assessment by CT. Pediatr. Radiol. 23(2), 120–123 (1993).
- Deutsch GH, Young LR, Deterding RR et al. Diffuse lung disease in young children: application of a novel classification scheme. Am. J. Respir. Crit. Care Med. 176(11), 1120–1128 (2007).
- Fan LL, Long MC, Wegener JS. The diagnostic valve of transbronchial, thorascopic and open lung biopsy in immunocompetent children with chronic interstitial lung disease. J. Pediatr. 131(4), 565–569 (1997).
- Lynch DA, Hay T, Nemel JD, Dirgi VD, Fan LL. Pediatric diffuse lung disease: diagnosis and classification using high resolution CT. AJR Am. J. Roentgenol. 173, 713–718 (1999).
- Copley SJ, Coren M, Nicholson AG, Rubens MB, Bush A, Hansell DM. Diagnostic accuracy of CT and chest radiography of pediatric interstitial lung disease. AJR Am. J. Roentgenol. 174(2), 549–554 (2000).
- Vrielynyk S, Mamou-Mani T, Lwand S, Schneimann P, Brunelle F, de Blie J. Diagnostic value of high resolution CT in the evaluation of chronic infiltrative disease in children. AJR Am. J. Roentgenol. 191(3), 914–920 (2008).
- Langston C, Dishop MK. Diffuse lung disease in infancy: a proposed classification applied to 259 diagnostic biopsies. Pediatr. Develop. Pathol. 12, 421–437 (2009).
- Brody AS, Guillerman RP, Hay TC et al. Neuroendocrine cell hyperplasia of infancy: diagnosis with high resolution CT. AJR Am. J. Roentgenol. 194(1), 238–244 (2010).
- Doan ML, Guillerman RP, Dishop MK. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax 63(4), 366–373 (2008).
- de Blic J. Pulmonary alveolar proteinosis in children. Paediatr. Respir. Rev. 5(4), 316–322 (2004).
- Lee EY, Boiselle PM, Shamberger RC. Multidetector computed tomography and 3-dimensional imaging: preoperative evaluation of thoracic vascular and tracheobronchial anomalies and abnormalities in pediatric patients. J. Pediatr. Surg. 45(4), 811–821 (2010).
- Kritsaneepaiboon S, Lee EY, Zurakowski D, Strauss KJ, Boiselle PM. MDCT pulmonary angiography evaluation of pulmonary embolism in children. Am. J. Roentgenol. 192(5), 1246–1252 (2009).
- Tomà P, Rizzo F, Stagnaro N, Magnano G, Granata C. Multislice CT in congenital bronchopulmonary malformations in children. Radiol. Med. 116(1), 133–151 (2011).
- Lee EY, Kritsaneepaiboon S, Zurakowski D, Arellano CM, Strauss KJ, Boiselle PM. Beyond the pulmonary arteries: alternative diagnoses in children with MDCT pulmonary angiography negative for pulmonary embolism. Am. J. Roentgenol. 193(3), 888–894 (2009).
- Papaioannou G, Young C, Owens C. Multidetector row CT for imaging the paediatric tracheobronchial tree. Pediatr. Radiol. 37(6), 515–529 (2007).
- Remy-Jardin M, Faivre JB, Pontana F et al. Thoracic applications of dual energy. Radiol. Clin. N. Am. 48(1), 193–205 (2010).
- Siegel MJ, Luker GD. Pediatric chest MR imaging. Noncardiac clinical uses. Magn. Reson. Imag. Clin. N. Am. 4(4), 599–613 (1996).
- Sacchetti A, Carraccio C, Giardino A, Harris RH. Sedation for pediatric CT scanning: is radiology becoming a drug-free zone? Pediatr. Emerg. Care 21(5), 295–297 (2005).
- Grasso SN, Keller MS. Diagnostic imaging in pediatric trauma. Curr. Opin. Pediatr. 10(3), 299–302 (1998).
- Scaife ER, Rollins MD. Managing radiation risk in the evaluation of the pediatric trauma patient. Semin. Pediatr. Surg. 19(4), 252–256 (2010).
- Moore MA, Wallace EC, Westra SJ. The imaging of paediatric thoracic trauma. Pediatr. Radiol. 39(5), 485–496 (2009).
- Goske MJ, Applegate KE, Boylan J et al. The ‘Image Gently’ campaign: increasing CT radiation in dose awareness through a national education and awareness program. Pediatr. Radiol. 38(3), 265–269 (2008).
- Kroft LJM, Roelofs JJH, Geleijns J. Scan time and patient does for thoracic imaging in neonates and small children using axial volumetric 320-detector row CT compared to helical 64-, 32-, and 16- detector row CT acquisitions. Pediatr. Radiol. 40, 294–300 (2010).
- Woodhouse N, Wild JM, van Beek EJ, Hoggard N, Barker N, Taylor CJ. Assessment of hyperpolarized 3He lung MRI for regional evaluation of interventional therapy: a pilot study in pediatric cystic fibrosis. J. Magn. Reson. Imaging 30(5), 981–988 (2009).
- Kritsaneepaiboon S, Lee EY, Zurakowski D, Strauss KJ, Boiselle PM. MDCT pulmonary angiography evaluation of pulmonary embolism in children. Am. J. Roentgenol. 192(5), 1246–1252 (2009).
- Lee EY. Advancing CT and MR imaging of the lungs and airways in children: imaging into practice. Pediatr. Radiol. 38(2), 208–212 (2008).
- Feuerstein DM, Jicha DL, Pass HI et al. Pulmonary metastases: MR imaging with surgical correlation – a prospective study. Radiology 182(1), 123–129 (1992).
- Montella S, Santamaria F, Salvatore M et al. Lung diseases assessment in primary ciliary dyskinesia: a comparison between chest high-field magnetic resonance imaging and high-resolution computed tomography findings. Ital. J. Pediatr. 35(1), 24 (2009).
- Chavhan GB, Babyn PS, Singh M, Vidarsson L, Shroff M. MR imaging at 3.0 T in children: technical differences, safety issues and initial experience. Radiographics 29(5), 1451–1466 (2009).
- Peprah KO, Andronikou S, Goussard P. Characteristic magnetic resonance imaging low T2 signal intensity of necrotic lung parenchyma in children with pulmonary tuberculosis. J. Thorac. Imaging doi:10.1097/ RTI.0b013e318211abfb (2011) (Epub ahead of print).
- Puderback M, Eichinger M, Haeselbarth J et al. Assessment of morphological MRI for pulmonary changes in cystic fibrosis (CF) patients: comparison to thin-section CT and chest x‑ray. Invest. Radiology 42(10), 715–725 (2007).
- Jaggers J, Balsara K. Mediastinal masses in children. Semin. Thorac. Cardiovasc. Surg. 16(3), 201–208 (2004).
- Williams HJ, Alton HM. Imaging of paediatric mediastinal abnormalities. Paediatr. Respir. Rev. 4(1), 55–66 (2003).
- Watt AJ. Chest wall lesions. Paediatr. Respir. Rev. 3(4), 328–338 (2002).
- Harty MP, Kramer SS, Fellows KE. Current concepts on imaging of thoracic vascular abnormalities. Curr. Opin. Pediatr. 12(3), 194–202 (2000).
- Hansell DM, Boiselle PM, Golding J et al. Thoracic imaging. Respirology 15, 393–400 (2010).
- Puderback M, Kauczor HU. Can lung MR replace lung CT? Pediatr. Radiol. 38(53), 5439–5451 (2008).
- Hirsch W, Sorge I, Kromer S, Weber D, Meier K, Holger T. MRI of the lungs in children. Eur. J. Radiol. 68, 278–288 (2008).
- Lee EY, Zurakowski D, Boiselle PM. Pulmonary embolism in pediatric patients of CT pulmonary angiography practices and policies. Acad. Radiol. 17(12), 1543–1549 (2010).
- Kauczor HU, Ley-Zaporozhon, Ley S. Imaging of pulmonary pathologies: focus on magnetic resonance imaging. Proc. Am. Thorac. Soc. 6, 458–463 (2009).
- Lee EY, Siegel MJ. MDCT of tracheobronchial narrowing in pediatric patients. J. Thorac. Imaging 22(3), 300–309 (2007).
- Puderbach M, Hintze C, Ley S, Eichinger M, Kauczor HU, Biederer J. MR imaging of the chest: a practical approach at 1.5 T. Eur. J. Radiol. 64, 345–355 (2007).
• • Issue of cancer induction was raised after calculations of the increasing frequency of use of CT in children in the USA was analyzed in conjunction with low-dose Hiroshima outcome models.
• • Minisymposium dedicated to significant issues unique to pediatric imaging with digital radiography, including the tendency to increase radiation dose to combat image noise.
• Large longitudinal study of prenatal sonographically detected congenital pulmonary cystic malformations, demonstrating the poor correlation between prenatal and subsequent pathological diagnoses. The study also evaluates sonographic findings that can be useful, as well as describing the ongoing debate over the potential need for resection of congenital pulmonary malformations.
• • One of a series of landmark articles summarizing the findings of a large group of pediatric pulmonologists, radiologists and pathologists, the so-called ‘chILD’ consortium, proposing a new approach to pediatric diffuse lung diseases that is based on a large series of biopsies and associated clinical and radiographic findings.
• Reviews the previous and current attempts at overall pediatric radiation dose reduction through a multidisciplinary group, ‘The Alliance For Radiation Safety in Pediatric Imaging’, created to advance the ‘Image Gently’ campaign.
• Summarizes our current CT and MR capabilities for imaging the pediatric lung, and encourages a transfer of imaging to MRI when indicated and feasible.
• • Reviews indications, techniques, artifacts and typical findings in many diseases as they appear on MRI of the lung in children.
• Reviews pulmonary MRI in general, including functional imaging using perfusion and ventilatory imaging techniques.