Review Article - Interventional Cardiology (2013) Volume 5, Issue 4
Contemporary management of axillosubclavian vein thrombosis
- Corresponding Author:
- Karl A Illig
University of South Florida Health/Morsani College of Medicine
Division of Vascular & Endovascular Surgery
2 Tampa General Circle, STC 7016, Tampa, FL 33606, USA
Tel: +1 813 259 0921
Fax: +1 813 259 0606
E-mail: killig@health.usf.edu
Abstract
Given the relative rarity of the condition, the current diagnostic and management algorithms utilized for effort thrombosis generally derive from single-center reports, retrospective reviews and expert surgical opinion. This article will review the most contemporary strategies employed in the management of primary effort thrombosis, with particular emphasis on costoclavicular junction (CCJ) pathology, the role of catheter-based thrombolytic therapy and the importance of prompt thoracic outlet decompression.
Keywords
axillosubclavian vein thrombosis;effort thrombosis;Paget–Schroetter syndrome;thoracic outlet decompression;venous thoracic outlet syndrome
Introduction
The first descriptions of Paget–Schroetter syndrome or primary axillosubclavian venous thrombosis, also known as ‘effort thrombosis’ owing to its relationship to activity, were provided by case reports from Paget and von Schroetter in 1875 and 1884, respectively [1–3]. With the assistance of modern diagnostic techniques, particularly duplex ultrasound and catheter-based venography, consensus now exists that the pathophysiologic mechanism of primary axillosubclavian vein thrombosis is related to extrinsic venous compression at the anterior portion of the bony thoracic outlet [4–6]. Primary axillosubclavian vein thrombosis is a distinct clinical entity that is defined as thrombosis in the absence of upper extremity immobility, coagulation disorders, the presence of an indwelling central venous catheter, or the systemic insults of elective surgery [7]. A population-based study estimated the incidence to be approximately two per 100,000 persons per year [8], although larger reviews have described upper extremity effortinduced thrombosis as primary in up to 4% of all venous thrombosis [9,10].
Given the relative rarity of the condition, the current diagnostic and management algorithms utilized for effort thrombosis generally derive from single-center reports, retrospective reviews and expert surgical opinion. This article will review the most contemporary strategies employed in the management of primary effort thrombosis, with particular emphasis on costoclavicular junction (CCJ) pathology, the role of catheter-based thrombolytic therapy and the importance of prompt thoracic outlet decompression.
Pathophysiology
Thoracic outlet syndrome (TOS) refers to three differing clinical syndromes, each defined by the anatomic structure that is primarily affected. In neurogenic TOS, the most common form, upper extremity motor and sensory deficits result from compression of the brachial plexus at the scalene triangle or pectoralis minor insertion site. The rarest form, arterial TOS, is defined as true injury to the axillosubclavian artery as it crosses the first rib. The neurogenic and arterial variants of TOS involve pathology in the scalene triangle, posterior to the anterior scalene, and involve the mid-to-posterior part of the first rib (or abnormal cervical rib/variant). By contrast, venous TOS (VTOS) is related to problems in the anterior part of the (bony) thoracic outlet, with axillosubclavian vein compression and damage occurring in the narrow window formed as the first rib and clavicle approximate anteromedially at the sternum.
VTOS itself may be classified as positional or intermittent venous obstruction without thrombosis, versus actual thrombotic occlusion of the vein (the subject of this monograph) [7]. The pathogenesis of both forms of VTOS results from venous injury due to the extrinsic mechanical compression at the anterior portion of the thoracic outlet [11], and familiarity with the thoracic outlet is important to properly understand and manage this problem.
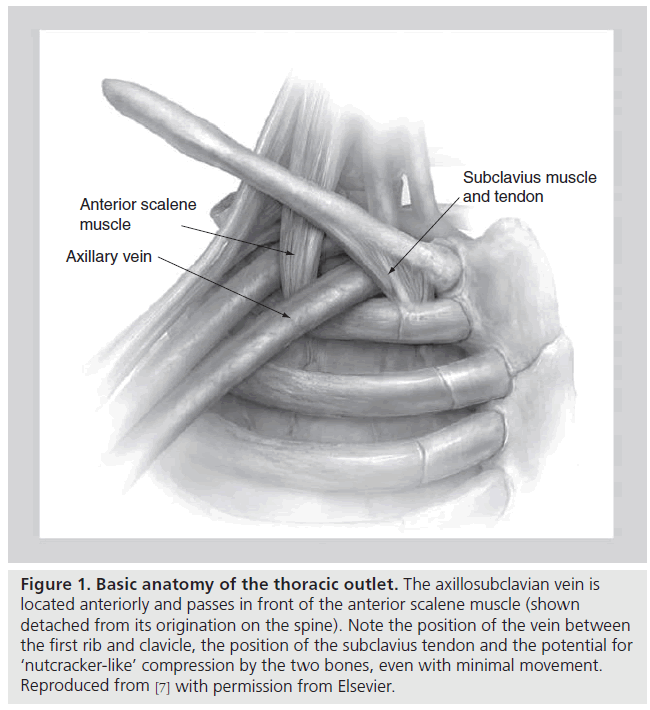
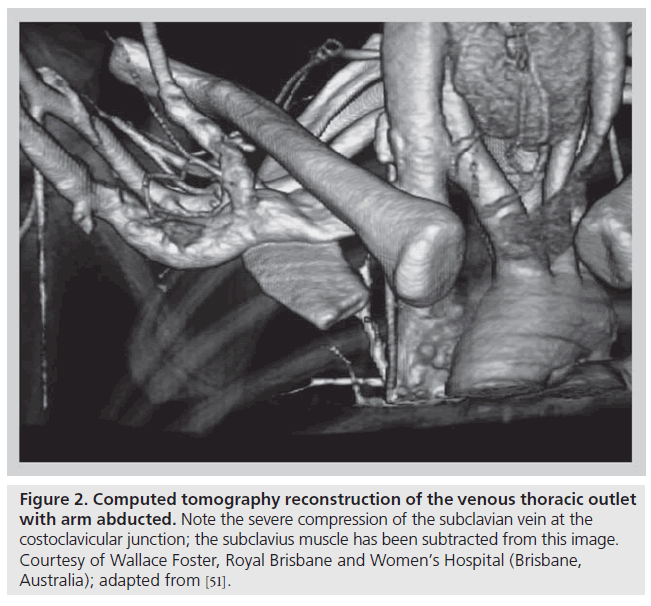
The thoracic outlet consists of two distinct anatomic areas (Figure 1). Posteriorly, the brachial plexus and axillosubclavian artery pass through a triangle bordered by the muscles of the anterior and middle scalene and the first rib. Compression of these structures within the scalene triangle produces the neurogenic or arterial forms of this syndrome. As discussed above, VTOS is caused by problems anterior to the scalene triangle in a space bordered by the bony and ligamentous structures of the CCJ, as well as the subclavius muscle and tendon. With arm abduction, the subclavian vein is compressed at this costoclavicular space, a finding that can often be easily demonstrated, even in patients without effort thrombosis (Figure 2) [12–17]. In addition, hypertrophy of the anterior scalene posteriorly or subclavius muscle anteromedially, further narrows the constrained costoclavicular space. The hypothesis that narrowing of the CCJ leads to effort thrombosis is supported by the relatively constant observation that VTOS often occurs in the setting of repetitive, vigorous athletic or occupational movements involving arm abduction or external rotation of the shoulder [18–22].
Figure 1: Basic anatomy of the thoracic outlet. The axillosubclavian vein is located anteriorly and passes in front of the anterior scalene muscle (shown detached from its origination on the spine). Note the position of the vein between the first rib and clavicle, the position of the subclavius tendon and the potential for ‘nutcracker-like’ compression by the two bones, even with minimal movement. Reproduced from [7] with permission from Elsevier.
Figure 2: Computed tomography reconstruction of the venous thoracic outlet with arm abducted. Note the severe compression of the subclavian vein at the costoclavicular junction; the subclavius muscle has been subtracted from this image. Courtesy of Wallace Foster, Royal Brisbane and Women’s Hospital (Brisbane, Australia); adapted from [51].
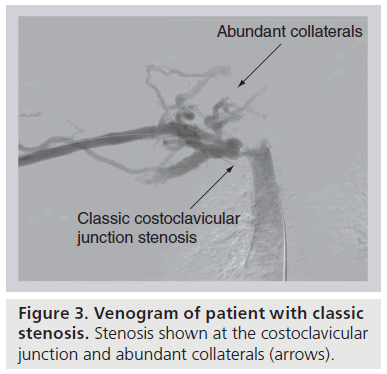
Chronic, repetitive venous compression at the CCJ initiates a cascade of perivenous inflammation leading to external fibrosis and, ultimately, fixation of the vein to the adjacent first rib or subclavius muscle [7,11,23]. Costochondral calcifications and occult first rib fractures with callous formation compound the localized inflammatory response. With this loss of mobility, the now scarencased vein is more prone to external injury and intimal hypertrophy with movements that alter the diameter of the costoclavicular space. This sequence of events fosters thrombosis of the subclavian vein as it passes adjacent to the CCJ. Prior to complete (or at least clinically relevant) thrombosis, there may be recurrent partial thrombosis with venous recanalization [13,24]. Asymptomatic or symptomatic partial thrombosis creates an inflammatory reaction and subsequent formation of venous intraluminal webs and fibrotic strictures. As the lumen of the subclavian vein narrows and flow is decreased, dense collaterals develop across the anterior chest wall, neck and axilla to facilitate upper extremity venous drainage (Figure 3) [25]. Ultimately, turbulent flow leads to a worsened stenosis and stasis leads to thrombosis of the strictured subclavian vein. Thrombus extension into the collateral network probably accelerates the clinical symptoms of effort thrombosis. What is most essential is the understanding that effort thrombosis is merely the sign of costoclavicular compression, and decompression of this area is required to prevent recurrence.
Figure 3: Venogram of patient with classic stenosis. Stenosis shown at the costoclavicular junction and abundant collaterals (arrows).
Diagnosis
Clinically, effort thrombosis occurs in young, predominantly right-handed men with a history of occupational or athletic overhead arm activity [20–22]. The presentation of VTOS varies with the acuity and degree of axillosubclavian vein thrombosis [26]. Patients with partial or intermittent thrombosis will present with episodic, transient upper extremity swelling and discoloration, often provoked or worsened by arm elevation. Partial or intermittent thrombosis may be asymptomatic, as a well-developed venous collateral network compensates for the impaired venous return [5,9,22,27]. In patients who present with intermittent obstruction only, a venogram may not reveal an abnormality unless the arm is abducted beyond 90 degrees. Nonetheless, partial thrombosis incites the cascade of inflammation and fibrosis, and predisposes to recurrent and potentially complete thrombosis.
By contrast, a patient with a previously normal vein who develops complete thrombosis more often presents with a distinct, abrupt onset of symptoms [26]. The classic scenario is a patient who complains of the acute development of an enlarged blue, heavy, painful arm combined with a history of upper extremity exercise within hours-to-days of symptom onset. In the absence of a central venous catheter, a patient with an acutely swollen blue arm should be considered to have effort thrombosis until definitively disproven via contrast venography. The clinical significance of this scenario cannot be over emphasized as a missed or delayed diagnosis alters the effectiveness of treatment strategies. Dilated veins of the neck, anterior chest or axilla suggest a more chronic occlusion and, thus, a more adherent and fibrotic thrombus [26].
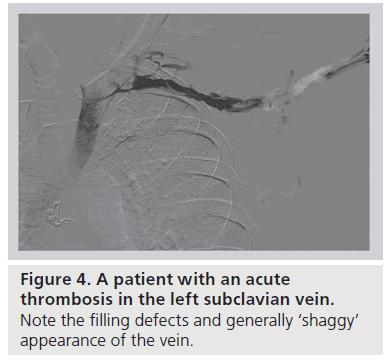
Clinical suspicion of effort thrombosis is confirmed radiographically, most conveniently by means of duplex ultrasound. This noninvasive, inexpensive technique, while not perfect, is associated with a greater than 80% sensitivity and specificity [28]. The absence of flow and a noncompressible vein signifies fresh venous thrombus. However, duplex ultrasonography is limited by its inability to visualize the central subclavian or innominate vein to fully assess a clot in a location obscured by depth or an overriding clavicle, and is unusually technician-dependent in this location. In the hands of a less experienced technician, enlarged collaterals can easily be mistaken for the subclavian vein, and a freshly clotted vein with pathology limited to the CCJ itself can be erroneously labeled as normal. With limited diagnostic accuracy and reported false-negative rates approaching 30%, a negative duplex ultrasound does not exclude the diagnosis of VTOS in the setting of high clinical suspicion [12]. Cross-sectional computed tomography or magnetic resonance venous-phase studies to further define the central venous circulation have been used in the setting of a nondiagnostic duplex ultrasound. The benefits of such axial studies are limited and the role undefined, especially considering the often pathognomonic clinical presentation and near universal need for prompt endovenous intervention. To date, the gold standard diagnostic study remains to be catheter-based venography [29,30]; as therapy for effort thrombosis almost always begins with catheter-directed thrombolysis (see the ‘Management’ section), venography is always required, at least in patients who present with acute thrombosis (Figure 4).
Management
When VTOS is suspected, patients should be started on systemic anticoagulation with unfractionated or low-molecular weight heparin to prevent extension of venous thrombus while awaiting diagnostic and therapeutic measures. Work up of inherited or genetic coagulation disorders may be performed during initial patient assessment as some evidence now suggests that patients may be more likely to have an underlying hypercoagulable condition [31]. However, as the pathology of VTOS is related to extrinsic mechanical venous compression, one should not delay definitive management while evaluating for a hypercoagulable state.
Before the ‘modern’ era, management of patients with effort thrombosis consisted solely of restrictions to physical activity, arm elevation and graded compression to the extremity [32]. Such conservative measures resulted in a high level of morbidity and disability from progressive venous obstruction and the sequelae of chronic venous congestion. In the 1950s, systemic anticoagulation was added. The reported success of anticoagulation therapy as the sole treatment of effort thrombosis has varied, although is largely disappointing. Anticoagulation therapy alone is associated with rates of residual upper extremity venous obstruction approaching 80%, symptoms of persistent venous congestion in as many as 90% of cases, a 6–15% incidence of pulmonary embolism and permanent disability in nearly 70% of cases [33–37]. The morbidities associated with anticoagulation alone result from failure to properly treat the inciting pathology of VTOS, that being the relief of extrinsic venous compression. In addition, although the use of systemic anticoagulation in the management of iliofemoral and popliteal deep venous thrombosis has been well documented, the duration and role of anticoagulation therapy in VTOS is unproven. Without surgical decompression, patients with VTOS would require lifelong systemic anticoagulation, accepting the medication risks and limitations to physical activity inherent to this treatment strategy. As the majority of patients with VTOS are younger, active individuals, resumption of a disability-free lifestyle is important and, as such, anticoagulation as sole therapy has been replaced by catheter-based thrombolysis followed by surgical decompression of the CCJ via first rib resection (Figure 5) [11].
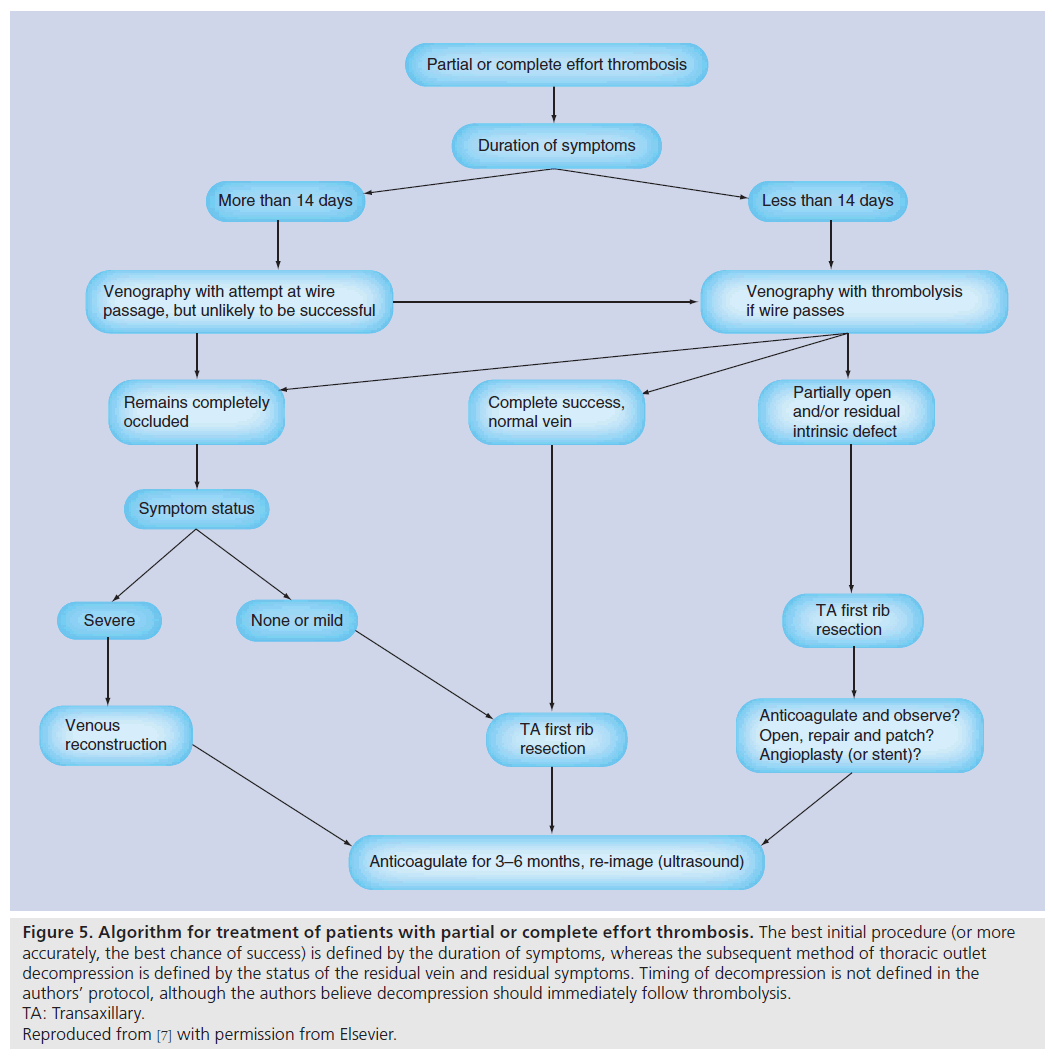
Figure 5: Algorithm for treatment of patients with partial or complete effort thrombosis. The best initial procedure (or more accurately, the best chance of success) is defined by the duration of symptoms, whereas the subsequent method of thoracic outlet decompression is defined by the status of the residual vein and residual symptoms. Timing of decompression is not defined in the authors’ protocol, although the authors believe decompression should immediately follow thrombolysis. TA: Transaxillary. Reproduced from [7] with permission from Elsevier.
▪ Catheter-based thrombolysis
Unless a contraindication to lytic therapy exists, catheter-directed lytic therapy is the standard initial step in VTOS management [38–40]. During the diagnostic venogram, a multiple sidehole catheter is placed directly within the axillosubclavian thrombus, with infusion of a thrombolytic agent for up to 48 h (it is important to stress that access should be obtained via the deep or basilic system and not through the cephalic vein, which does not allow treatment of the vein peripheral to its insertion into the deep system). During and following this infusion period, repeat venography is performed to assess the success of thrombolysis. Such imaging will identify any residual intraluminal venous defects, as well as allow for the assessment of the degree of venous compression at the CCJ. Successful lytic therapy, measured by restoration of unobstructed central venous inflow, is correlated with a 30% greater likelihood of freedom from symptoms of venous congestion, as compared with partial or unsuccessful thrombolysis [41]. Furthermore, both the duration from symptom onset to the initiation of lytic therapy and the extent of thrombus burden are also correlated with the success of thrombolysis [42]. With a prompt diagnosis of effort thrombosis and expedited initiation of lytic therapy against a smaller thrombus burden, the chance of success in relieving the obstructed venous segment is high. Multiple reports have demonstrated failure of thrombolytic therapy if initiated beyond 10–14 days after symptom onset [43,44]. The authors’ experience has resulted in a >80% relief of obstruction with lytic therapy started within a 2-week window from symptom onset [42]. The time-sensitive success of thrombolysis reinforces the ideal that, with time, the thrombus itself will solidify and stabilize in the fibrotic vein and thus be less susceptible to lytic dissolution. Unsuccessful venous thrombolysis may alter or limit the options for venous reconstruction to restore central inflow at the time of surgical decompression. Finally, an evolving alternative option is on-table pharmacomechanical thrombolysis, which can be performed in the operating room at the same time as thoracic outlet decompression.
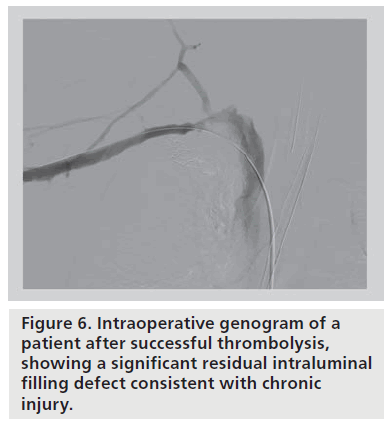
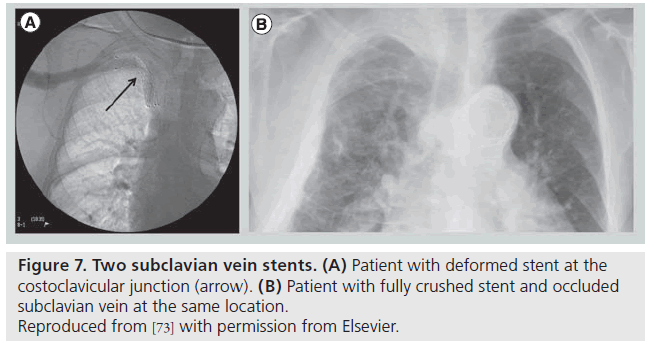
Following thrombolysis, the venogram usually reveals some degree of intrinsic venous defects, residual thrombus or focal stenosis [45]. It should again be stressed that most residual venous defects represent perivenous inflammatory and fibrotic injury resulting from repetitive extrinsic mechanical compression at the CCJ (Figure 6). Such findings simply reinforce the second guiding principle in VTOS management, that acute treatment of the clot is useless without correction of the causative factor, and surgical decompression of the thoracic outlet must follow lytic therapy. No medical or percutaneous management strategy provides a more sustainable reduction in rethrombosis or extremity disability than surgical decompression [46]. The risk of recurrent thrombosis after thrombolysis, if first rib resection is not performed, has been reported to be as high as 50–70% [45]. Transluminal balloon angioplasty is marginally better than anticoagulation alone in the absence of decompression, but is also associated with a high incidence of recurrent thrombosis [47,48]. Angioplasty of a residual stenosis should not be viewed as a definitive treatment, but may be performed to improve extremity venous drainage and associated symptoms while awaiting surgical decompression. Placement of endovascular stents is essentially contraindicated unless decompression has been performed (and is discouraged even if it has) due to high rates of stent fracture and thrombosis [48,49]. The anatomic constraints of chronic fibrosis and fixed bony structures at the CCJ will easily overwhelm the radial force of any stent (Figure 7).
▪ Thoracic outlet decompression
Definitive management of VTOS ultimately depends upon decompression of the thoracic outlet, relieving extrinsic compression on the axillosubclavian venous segment. While thrombolysis usually alleviates the symptoms by removing the acute complication (clot), decompression of the thoracic outlet treats the underlying disease process. Failure to decompress the thoracic outlet following thrombolytic therapy leads to rethrombosis and persistent symptomatology in nearly a third of patients [50]. A number of surgical approaches may be used for adequate exposure and decompression of pathology at the CCJ (for a detailed description see [51]). Whether performed via a transaxillary, or supra-, para- or infra-clavicular incision, the surgeon should be aware of the benefits and limitations of each surgical approach as the operative plan depends on the underlying pathology. The anticipated need for extensive venous reconstruction, especially in the setting of a residual or chronic venous obstruction, is often the critical factor affecting choice of surgical exposure.
Transaxillary first rib resection (with partial scalenectomy) was the first reported, and remains the most common, approach for VTOS (Figure 8) [52]. Excellent exposure to the anterior portion of the first rib and the insertion of the anterior and middle scalene muscles is possible. For truly complete venous decompression, one should also excise the potentially bulky subclavius muscle and tendon at the CCJ, as well as perform a circumferential venolysis centrally to the confluence with the internal jugular vein [53,54]. However, a drawback of the transaxillary approach is the inability to perform any venous reconstruction. An incision centered over the clavicle with medial or total claviculectomy provides unobstructed venous exposure, at the expense of some instability to the shoulder joint and loss of bony protection to the upper extremity neurovascular bundle. Combining incisions above, on top or just below the clavicle also provides excellent visualization of the CCJ pathology [55–58]. Accepting a higher degree of patient morbidity and potential wound complications, excellent surgical exposure can also be gained with sternal disarticulation and first rib resection [59]. It is important to impart on these often young, active patients that surgical removal of the first rib, scalene muscles and the subclavius muscle/tendon complex does not ultimately hinder one’s potential to engage in highly competitive athletics [12].
▪ Necessity & timing of decompression
Over the last several decades there has been increasing consensus as to the necessity and timing of thoracic outlet decompression for VTOS. A vocal minority of surgeons continue to recommend only selective first rib excision following thrombolysis. A single-center review of 50 patients treated with anticoagulation therapy and observation after successful thrombolytic therapy describes long-term symptom-free rates approaching 90%, although these results have been disseminated in chapter and oral form only [60,61]. Similarly, a smaller retrospective review evaluated the long-term outcomes of selective thoracic outlet decompression following successful thrombolysis. Of 64 patients managed nonoperatively, 29 patients required first rib resection within 3 months of thrombolysis for persistent symptoms. In addition, of the 35 patients initially treated successfully with thrombolysis followed by anticoagulation, eight patients later required rib resection for persistent symptomatology or rethrombosis [62]. What is not stressed in the original article is that the requirement of first-rib resection for symptoms or rethrombosis actually denotes a treatment failure in 58% of patients when managed nonoperatively. Recurrent thrombosis is less amenable to thrombolytic therapy and may compromise the accessory collateral venous drainage. Furthermore, the presence of residual thrombus portends a poorer symptom-free prognosis with increased morbidity and disability, and can significantly limit the options of surgical venous reconstruction if an obstruction to axillary venous return exists. Finally, logic does not support this algorithm, which essentially states that the rib does not need to be removed after one clot, but does after a second, a difference that appears to be very arbitrary. Data supporting selective first rib excision are sparse, and when the excellent outcomes with thoracic outlet decompression are considered, it is difficult to justify nonobligatory decompression in patients with an acceptable operative risk. Large retrospective reviews highlight the ability to perform postlytic first rib resection with freedom from rethrombosis or recurrent symptoms in 95–100% of patients [19,63].
The question of when to decompress the thoracic outlet depends on the balance between the benefits of definitive correction of the CCJ pathology and return to predisease lifestyle against the risks of perioperative bleeding complications, and the risk of rethrombosis during the interval prior to first rib resection. In an effort to avoid iatrogenic venous injury or bleeding complications after thrombolysis, the earliest algorithms recommended a 3-month period of anticoagulation prior to thoracic outlet decompression. Such an approach includes at least a 10% risk of rethrombosis while awaiting definitive repair, with added morbidity and disability inherent to this problem [41,64]. More recently, numerous single-institution retrospective reviews demonstrate the efficacy, low complication rates and freedom from symptom recurrence with immediate postlytic thoracic outlet decompression [65–67]. The technical successes of early decompression are paralleled by equal success in the metrics of freedom from disability and return to a productive work life [68]. Most clinicians who routinely treat this disorder now advocate immediate (hours-to-days) first rib resection once the vein has been recanalized, although it must be noted that no prospective data exist.
▪ Residual & chronic obstruction
As described earlier, venography following thrombolysis usually reveals residual venous fibrotic injury. How best to treat these residual defects following thrombolysis and thoracic outlet decompression is, however, unknown. A nonoperative approach with continued anticoagulation may be the most prudent choice, allowing the patent decompressed vein lumen to increase in diameter and collateral channels to enlarge; such patients who undergo this approach do very well. Similarly, venography and transluminal balloon venoplasty at the time of (or following) rib excision is an alternative low-risk strategy. Ultimately, the symptomatology of the patient should dictate the aggressiveness of further interventions; those with minimal symptoms will probably not need formal reconstruction, while those with severe obstructive symptoms, even following decompression, will. The most cited and largest clinical series continues to recommend direct subclavian reconstruction as the optimal approach to symptomatic residual or chronic venous obstructions.
Less invasive interventions to improve venous patency and central inflow have been suggested. One report investigated the placement of intraluminal stents across residual venous defects, even after the rib had been removed, with observations of decreased 4-year patency rates as compared with venoplasty alone [69]. The most aggressive approach to residual venous defects entails surgical reconstruction of the involved venous segment, with the creation of a distal arteriovenous fistula to elevate venous return [70,71]. The authors own approach is to reserve extensive reconstruction for patients with persistent upper extremity symptomatology, irrespective of the venographic picture of luminal narrowing after decompression [7].
Finally, patients with chronic occlusion of the axillosubclavian vein should probably be managed in a similar fashion to those with residual defects. Some evidence supports first rib resection even if the vein cannot be reopened, as this has been associated with spontaneous recanalization over time [72]. Again, in the absence of severe symptoms, clinical surveillance and systemic anticoagulation alone is an accepted management plan for patients with a chronically occluded vein.
Dialysis-associated VTOS
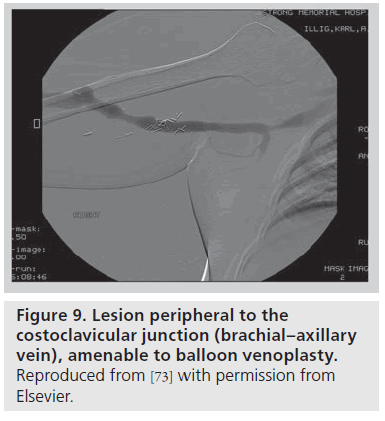
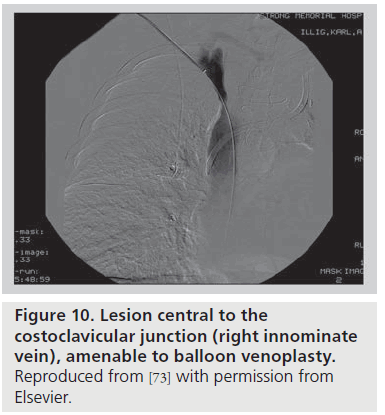
In the setting of an upper arm dialysis arteriovenous fistula (AVF), stenosis or obstruction of venous return at the CCJ serves as a functional venous outflow obstruction [73]. While such a problem has typically been thought to be due to injury from indwelling subclavian catheters, there are many people with this issue who have never had such a catheter, and we suggest that the turbulence created by the high flow through this area creates a positive feedback loop that makes this location highly susceptible to stenosis. Such stenosis can lead to failure of AVF maturation or loss of functional patency of the access. AVF venous outflow stenoses are not all the same entity; the anatomic location of the venous segment should guide definitive management. A stenosis peripheral (brachial or axillary veins; Figure 9) or central (innominate vein or superior vena cava; Figure 10) to the bony CCJ responds well to endovascular intervention. By contrast, lesions at the CCJ are the result of bony compression and will not respond to venoplasty or stenting, as discussed above.
The authors’ approach to these lesions is similar to their management of effort-induced thrombosis. In a patient with a dysfunctional access (prolonged bleeding, high pressures, and inefficient dialysis/recirculation) and a stenosis at the CCJ, we aggressively decompress the thoracic outlet by means of first rib resection. If no immediate imaging is needed, the transaxillary approach with external venolysis and follow-up of the patient’s symptoms, access function and flow volumes on duplex ultrasound is preferred. However, we now prefer to image the vein prior to incision and, as such, place the patient supine and plan on an infraclavicular approach. This allows excellent access to the anterior portion of the first rib; we can usually divide the rib posterior to the scalene tubercle (thus resecting the anterior scalene). We are quite aggressive with the subclavius muscle and costoclavicular tendon; the end goal being to have the vein completely freed up from the underlying and constricting tissues on all sides. When more aggressive reconstruction is needed, full venous exposure can be obtained by means of claviculectomy or rotation of the sternoclavicular joint superiorly. At the final formal analysis, we achieved fistula salvage in 75% of a cohort of 12 patients [73]; we have since added another 14 patients, with all but one maintaining patency. It should be noted that we have not observed any difference in bleeding, either subjectively at operation or noted by complication rates in these patients; their procedures are indistinguishable from those in patients with conventional VTOS.
▪ Postoperative management
The underlying pathology of VTOS is extrinsic mechanical compression and not a hypercoagulable or prothrombotic state. Following relief of extrinsic mechanical compression, systemic anticoagulation is administered for only 3–6 months postoperatively, it is then stopped if the duplex looks favorable (it is stressed that this time frame is, at present, arbitrary). Clinical and venous duplex examinations are performed at 1, 6 and 12 months following surgery, with annual examinations thereafter. Most patients with intrinsic damage will continue to demonstrate chronic filling defects, but rethrombosis is extremely rare after first rib excision.
▪ Outcomes
It is quite difficult to weigh the comparative successes of different treatment strategies for a rare, under-recognized and anecdotally reported disease process. Expert consensus opinion and a recent meta-analysis suggest that ‘good’ results should be achieved in approximately 50% of patients treated with anticoagulation alone, 80–90% of patients managed with thrombolysis and delayed first rib resection, and more than 95% of those treated with thrombolysis followed by immediate thoracic outlet decompression [7,46].
Conclusion
VTOS is a disease process that, with prompt recognition, can be managed with high technical success rates, low complication rates and excellent long-term outcomes. Despite ongoing debates to varying degrees regarding multiple issues, the general consensus has evolved that this disease process, especially if presenting acutely, is best treated with catheter-based thrombolysis with subsequent (early) thoracic outlet decompression (first rib resection, scalenectomy and external venolysis) and possible subclavian vein reconstruction. Some evidence also suggests that first rib resection is beneficial, even in patients with chronic thrombus and veins that cannot be reconstructed. The use of intraluminal stents should be avoided owing to unacceptably high failure rates, even if the rib has been removed (and certainly if it has not). Using this algorithm, good long-term outcomes should be expected in over 90% of affected patients. If the diagnosis is missed, however, a substantial proportion of patients suffer ongoing, long-term disability.
Future perspective
Despite the recent evolution in the diagnostic and therapeutic strategies utilized in the treatment of VTOS, multiple unanswered questions remain, or at least issues exist that have no real data supporting their effectiveness. The authors understanding of the optimal management strategy for VTOS will be redefined with future research and clinical experience directed towards addressing the following:
Executive summary
Diagnosis
▪ Effort thrombosis is the most common and most extreme presentation of venous thoracic outlet syndrome (VTOS).
Management
▪ The modern consensus is that the management of VTOS requires:
– Prompt catheter-based thrombolysis (ideally within 14 days of thrombosis/symptom onset); followed by
– Thoracic outlet decompression via first rib resection, scalenectomy and external venolysis (ideally at the same admission).
▪ Placement of intraluminal stents, either pre- or post-decompression, is associated with poorer outcomes and limits future venous reconstruction.
▪ Dialysis-associated VTOS is venous outflow obstruction related to the pathology at the costoclavicular junction, and probably threatens access patency if the first rib is not removed (although currently, no solid data exist).
▪ Randomized controlled trials are needed to better define management algorithms in VTOS.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Paget J. Clinical Lectures and Essays.Longmans, Green & Co., London, UK, (1875).
- von Schroetter L. Erkrankungen der Gefasse. In: Nothnagel Handbuch der Pathologie und Therapie. Wein, Holder, Germany (1884).
- Hughes ES. Venous obstruction in upper extremity. Br. J. Surg. 36, 155–163 (1948).
- Landry GJ, Liem TK. Endovascular management of Paget–Schroetter syndrome. Vascular 15, 290–296 (2007).
- Shebel ND, Marian A. Effort thrombosis (Paget–Schroetter syndrome) in active young adults: current concepts in diagnosis and treatment. J. Vasc. Nurs. 24, 116–126 (2006).
- Swinton NW Jr, Edgett JW Jr, Hall RJ. Primary subclavian-axillary vein thrombosis. Circulation 38, 737–745 (1968).
- Illig KA, Doyle AJ. A comprehensive review of Paget–Schroetter syndrome. J. Vasc. Surg. 51(6), 1538–1547 (2010).
- Lindbald B, Tengborn L, Bergqvist D. Deep vein thrombosis of the axillary- subclavian veins: epidemiologic data, effects of different types of treatment and late sequelae. Eur. J. Vasc. Surg. 2, 161–165 (1988).
- Prandoni P, Bernardi E. Upper extremity deep vein thrombosis. Curr. Opin. Pulm. Med. 5, 222–226 (1999).
- Sternbach Y, Green RM. Endovascular and surgical management of acute axillary-subclavian venous thrombosis. In: Handbook of Venous Disease (2nd Edition).Gloviczki P, Yao JST (Eds). Hodder Arnold, London, UK, 209–213 (2009).
- Thompson RW. Comprehensive management of subclavian vein effort thrombosis. Semin. Intervent. Radiol. 29, 44–51 (2012).
- Melby SJ, Vedantham S, Narra VR et al. Comprehensive surgical management of the competitive athlete with effort thrombosis of the subclavian vein (Paget–Schroetter syndrome). J. Vasc. Surg. 47(4), 809–820 (2008).
- Adams JT, DeWeese JA, Mahoney EB, Rob CG. Intermittent subclavian vein obstruction without thrombosis. Surgery 68, 147–165 (1968).
- Gould PE, Patey DH. Primary thrombosis of axillary vein: a study of eight cases. Br. J. Surg. 16, 208–213 (1928).
- Falconer MA, Weddell GL. Costoclavicular compression of subclavian artery and vein. Lancet 242, 539 (1943).
- Adams JT, McEvoy RK, DeWeese JA. Primary deep venous thrombosis of upper extremity. AMA Arch. Surg. 91, 29–42 (1965).
- Sampson JJ, Saunders JB, Capp CS. Compression of the subclavian vein by first rib and clavicle. Am. Heart J. 19, 292 (1940).
- Heron E, Lozinguez O, Emmerich J, Laurian C, Fiessinger JN. Long-term sequelae of spontaneous axillary-subclavian venous thrombosis. Ann. Intern. Med. 131, 510–513 (1999).
- Urschel HC Jr, Razzuk MA. Paget–Schroetter syndrome: what is the best management? Ann. Thorac. Surg. 69, 1663–1668 (2000).
- Adams JT, DeWeese JA. ‘Effort’ thrombosis of the axillary and subclavian veins. J. Trauma 11, 923–930 (1971).
- Tilney NL, Griffiths HJG, Edwards EA. Natural history of major venous thrombosis of the upper extremity. Arch. Surg. 101, 792–796 (1970).
- Horattas MC, Wright DJ, Fenton AH, Evans DM, Oddi MA. Changing concepts of deep venous thrombosis of the upper extremity – report of a series and review of the literature. Surgery 104, 561–567 (1988).
- Aziz S, Strachley CJ, Whealen TJ Jr. Effort-related axillo-subclavian vein thrombosis. A new theory of pathogenesis and a plea for direct surgical intervention. Am. J. Surg. 152, 57–61 (1986).
- Inahara T. Surgical treatment of ‘effort’ thrombosis of the axillary and subclavian veins. Am. Surg. 34, 479–483 (1968).
- Adams JT, McEvoy RK, DeWeese JA. Primary deep venous thrombosis of upper extremity. AMA Arch. Surg. 91, 29–42 (1965).
- Sanders RJ, Hammond SL. Venous thoracic outlet syndromes. Hand Clin. 20(1), 113–118 (2004).
- Sharafuddin MJ, Sun S, Hoballah JJ. Endovascular management of venous thrombotic disease of the upper torso and extremities. J. Vasc. Interv. Radiol. 13, 975–990 (2002).
- Chin EE, Zimmerman PT, Grant EG. Sonographic evaluation of upper extremity deep venous thrombosis. J. Ultrasound Med. 24, 829–838 (2005).
- Green RM, Rosen R. The management of axillo-subclavian venous thrombosis in the setting of thoracic outlet syndrome. In: Handbook of Venous Disorders (3rd Edition).Gloviczki P (Ed.), Hodder Arnold, London, UK, 292–298 (2008).
- Polak JF, Yucel EK, Bettman MA et al. Suspected upper extremity deep venous thrombosis (DVT). In: Online publication, American College of Radiology (ACR). NationalGuideline Clearinghouse, Reston, VA (2005).
- Cassada DC, Lipscomb AL, Stevens SL, Freeman MB, Grandas OH, Goldman MH. The importance of thrombophilia in the treatment of Paget–Schroetter syndrome. Ann. Vasc. Surg. 20, 596–601 (2006).
- Hughes ES. Venous obstruction upper extremity: review of 320 cases. Int. Abst. Surg. 88, 89–127 (1949).
- Heron E, Lozinguez O, Emmerich J, Laurian C, Fiessinger JN. Long-term sequelae of spontaneous axillary-subclavian venous thrombosis. Ann. Intern. Med. 131, 510–513 (1999).
- Becker DM, Phillbirck JT, Walker FB. Axillary and subclavian venous thrombosis. Prognosis and treatment. Arch. Intern. Med. 151, 1934–1943 (1991).
- Monreal M, Lafoz E, Ruiz J, Valls R, Alastrue A. Upper-extremity deep venous thrombosis and pulmonary embolism. A prospective study. Chest 99, 280–283 (1991).
- Hingorani A, Ascher E, Lorenson E, DePippo P, Salles-Cunha S, Scheinman M. Upper extremity deep venous thrombosis and its impact on morbidity and mortality rates in a hospital-based population. J. Vasc. Surg. 26, 853–860 (1997).
- AbuRahma AF, Sadler D, Stuart P, Khan MZ, Boland JP. Conventional versus thrombolytic therapy in spontaneous (effort) axillary-subclavian vein thrombosis. Am. J. Surg. 161, 459–465 (1991).
- Druy EM, Trout H, Giordano JM, Hix WR. Lytic therapy in treatment of axillary and subclavian vein thrombosis. J. Vasc. Surg. 2, 821–827 (1985).
- Taylor LM, McAllister WR, Dennis DL, Porter JM. Thrombolytic therapy followed by first rib resection for spontaneous (‘effort’) subclavian vein thrombosis. Am. J. Surg. 149, 644–647 (1985).
- Becker GJ, Holden RW, Rabe FE et al. Local thrombolytic therapy for subclavian and axillary vein thrombosis. Treatment of the thoracic inlet syndrome. Radiology 149, 419–423 (1983).
- Machleder HI. Evaluation of a new treatment strategy for Paget–Schroetter syndrome: spontaneous thrombosis of the axillary-subclavian vein. J. Vasc. Surg. 17, 305–315 (1993).
- Doyle A, Wolford HY, Davies MG, Adams JT, Singh MJ, Saad WE. Management of effort thrombosis of the subclavian vein: today’s treatment. Ann. Vasc. Surg. 21, 723–729 (2007).
- Adelman MA, Stone DH, Riles TS, Lamparello PJ, Giangola G, Rosen R. A multidisciplinary approach to the treatment of Paget–Schroetter syndrome. Ann. Vasc. Surg. 11, 149–154 (1997).
- Wilson JJ, Zahn CA, Newton H. Fibrinolytic therapy for idiopathic subclavian-axillary vein thrombosis. Am. J. Surg. 159, 208–210 (1990).
- Porter JM, Bergan JJ, Goldstone J, Greenfield LJ. Axillary-subclavian vein thrombosis. Perspect. Vasc. Surg. 4, 85–89 (1991).
- Lugo J, Armstrong P, Back M et al. Does the first rib need to be removed after thrombolysis? A meta-analysis. Presented at: 2013 International Society for Vascular Surgery.FL, USA, 14–16 February 2013.
- Meier GH, Pollak JS, Rosenblatt M, Dickey KW, Gusberg RJ. Initial experience with venous stents in exertional axillary-subclavian vein thrombosis. J. Vasc. Surg. 24, 974–981 (1996).
- Strange-Vognsen HH, Hauch O, Anderson J, Struckman J. Resection of the first rib, following deep arm vein thrombolysis in patients with thoracic outlet syndrome. J. Cardiovasc. Surg. (Torino) 30, 430–433(1989).
- Urschel HC, Patel AN. Paget–Schroetter syndrome therapy: failure of intravenous stents. Ann. Thorac. Surg. 75(6), 1693–1696 (2003).
- Machleder HI. Upper extremity venous occlusion. In: Current Therapy in Vascular Surgery (3rd Edition). Ernst CB, Stanley JC(Eds). Mosby-Year Book Inc., MO, USA, 958–963 (1995).
- Illig KA, Thompson R, Freischlag J, Donahue D, Jordan S, Edgelow P (Eds). Thoracic Outlet Syndrome. Springer, NY, USA(2013).
- Roos DB. Transaxillary approach for first rib resection to relieve thoracic outlet syndrome. Ann. Surg. 163, 354–358 (1966).
- Freischlag J. Venous thoracic outlet syndrome: transaxillary approach. Operative Techniques General Surgery 10, 122–130(2008).
- Thompson RW, Schneider PA, Nelken NA, Skioldebrand CG, Stoney RJ. Circumferential venolysis and paraclaviclar thoracic outlet decompression for ‘effort thrombosis’ of the subclavian vein. J. Vasc. Surg. 16, 723–732 (1992).
- Azakie A, McElhinney DB, Thompson RW, Raven RB, Messina LM, Stoney RJ. Surgical management of the subclavian-vein effort thrombosis as a result of thoracic outlet decompression. J. Vasc. Surg. 28, 777–786 (1988).
- Molina JE. Operative technique of first rib resection via a subclavicular approach. Vasc. Surg. 27, 667–672 (1993).
- Molina JE. Approach to the confluence of the subclavian and internal jugular veins without claviculectomy. Semin. Vasc. Surg. 13, 10–19 (2000).
- Thompson RW. Venous thoracic outlet syndrome: paraclavicular approach. Operative Techniques General Surgery. 10, 113–121(2008).
- Green RM, Waldman D, Ouriel K, Riggs P, DeWeese JA. Claviculectomy for subclavian venous repair: long-term functional results. J. Vasc. Surg. 32, 315–321 (2000).
- Johansen KH. Thoracic outlet syndrome: too many operations or too few? In: Vascular Surgery: Therapeutic Strategy. Pearce W, Yao J,Matusumura J, Eskandari M (Eds). Peoples’ Medical Publishing, IL, USA, (2009).
- Johansen KH. Thoracic outlet syndrome: challenges, controversies, and consensus. Presented at: Washington University, MO, USA, 23–24 October 2009.
- Lee JT, Karkowski JK, Harris EJ, Haukoos JS, Olcott C. Long-term thrombotic recurrence after nonoperative management of Paget–Schroetter syndrome. J. Vasc. Surg. 43, 1236–1243 (2006).
- Molina JE, Hunter DW, Dietz CA. Paget–Schroetter syndrome treated with thrombolytics and immediate surgery. J. Vasc. Surg. 45, 328–334 (2007).
- Kunkel JM, Machleder H. Treatment of Paget–Schroetter syndrome: a staged, multidisciplinary approach. Arch. Surg. 124, 1153–1158 (1989).
- Angle N, Gelebert HA, Farooq MM, Ahn S, Caswell D, Freischlag J. Safety and efficacy of early surgical decompression of the thoracic outlet for Paget–Schroetter syndrome. Ann. Vasc. Surg. 15, 37–42 (2001).
- Caparelli DJ, Freischlag J. A unified approach to axillosubclavian venous thrombosis in a single hospital admission. Sem. Vasc. Surg. 18, 153–157 (2005).
- Urschel HC, Razzuk MA. Improved management of the Paget–Schroetter syndrome secondary to thoracic outlet compression. Ann. Thor. Surg. 52, 1217–1221 (1991).
- Divi V, Proctor M, Axelrod D, Greenfield LJ. Thoracic outlet decompression for subclavian vein thrombosis: experience in 71 patients. Arch. Surg. 140, 54–57 (2005).
- Kreienberg PB, Chang BB, Darling RC, Roddy SP, Paty PS, Lloyd WE. Long-term results in patients treated with thrombolysis, thoracic inlet decompression, and subclavian vein stenting for Paget–Schroetter syndrome. J. Vasc. Surg. 33(Suppl. 2), S100–S105(2001).
- Thompson RW, Petrinec D, Toursarkission B. Surgical treatment of thoracic outlet compression syndromes II: supraclavicular exploration and vascular reconstruction. Ann. Vasc. Surg. 11, 442–451 (1997).
- Melby SJ, Vedantham S, Narra VR et al. Comprehensive surgical management of the competitive athlete with effort thrombosis of the subclavian vein (Paget–Schroetter syndrome). J. Vasc. Surg. 47, 809–820 (2008).
- de León R, Chang DC, Busse C et al. First rib resection and scalenectomy for chronically occluded subclavian veins: what does it really do? Ann. Vasc. Surg. 22, 395–401 (2008).
- Illig KA. Management of central vein stenoses and occlusions: the critical importance of the costoclavicular junction. Semin. Vasc. Surg. 24, 113–118 (2011).
- Illig KA. An improved method of exposure for transaxillary first rib resection. J. Vasc. Surg. 52(1), 248–249 (2010).
▪ Broad review of Paget–Schroetter syndrome and its management as of 2010.
▪ Broad review by one of the experts in this field (venous thoracic outlet syndrome).
▪▪ One of the early seminal reviews of effort thrombosis.
▪▪ One of the first series describing thrombolysis as a treatment for effort thrombosis.
▪▪ Another early report of thrombolysis.
▪▪ First multiauthor comprehensive textbook covering thoracic outlet syndrome.