Image - Imaging in Medicine (2009) Volume 1, Issue 1
Contrast agent evaluation
Akihiro Tanimoto*Department of Diagnostic Radiology, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan
- Corresponding Author:
- Akihiro Tanimoto
Department of Diagnostic Radiology
Keio University School of Medicine
35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan
Tel: +81 3 3225 5715
Fax: +81 3 3225 5715
E-mail: t-mri@tt.rlm.or.jp
Abstract
Keywords
contrast enhancement ▪ gadoxetic acid ▪ Gd-EOB-DTPA ▪ hepatocellular carcinoma ▪ liver ▪ MRI
Epidemiology & etiology of hepatocellular carcinoma
Hepatocellular carcinoma (HCC), which accounts for 80–90% of primary liver cancers, is the fourth most common malignancy worldwide, although there are distinct differences in the prevalence in different continents. The scale of the problem is indicated by the fact that, in 2005, it has been estimated that there were 671,000 new cases throughout the world [1]. The American Cancer Society has proposed that 22,620 new cases of primary liver cancer and intrahepatic bile duct cancer will be diagnosed in the USA alone during 2009 [101]. The incidence of liver cancer in the USA has been gradually increasing in recent years [101]: in 2008, for instance, the estimate was that there would be 21,739 cases. HCC poses a particular healthcare problem in both Japan and Korea, where the incidence exceeds 25 cases/100,000 of the population per year [2].
The primary risk factor of HCC is liver cirrhosis. In Europe and the USA, cirrhosis is principally due to alcohol abuse [102]. By contrast, in China, Korea and Africa, the main cause is chronic hepatitis B virus infection. In Japan, hepatitis C virus infection is the primary causative factor. Hepatitis C virus is being increasingly implicated in the USA and Europe, where the infection frequently goes undiagnosed, thus allowing it to be transmitted to others. As a consequence, the scale of the problem is likely to escalate in the future. In the USA, ethnic differences are apparent and HCC occurs most frequently in people of Chinese origin [102]. Its prevalence has increased strikingly in recent years in this ethnic group, and this is largely attributed to an increase in the incidence of hepatitis viral infections [103]. The annual risk of developing HCC in subjects with cirrhosis is between 1 and 6% [101]. The predisposing factors for developing HCC in the cirrhotic subject are male gender, with approximate 2.5 cases in men to every one case in women [101], and older age: it being most commonly diagnosed between the ages of 50 and 60 years [3]. If not detected early, the prognosis is poor, and without removal of the malignancy, death usually occurs within 6 months of diagnosis. The incidence of mortality due to HCC is high [102]. The American Cancer Society predicts that there will be 18,160 deaths from liver cancer in 2009 in the USA [101]. The scale of the US problem, however, pales into insignificance in comparison to that experienced by many far eastern countries. In Korea and Japan, after stomach and lung cancer, HCC is the third most common cause of death due to cancer. An encouraging recent improvement in the incidence of mortality due to HCC in Korea and Japan may be partly attributed to aggressive vaccination programs against hepatitis B and C [2]. In addition, close monitoring of subjects with predisposing factors has proved highly beneficial.
Noninvasive identification of HCC
The development of HCC in the cirrhotic liver is a multistep process, progressing from low-grade dysplastic nodules, through to high-grade dysplastic nodules and early HCC, well-differentiated HCC and nodule-in-nodule HCC, and ultimately to undifferentiated HCC. Distinguishing between the non-malignant focal lesions and HCC is important. The invasive nature of biopsy carries inherent risks and may not prove effective in the detection of early, small malignant lesions. Regular noninvasive HCC surveillance is important in all atrisk patients for the detection of carcinogenetic changes and early identification of small-diameter cancerous lesions [3]. In 2003, the British Society of Gastroenterology recommended surveillance of cirrhotic patients every 6 months [4]. Similar frequencies were proposed by the European Association for the Study of the Liver in 2003 [5] and by the American Association for the Study of Liver Diseases in 2005 [6]. The recently published Japanese evidence-based guidelines, which are considered equally applicable to western countries, recommend that imaging is performed every 3–4 months in very high-risk individuals and every 6 months in high-risk subjects [7]. Detection of small focal liver lesions (<1 cm in diameter) is crucial to provide optimal prognosis and the early identification of potential candidates for liver transplantation. However, the underlying liver cirrhosis may complicate the detection of such lesions.
The major guidelines consistently recommend that imaging is initially performed using ultrasonography [5–7], but an important limitation in its performance is that it is operator dependent. In addition, the sensitivity of ultrasonography to detect lesions less than 2 cm in diameter in cirrhotic liver has been reported to be only approximately 30% [8]. The relatively recent development of microbubble contrast agents has improved the previously limited ability of ultrasonography to detect small focal liver lesions [9]. In patients with advanced liver cirrhosis or obesity, evaluation of dynamic lesion enhancement using contrast-enhanced computed tomography (CT) or MRI is regarded as being more effective, and the recent Japanese guidelines proposed that such techniques should be employed in further investigations to provide a differential diagnosis [7]. Because of the more invasive nature of CT arterio-portography and arteriography, and the high false-positive rates without a substantial increase in sensitivity [10], these imaging techniques have become largely superseded by contrast-enhanced spiral CT. The risk of repeated exposure to radiation using CT when the imaging technique is used for regular surveillance is overcome by the use of MRI [11]. Contrast-enhanced MRI using T1-weighted images is now acknowledged as providing superior diagnostic specificity and sensitivity, compared with CT, in the evaluation of carcinogenesis in the cirrhotic liver and the detection of HCC [12,13].
Dynamic MRI using gadolinium-based extracellular f luid contrast agents, such as gadobutrol, gadodiamide, gadolinium diethylaminepentaacetic acid (Gd-DTPA, also known as gadopentetate dimeglumine), gadobenate meglumine and gadoteridol, allows the detection of the hypervascular HCC lesions (which appear hyperintense during the arterial phase and hypointense during the portal-venous or delayed phase due to washout of the contrast medium). Thus, HCC may be distinguished from any nonhypervascular (i.e., iso- or hypovascular) benign lesions on the basis of the time course of enhancement. In addition to avoiding any potential risk of repeated radiation exposure, smaller volumes of contrast medium are required for MRI compared with contrast-enhanced CT, and adverse reactions are less frequent [14]. Dynamic contrast-enhanced MRI and contrastenhanced CT are principally useful for the diagnosis of hypervascular HCC displaying typical enhancement patterns [15]. However, it has been demonstrated that not all HCCs display typical arterial-phase enhancement and washout in the portal-venous phase; this can make diagnosis difficult [15].
The reticuloendothelial system-specif ic superparamagnetic iron oxide contrast agents, which include ferucarbotran and ferumoxide, allow the detection of nonhypervascular lesions that are difficult to see using dynamic contrast-enhanced MRI employing any of the extracellular gadolinium chelates [15]. However, owing to only weak (in the case of ferucarbotran) or the lack of (in the case of ferumoxide) dynamic-phase imaging, characterization of lesions is not always possible and distinguishing between well-differentiated HCC and dysplastic nodules is difficult [15].
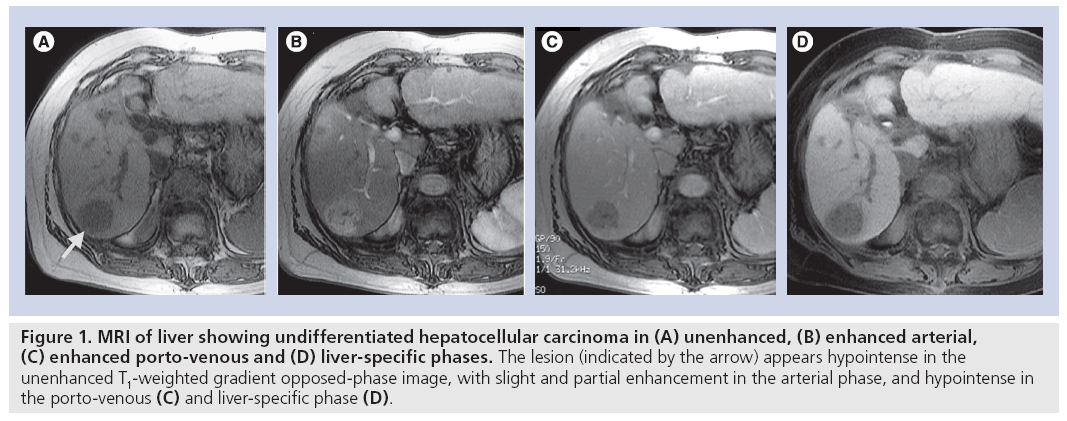
The use of a hepatocyte-specific contrast agent allows the differentiation of normal liver from hepatocyte-free lesions, the T1-weighted images of the former appearing hyperintense and of the latter hypointense. The hepatocytespecific agent mangafodipir trisodium, however, has only limited ability to differentiate between various focal liver lesions as it lacks the dynamic phase provided by conventional extracellular gadolinium-based contrast media and is more effective in the detection of colorectal liver metastases. The use of a hepatocyte-specific agent that allows both dynamic MRI plus delayed hepatobiliary-phase imaging has the potential to provide not only improved detection, but also characterization of the focal liver lesions. In addition, such an agent may allow the 3D mapping of the vasculature in relation to the lesion using a single procedure [15]. This provides valuable information for the surgeon on potential vascular complications. Gadoliniumethoxybenzyl- diethyleneaminepentaacetic acid (Gd-EOB-DTPA), also known as gadoxetic acid disodium (Primovist®, Bayer Schering Pharma AG, Berlin, Germany; Eovist®, Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ, USA; EOB Primovist®, Bayer Yakuhin, Osaka, Japan), is a liver-specific magnetic resonance contrast agent developed for combined T1-weighted dynamic and hepatocyte-specific MRI in a single examination. In common with extracellular gadolinium-based contrast agents, Gd-EOB-DTPA provides information on vascular distribution during the arterial phase (~25 s postinjection), portal-venous phase (~60–70 s postinjection) and equilibrium phase (~120 s postinjection) and provides additional information during the later (hepatocytespecific) phase (10–20 min postinjection). For optimal contrast enhancement in the dynamic phase, bolus-timing techniques and an injection rate of 1–2 ml/s should be used, followed by a saline flush of 20–30 ml. For dynamicphase imaging, T1-weighted 3D gradient echo sequences are recommended. T2-weighted imaging can be performed before or after Gd-EOB-DTPA injection to save examination time [16]. The absence of functional hepatocytes in HCC results in little or no uptake of Gd-EOB-DTPA in the hepatocyte-specific late phase; thus, undifferentiated HCC appears hypointense compared with the hyperintense normal liver parenchyma (Figure 1). Recent studies suggest a relationship between the differentiation grade of HCC and hepatocyte-specific uptake of Gd-EOB-DTPA. In a rat model, it was shown that two of 86 experimental tumors (HCC grade I–IV) appeared hyperintense using Gd-EOB-DTPA-enhanced MRI [17]. The two tumors that took up Gd-EOB-DTPA were highly differentiated (grade I) HCCs. This uptake may reasonably be attributed to residual hepatocyte function and impaired biliary excretion in grade I HCCs. No grade II–IV HCCs displayed Gd-EOB-DTPA uptake in this animal study [17]. In a retrospective evaluation of 30 patients with 32 HCCs, the lesion–liver contrast- to-noise ratios of well-differentiated HCCs (n = 7) were significantly higher compared with those of moderately (n = 20) and poorly differentiated HCCs (n = 5) [18]. It was concluded that Gd-EOB-DTPA-enhanced MRI may be helpful for distinguishing between well-differentiated and moderately to poorly differentiated HCC. Further research is needed.
Figure 1: MRI of liver showing undifferentiated hepatocellular carcinoma in (A) unenhanced, (B) enhanced arterial, (C) enhanced porto-venous and (D) liver-specific phases. The lesion (indicated by the arrow) appears hypointense in the unenhanced T1-weighted gradient opposed-phase image, with slight and partial enhancement in the arterial phase, and hypointense in the porto-venous (C) and liver-specific phase (D).
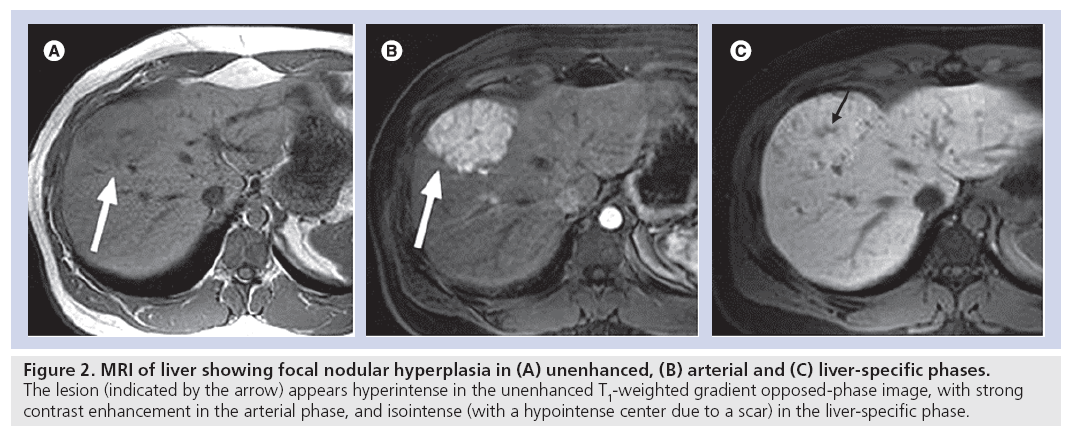
Benign liver lesions, such as focal nodular hyperplasia, also display a typical enhancement pattern in Gd-EOB-DTPA-enhanced MRI, in other words, iso- to hyperintensity compared with normal liver tissue in the hepatocyte-specific late phase [19]. The appearance of focal nodular hyperplasia in the arterial and hepatocyte-specific late phases is shown in Figure 2. Liver metastases always appear hypointense in the hepatocyte-specific late phase [20].
Figure 2: MRI of liver showing focal nodular hyperplasia in (A) unenhanced, (B) arterial and (C) liver-specific phases. The lesion (indicated by the arrow) appears hyperintense in the unenhanced T1‑weighted gradient opposed-phase image, with strong contrast enhancement in the arterial phase, and isointense (with a hypointense center due to a scar) in the liver-specific phase.
Chemistry & physical properties of Gd-EOB-DTPA
Gd-EOB-DTPA is derived from Gd-DTPA, the additional presence of the lipophilic EOB moiety facilitating specific uptake by hepatocytes and, hence, liver-specific contrast enhancement. Gd-EOB-DTPA is a low-molecular-weight, hydrophilic gadolinium chelate (Figure 3) that is supplied as a clear, ready-to-use solution at a concentration of 0.25 mmol/ml [21]. The solution, which is highly stable in vitro and in vivo, exhibits low viscosity and osmolality and shortens the T1-relaxation time, leading to an increase in signal intensity [22]. In plasma at 37°C, r1 relaxivity is 7.41 l mmol-1 s-1 at 1.5 T [22]. Another study comparing r1 relaxivities in plasma at 1.5 T reported that for Gd-EOBDTPA it was 6.9 l mmol-1s-1, which was higher than that of extracellular agents such as gadobenate dimeglumine (6.3 l mmol-1s-1) and Gd-DTPA (4.1 l mmol-1s-1) [23]. T1 relaxivity of Gd-EOB-DTPA in hepatocytes is even higher, with enhancement in normal liver tissue lasting at least 2 h [24]. Furthermore, compared with both gadobenate dimeglumine and Gd-DTPA, Gd-EOB-DTPA is more stable in human serum (pH 7.4) at 37°C with less gadolinium release [25].
Pharmacokinetics/pharmacodynamics of Gd-EOB-DTPA
Following intravenous bolus injection, Gd-EOB-DTPA is rapidly distributed throughout the bloodstream and extracellular fluid. The EOB moiety promotes the subsequent uptake of Gd-EOB-DTPA into normal hepatocytes by an organic anion-transporting polypeptide (OAPT) on the cell membrane [26]. Gd-EOBDTPA is not metabolized and is completely eliminated from the body within 24 h in almost equal quantities via the kidneys in urine (43.1–53.2%) and via the biliary system in feces (41.6–51.2%) [27].
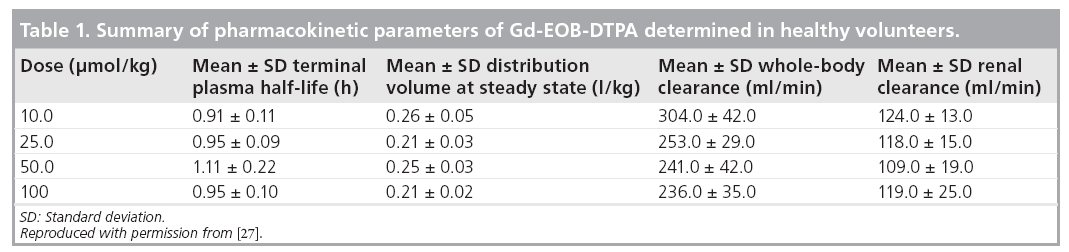
Based on observations in 44 healthy volunteers, serum AUC and maximum serum concentration (Cmax) when Gd-EOB-DTPA was administered at doses in the range of 10 to 100 μmol/kg bodyweight are dose dependent, but the plasma elimination half-life (T1/2) of 60 min is dose independent (Table 1); thus, the hepatocellular uptake of Gd-EOB-DTPA is not saturable in this dose range [27]. Renal Gd-EOBDTPA clearance was found to be virtually identical (Table 1) to the glomerular filtration rate of 120 ml/min observed in healthy humans, suggesting no tubular secretion or reabsorption by the kidneys [27]. This Phase I study also showed that the homogeneous enhancement of liver parenchyma started immediately after intravenous injection of Gd-EOB-DTPA and liver signal intensity became maximal after 20 min, and thereafter plateaued for approximately 2 h [24,27]. Another study of the pharmacokinetics and pharmacodynamics of Gd-EOB-DTPA in human volunteers with varying degrees of hepatic or renal impairment demonstrated that the intact excretory route compensates for the impairment of the other organ [Bayer Schering Pharma, Data on File].
Clinical efficacy of Gd-EOB-DTPA
Extensive studies in rats and dogs have demonstrated the potential role of Gd-EOB-DTPA in contrast-enhanced MRI of the liver [28–38].
Phase II studies
Phase II studies were conducted in adult patients with known solid liver lesions using Gd-EOBDTPA at intravenous bolus doses of 12.5, 25.0 and 50.0 μmol/kg bodyweight (corresponding to injection volumes of 3.5, 7.0 and 14 ml in a 70-kg subject) [24,39–43]. In a double-blind study, enhancement characteristics of focal liver lesions in 31 patients (liver metastases [n = 23], HCC [n = 4] and hemangioma [n = 4]) were evaluated using a breath-hold, T1-weighted, fast low-angle (FLASH) pulse sequence at a field strength of 1 T with and without fat saturation [40]. The T2-weighted sequences were acquired either before or after the administration of Gd-EOBDPTA. No influences on lesion–tumor contrast- to-noise ratio and lesion conspicuity were detected [16]. Malignant tumors devoid of hepatocytes, such as metastases and undifferentiated HCCs, appeared dark against the bright background of the liver in the hepatocyte-specific late phase. Tumors with hepatocellular elements (e.g., highly differentiated HCC) displayed uptake of Gd-EOB-DTPA and increased signal intensity in the hepatocyte-specific late phase. Lesions with a relevant blood pool (e.g., hemangiomas) displayed enhancement, which persisted for less than 10 min and appeared hypointense in the hepatocyte-specific late phase. Furthermore, Gd-EOB-DTPA was shown to significantly increase liver signal intensity and the lesion– liver contrast-to-noise ratio with improved lesion detection 20 and 45 min after Gd-EOB-DTPA administration [40].
Comparison of Gd-EOB-DTPA with the superparamagnetic iron oxide ferucarbotran in a total of 66 patients showed that a bolus injection of Gd-EOB-DTPA allowed not only hepatocyte enhancement, but also the detection of the earlier dynamic enhancement patterns [41]. This additional feature of Gd-EOBDTPA facilitated tumor characterization, and the study confirmed that, compared with other T1-weighted techniques with or without fat saturation, a breath-hold FLASH pulse sequence provided superior images.
The diagnostic capabilities of Gd-EOBDTPA- enhanced MRI were directly compared with Gd-DTPA in another study of 31 patients with focal liver lesions, including 12 with HCC, undergoing T2- and T1-weighted spin-echo imaging and FLASH 2D imaging at 1.5 T [24]. Similar dynamic enhancement was observed using either Gd-EOB-DTPA (25 μmol/kg) or Gd-DTPA (100 μmol/kg) with a saline flush of 20 ml. During the hepatobiliary phase, Gd-EOB-DTPA significantly improved the detection of focal liver lesions compared with unenhanced or Gd-DTPA-enhanced images obtained 10 min postinjection.
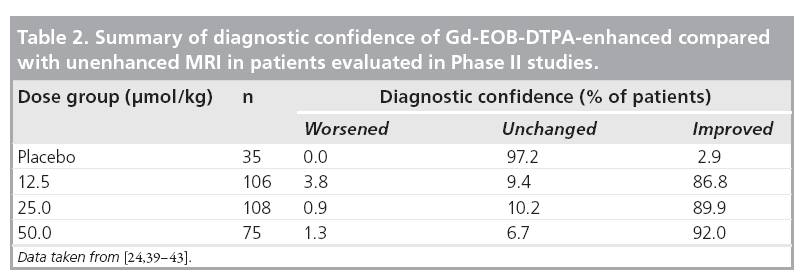
The combined data from Phase II studies showed that, in comparison with unenhanced MRI, the diagnostic confidence was improved by use of Gd-EOB-DTPA (Table 2) [24,39–43]. At a dose of 25 μmol/kg bodyweight, investigators showed an improvement in diagnostic confidence for 89.9% of patients. Although diagnostic confidence is a subjective measure provided by the investigating radiologist, it is relevant to routine clinical practice when determining appropriate therapy for the patient.
Phase III studies
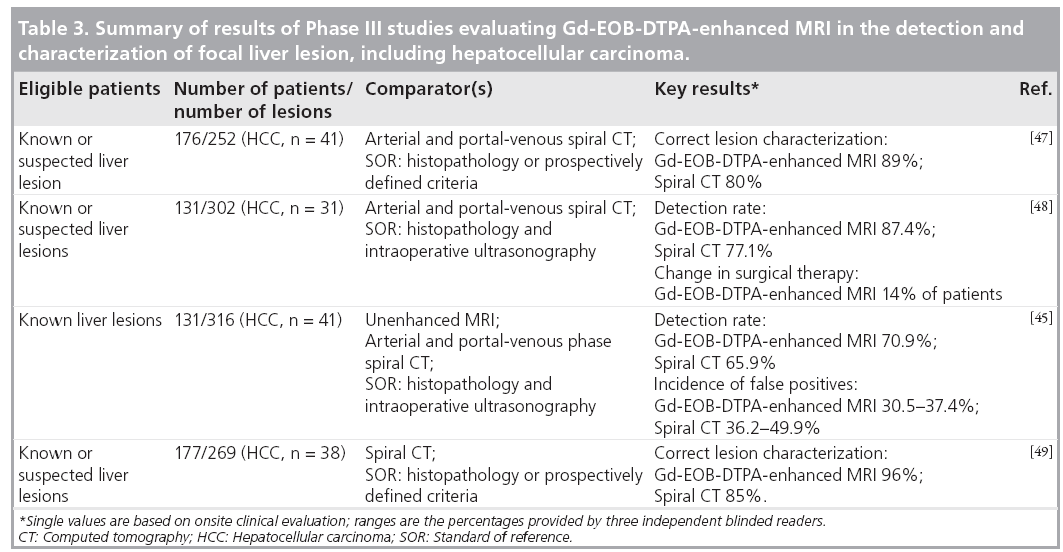
Based on the Phase II dose-ranging studies, in Phase III studies, Gd-EOB-DTPA solution (concentration 0.25 mmol/ml) was administered as an intravenous bolus injection at a flow rate of 2 ml/s and a dose of 0.1 ml/kg bodyweight (equivalent to 25 μmol/kg bodyweight). A subsequent saline flush was used: the volume was sufficient to clear the intravenous line of all contrast agent and was typically 20–30 ml. Patients with known or suspected focal liver lesions that included HCC were evaluated in four Phase III studies conducted in Europe and the USA [19,44–49]. The focus was either on the detection or characterization of focal liver lesions. The principal findings are reported in Table 3. An analysis of the subgroup of patients in these trials with focal nodular hyperplasia has also been reported [19]. The relatively high incidence of liver metastases as opposed to primary liver cancer in patients living in the USA and Europe is reflected in the relatively small numbers of patients with HCC who were evaluated during these studies. However, in the recently conducted Japanese Phase III trial on the detection and characterization of focal liver lesions, the majority of the patient population were suffering from HCC [Bayer Schering Pharma, Data on File].
In all Phase III studies, MRI was performed using high-field-strength (1.0 or 1.5 T) machines, spoiled gradient-recalled echo sequences and dedicated phased array coils. Unenhanced T1-weighted imaging plus T2-weighted fast-spin echo, turbo spin-echo or fat-suppressed half- Fourier single-shot turbo spin-echo sequence imaging was performed first. After the injection of Gd-EOB-DTPA, T1-weighted images were obtained in the dynamic arterial (10–20 s postinjection), portal-venous (50–60 s postinjection) and late (~120 s postinjection) phases, and in the hepatocyte-specific phase (20 min postinjection), using breath-hold acquisition. Spiral CT scans were performed within 6 weeks of the MRI procedure in the same patients. A nonionic iodinated contrast medium (100–200 ml) was used that was injected intravenously at a rate of 3–5 ml/s. Arterial (25–35 s postinjection) and portal-venous (45–70 s postinjection) images were obtained. As well as clinical onsite evaluation of images, all images were reviewed by independent blinded readers with no knowledge of each patient’s diagnosis. Histopathological confirmation of the diagnosis was obtained in all the detection studies and for most of the lesions in the characterization studies.
The findings of a multicenter European study conducted in 176 patients with a total of 104 malignant and 148 benign lesions confirmed that Gd-EOB-DTPA-enhanced MRI, when combined with data from unenhanced MRI, provides reliable classification (benign/malignant) and characterization (lesion type diagnosis) of focal liver lesions [47]. For all three blinded readers, the numbers of correctly classified lesions was lower with spiral CT than with Gd-EOB-DTPAenhanced MRI, and there were fewer false-positive identifications with the contrast-enhanced MRI. More lesions were correctly characterized using Gd-EOB-DTPA; the superiority of MRI compared with CT was statistically significant in the onsite clinical evaluation (p = 0.0018) and for two of the three readers in the blinded offsite evaluation (p = 0.025 and 0.014). The readers’ confidence in lesion characterization, based on a five-point scale, was greater with MRI than with CT. The percentage of lesions with highconfidence ratings was higher, and the percentage of lesions with low-confidence ratings was lower using MRI.
A similar trend in favor of Gd-EOB-DTPAenhanced MRI was observed in a second European study, which focused on lesion detection [48]. There were 131 patients with a total of 302 liver lesions eligible for surgery. Liver specimens were pathologically evaluated, and intraoperative ultrasonography was performed to confirm the diagnosis. The use of Gd-EOBDTPA enhancement resulted in a 10.44% greater frequency in the correct detection of lesions. On the basis of the information provided by the Gd-EOB-DTPA-enhanced images, there was a change in surgical therapy in 14.5% of the patients. The number of correctly detected and characterized lesions using Gd-EOB-DTPAenhanced MRI was 82.1%, as opposed to 71.0% using CT. In this study, it was especially shown that Gd-EOB-DTPA-enhanced MRI was superior to spiral CT in the correct detection of small lesions (<1 cm in diameter).
One US multicenter study on lesion detection identified a total of 316 lesions [45]. Enhanced MRI with Gd-EOB-DTPA combined with precontrast MRI detected more lesions than unenhanced MRI – the higher detection rate using Gd-EOB-DTPA-enhanced MRI for the blinded readings provided by two of the three offsite readers was statistically significant (p = 0.027 and 0.012). Although the sensitivity of combined pre- and postcontrast MRI was comparable to that of spiral CT, Gd-EOB-DTPA-enhanced MRI resulted in fewer false-positive lesions being identified by the blinded readers.
The second US study concentrated on evaluating the efficacy of Gd-EOB-DTPA-enhanced MRI in comparison with unenhanced MRI or contrast-enhanced spiral CT in the characterization of focal liver lesions [49]. The proportion of lesions (96%) correctly characterized using a combination of unenhanced and Gd-EOBDTPA- enhanced MRI in the clinical evaluation was significantly higher (p < 0.0008) than with either unenhanced MRI (84%) or contrast- enhanced spiral CT (85%). In the blinded evaluation carried out by three readers, the percentage of lesions correctly characterized using combined unenhanced and contrast-enhanced MRI was consistently higher (61–76%) compared with unenhanced MRI (48–65%); for two of the three readers this difference was statistically significant (p < 0.012). Lesions were correctly characterized by the blinded readers in 62–77% of cases using combined MRI and in 57–76% using enhanced spiral CT; the differences between the two imaging techniques were not statistically significant.
The findings of the yet to be published Japanese Phase III study performed predominantly in patients with HCC confirmed the diagnostic ability of Gd-EOB-DTPA-enhanced MRI to detect and characterize focal liver lesions [ichikawa t et al. (2009), submitted].
Phase IV studies
Multidetector CT (MDCT) is the most recent advance in CT imaging technology, providing improved lesion detection of both benign and malignant abdominal tumors, including focal liver lesions compared with spiral CT [50]. Three Phase IV studies have compared the efficacy of Gd-EOB-DTPA-enhanced MRI with MDCT for the detection of HCC [51–53].
In a study conducted in Korea, 62 patients with 83 HCCs were imaged no more than 5 days before resection. Arterial, portal-venous and equilibrium phase images were obtained using 64-slice MDCT and Gd-EOB-DTPA-enhanced MRI at 3 T and were assessed by three readers [51]. The diagnostic accuracy was evaluated using alternative free-response receiver operating characteristic (ROC) analysis, with results being expressed as the areas under the ROC curve. For the three readers, area under the ROC curves were in the range of 0.959 to 0.971 for contrastenhanced MRI and 0.943–0.950 for MDCT. All readers detected more lesions less than 1 cm in diameter using Gd-EOB-DTPA-enhanced MRI than with MDCT, although the numbers were too small to establish statistical significance.
An Italian study of 37 patients with 67 HCC nodules found that the area under the ROC curve was significantly higher with Gd-EOBDTPA- enhanced MRI than with MDCT (0.88 vs 0.77; p < 0.05) [52]. In addition, sensitivity of Gd-EOB-DTPA-enhanced MRI was superior (81.1 vs 65.5%; p < 0.05), as was the specificity (94.7 vs 84.2%; p < 0.05).
Another Italian study of 110 cirrhotic patients with 185 HCC lesions also showed that the overall sensitivity of Gd-EOB-DTPA-enhanced MRI was superior to that of three-phase MDCT (96 vs 84%; p = 0.009) [53]. The sensitivities of the two imaging techniques to detect the main HCC were similar, but Gd-EOB-DTPA-enhanced MRI was superior to MDCT in the detection of small secondary HCC nodules: sensitivities were 95 and 65%, respectively (p < 0.005).
A recently reported study compared the vascular enhancement achievable with Gd-EOBDTPA (25 μmol/kg bodyweight) or with Gd-DTPA (100 μmol/kg bodyweight) using a 3D gradient echo sequence in a pig model [54]. Despite the gadolinium dose for Gd-EOB-DTPA being one-quarter of that for Gd-DTPA, dynamic arterial enhancement was comparable for the two contrast agents at optimized injection flow rates.
The feasibility of using a lower injection rate of 1 ml/s, as opposed to the rate of 2 ml/s used in Phase III studies, has been investigated in this study. The slower injection rate of 1 ml/s for Gd-EOB-DTPA provided better arterial enhancement than 2 ml/s [54]. The maximal signal-to-noise ratio for Gd-EOB-DTPA (0.025 mmol/kg bodyweight) at a flow rate of 1 ml/s was 25.21, which was comparable to a ratio of 25.37 for Gd-DTPA (0.1 mmol/kg bodyweight) at a flow rate of 2 ml/s. This may be explained by the broader peak enhancement of the bolus, resulting in broader peak. The lower injection rate, however, had no effect on portal vein or liver parenchyma enhancement.
The feasibility of using respiratory triggered T1-weighted MRI, as opposed to the breathhold images obtained in the Phase III studies, to provide high-spatial-resolution images using Gd-EOB-DTPA has been recently examined [55].
High-resolution images are crucial in the identification of very small liver lesions and establishing the pathological changes in the small branches of the biliary tract. An inversion recovery-prepared spoiled gradient echo sequence with respiratory triggering was evaluated qualitatively using a fivepoint scale and quantitatively by the determination of signal intensities of lesion versus liver parenchyma, contour sharpness index of the biliary tract and the signal-to-noise ratio. The imaging, which was performed in the hepatobiliary phase (starting 10 min after Gd-EOB-DTPA injection), was carried out in 20 patients with a total of 41 focal liver lesions. The signal-to-noise ratio was determined in a volunteer to avoid the examination time having to be doubled in a patient. The images obtained using respiratory triggering were compared with axial and coronal breath-hold spoiled gradient echo sequence images. The provision of high-spatial-resolution 3D images proved technically feasible using the respiratory-triggered sequence. Liver–lesion contrast, contour sharpness index and score for the depiction of focal liver lesions were all significantly higher using respiratory triggering, and there was no increase in the incidence of imaging artifacts. However, superior contrast between the common bile duct and the liver parenchyma was achieved using the coronal breath-hold gradient echo sequence.
The possibility of reducing the total examination time using Gd-EOB-DTPA was examined in 265 Japanese patients who had a total of 495 malignant liver lesions [56]. In Phase III studies, the hepatocyte-specific phase images were typically obtained 20 min postinjection and this is the currently recommended examination process. The 20-min images were compared in this Phase IV study with those acquired only 10 min after Gd-EOB-DTPA administration using a four-point scale to quantify liver enhancement, and the visual liver–spleen contrast ratio was evaluated. In addition, a quantitative liver–spleen contrast ratio was determined. Two radiologists also assessed the sensitivity of lesion detection. The authors concluded that, in 61% of their patient population, it was feasible to reduce the total examination time by omitting 20-min imaging. The selective use of shorter imaging times, especially in noncirrhotic livers, could result in lower costs and greater throughput in high-demand MRI facilities.
Safety of Gd-EOB-DTPA
The safe pharmacological and toxicological profile of Gd-EOB-DTPA has been demonstrated in animal models [28,57–59]. Preclinical studies provided no evidence of cardiovascular intolerance, with the absence of any effects on arterial blood pressure, contractility, heart rate, left ventricular diastolic pressure, central venous pressure or cardiac output. No effects on ECG were detected in conscious dogs [28]. Other animal studies did not identify any significant effects on erythrocytes, platelets and other blood cells [Bayer Schering Pharma, Data on File]. A slight increase in the bleeding time was observed in rats receiving 20 times the recommended diagnostic dose of Gd-EOB-DTPA. Preclinical studies also established that the safety margin for biochemical effects, such as histamine release, complement activation, hemolysis and lysozyme inhibition, was high. There was also no evidence of in vivo interaction of Gd-EOB-DTPA with prednisolone, doxorubicin, cisplatin, propranolol, scopolamine, theophylline, ampicillin, cefotaxime, verapamil or diazepam. Hepatic uptake of Gd-EOB-DTPA was competetively inhibited by rifamycin, and a significant decrease in enhancement was observed. In a rat model of severe renal impairment, Gd-EOB-DTPA was still rapidly eliminated owing to full compensation by the alternative, normally functioning hepatic elimination pathway [60]. Similarly, in rats with experimentally induced hepatic impairment, Gd-EOB-DTPA was effectively eliminated in urine [60].
Phase I evaluation confirmed the preclinical findings, with few adverse events occurring in the 72 h, which corresponds to approximately 48 half-lifes, after bolus intravenous injection of 10–100 μmol/kg Gd-EOB-DTPA [27]. A total of five definite drug-related transient events (injection site pain, parosmia, taste perversion, paresthesia and nausea) occurred in one Phase I study of 44 volunteers receiving receiving Gd-EOB-DTPA 10–100 μmol/kg bodyweight; except for pain at the injection site following administration of 10 μmol/kg, the other adverse events occurred with the 100 μmol/kg dose [27]. Gd-EOB-DTPA was found to have no relevant or clinically significant effects on urinary and hepatic parameters in healthy volunteers in comparison with placebo [27].
Gd-EOB-DTPA has proved safe and well tolerated in Phase II and III studies [19,24,39–49,61]. During Phase II and III studies in which 580 and 1175 patients, respectively, were evaluated, a total of 76 patients experienced one or more adverse events (all of mild-to-moderate severity) that were considered possibly, probably or definitely related to Gd-EOB-DTPA. The most common drug-related events were (incidence) feeling hot (0.7%), nausea (0.7%), headache (0.6%) and taste perversion (0.3%). There were no serious adverse events (i.e., ones that resulted in death, or were life-threatening, required inpatient hospitalization or prolongation of existing hospitalization, or resulted in persistent or significant disability/incapacity) related to Gd-EOB-DTPA.
A Phase III study confirmed the rat model, which showed that impairment of hepatic or renal function is compensated [60], and demonstrated that there is no increase in the incidence of adverse drug reactions in patients with mild, moderate or severe hepatic impairment [61]. Some gadolinium-based contrast agents for use in MRI have been associated with nephrogenic systemic fibrosis in patients with severe acute or chronic renal impairment or acute renal insufficiency due to hepatorenal syndrome or during the perioperative liver transplantation period [62]. Although there are no reports of nephrogenic systemic fibrosis related to the administration of Gd-EOB-DTPA, as a precautionary measure, all patients should be screened for renal dysfunction; in particular, for patients older than 65 years, medical history and laboratory testing should be performed. Careful risk–benefit assessment should be conducted in patients with severe renal impairment or acute renal insufficiency, and Gd-EOB-DTPA should only be administered in these patients if diagnostic information is essential and cannot be provided by unenhanced MRI. If use of Gd-EOB-DTPA is considered crucial in a patient with severe renal impairment, hemodialysis shortly after imaging may be useful to remove Gd-EOB-DTPA.
ECGs revealed transient QT prolongation in some patients [Bayer Schering Pharma, Data on File], but there were no associated clinical adverse events. Caution should be exercised in patients with severe cardiovascular problems, especially those with a known or family history of congenital long QT syndrome, known previous arrhythmias when in receipt of a drug that prolongs cardiac repolarization or if currently being treated with a drug that prolongs cardiac repolarization, such as a class III anti-arrhythmic agent.
Gd-EOB-DTPA had no clinically significant effect on the energy status and function of hepatocytes. No changes in creatinine clearance were detected during Phase I. Urine chemistry remained stable in patients evaluated in Phase III studies, including those with impaired renal or hepatic function. There were no clinically relevant changes in hematology and clotting status associated with Gd-EOB-DTPA during the clinical trials.
Postmarketing spontaneous reporting has identified isolated incidences of restlessness and tachycardia. Allergy-like reactions, including shock, are rare events associated with administration of gadolinium-based contrast agents. The risk of hypersensitivity is increased in a patient with a prior reaction to a contrast agent or with a history of bronchial asthma or allergic disorders. Hypersensitivity can be more intense in patients being treated with β-blockers. It should be noted that such patients may not respond to the standard therapy for hypersensitivity reactions of β-agonists.
Regulatory affairs
The ready-to-use solution of Gd-EOB-DTPA is supplied in a 10-ml prefilled syringe; the solution of Gd-EOB-DPTA should be administered undiluted as an intravenous bolus injection. The product was first launched in Sweden in 2004. Between then and the end of 2007, Gd-EOBDTPA became available in other European countries, Australia, Indonesia, Korea, Malaysia, Philippines, Thailand, Singapore and Japan. The summer of 2008 saw the approval of Gd-EOBDTPA by the US FDA for intravenous use in T1-weighted MRI of the liver to detect and characterize lesions in adults with known or suspected focal liver disease. In total, Gd-EOB-DTPA is now approved in more than 40 countries.
Future perspective
Contrast-enhanced magnetic resonance cholangiography (MRC) may provide a noninvasive approach to the evaluation of biliary morphology and function in patients with signs of biliary disease. If used before endoscopic retrograde cholangiopancreatography, the more common complications associated with the procedure, namely pancreatitis and sepsis, may be reduced. T2-weighted MRC is not always conclusive because of limited spatial resolution, and it is highly prone to movement artifacts. Furthermore, MRC does not provide information on hepatobiliary function. The hepatobiliary excretion of Gd-EOB-DTPA suggests that it may provide a promising alternative approach to the evaluation of hepatobiliary function. In 10 healthy volunteers, Gd-EOB-DTPA given at the recommended dose of 25 μmol/kg bodyweight yielded earlier onset of contrast between the common hepatic duct and liver parenchyma that lasted for longer than that produced by gadobenate dimeglumine at a dose of 100 μmol/kg bodyweight [63]. Gadobenate dimeglumine differs from Gd-EOB-DTPA in that only 3–5% of the injected dose is excreted via the bile, as opposed to 50% of the Gd-EOB-DTPA injected dose. In the case of Gd-EOB-DTPA, biliary enhancement was clearly apparent after 10 min. The enhancement achieved with Gd-EOB-DTPA at 20 min was comparable to that observed with gadobenate dimeglumine at 40 min after administration. Thereafter (130–300 min postinjection), the contrast between the common hepatic duct and liver parenchyma was significantly superior (p < 0.002) using Gd-EOB-DTPA. The functional information from the assessment of biliary dynamic enhancement demonstrates the benefit of Gd-EOB-DTPA in examining hepatobiliary excretion by allowing a more flexible time window for imaging. This should facilitate scheduling of imaging in an MRI unit.
The occasional paradoxical uptake of Gd-EOB-DTPA during the hepatobiliary phase in some HCC lesions, making them appear isoor hyperintense compared with liver parenchyma as opposed to the typical hypointense appearance, may potentially be used to predict the efficacy of anticancer drugs. Gd-EOB-DTPA uptake is mediated by OATPs located on the surface of hepatocytes [27]. In a retrospective analysis of 22 patients who had undergone Gd-EOBDTPA- enhanced MRI prior to surgery, the extent of enhancement (expressed as an enhancement ratio) was determined and the expression of OATP1B3 measured [64]. The analysis showed that OATP1B3 levels correlated with enhancement ratios (r = 0.91; p < 0.0001). In addition to the ability of OATP1B3 to transport Gd-EOBDTPA, this cell-membrane polypeptide is able to transport anticancer drugs such as methotrexate, paclitaxel and docetaxel [65,66].
An initial study in healthy volunteers has demonstrated the possibility of using Gd-EOBDTPA- enhanced MRI to assess liver function by the measurement of hepatic extraction fraction and input relative blood flow, as markers of parenchymal function [67]. Hepatic extraction fraction and input relative blood flow were calculated for each liver segment in this preliminary study. The generation of liver maps demonstrating segmental liver function capacity may provide important information, especially for presurgical evaluation to reduce the incidence of postoperative liver failure.
The use of Gd-EOB-DTPA-enhanced MRI may be more cost effective than other imaging techniques for patient work-up when deciding on the most appropriate course of treatment. Taking into account all diagnostic work-ups, intraoperative treatment changes and unnecessary surgery in the management of patients with suspected colorectal metastases, data amassed from a health-economic evaluation for three European countries (Germany, Italy and Sweden) showed that Gd-EOB-DTPA-enhanced MRI was more cost effective than MRI using extracellular gadolinium-based contrast agents [68]. This was due to greater diagnostic accuracy of Gd-EOB-DTPA-enhanced MRI and avoiding the need for additional imaging techniques and surgical changes. This evaluation also demonstrated that, overall, Gd-EOB-DTPA-enhanced MRI was no more expensive than three-phase MDCT. The same may hold true for the different imaging techniques when used for the detection and management of HCC. Future clinical trials in patients with HCC should be designed to include health-economic data.
Conclusion
The hepatocyte-specific MRI contrast medium Gd-EOB-DTPA combines the features of the well-established extracellular gadoliniumcontaining contrast media, which only allow the visualization during the dynamic arterial, portal-venous and equilibrium phases of distribution, together with its ability to be taken up subsequently by hepatocytes. This facilitates distinguishing between normal liver parenchyma and focal liver lesions of hepatocyte and nonhepatocyte origin. Clinical trials have consistently shown that the combined features of Gd-EOB-DTPA improve the detection of HCC, including tumors less than 1 cm in diameter, using T1-weighted imaging. The total imaging time that includes T1-weighted unenhanced imaging and T2-weighted imaging (performed either before or after the administration of GD-EOBDTPA) is approximately 30 min. There is the potential to reduce the overall examination time by 10 min in a high proportion of patients. Gd-EOB-DTPA also improves the characterization of focal liver lesions and is able to differentiate between benign lesions and the presence of malignancies in the cirrhotic liver. The diagnostic capability of Gd-EOB-DTPA-enhanced T1-weighted MRI when performed in conjunction with unenhanced MRI in a single procedure allows the early detection of HCC in highrisk patients, such as those with alcohol-related cirrhosis or chronic hepatitis B and/or C viral infections. Early detection of the continuum of carcinogenetic changes increases the therapeutic options and maximizes the prognosis. Gd-EOBDTPA is well-tolerated, with a similar safety profile to those of other gadolinium chelates, and only minor adverse events being observed in clinical trials. Postmarketing surveillance confirms the good safety profile of Gd-EOB-DTPA. Its unique pharmacokinetic/pharmacodynamic features suggest that the use of Gd-EOB-DTPA may not be restricted in the future to the classification and characterization of focal liver lesions; Gd-EOB-DTPA may also play a role in the evaluation of biliary disease and the assessment of hepatic function.
Financial & competing interests disclosure
This work was supported by Bayer Schering Pharma AG. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing assistance was utilized in the production of this manuscript. Medical writing support was given by Parexel.

References
- Motola-Kuba D, Zamora-Valdes D, Uribe M, Mendez-Sanchez N: Hepatocellular carcinoma. An overview. Ann. Hepatol. 5, 16–24 (2006)
- Kim SR, Kudo M, Hino O, Han KH, Chung YH, Lee HS; For the Organizing Committee of Japan–Korea Liver Symposium (JKLS): Epidemiology of hepatocellular carcinoma in Japan and Korea; a review. Oncology 75(Suppl. 1), 13–16 (2008)
- Russo MW, Jacobson IM: Hepatocellular cancer: screening, surveillance, and prevention. In: Gastrointestinal Oncology: Principles and Practices. Kelsen DP, Daly JM, Kern SE et al. (Eds). Lippincott, Williams and Wilkins, PA, USA 559–568 (2002)
- Ryder SD: Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 52(Suppl. III), iii1–iii8 (2003)
- Bruix J, Sherman M, Llovet JM et al.: Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J. Hepatol. 35, 421–430 (2003).
- Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases: Management of hepatocellular carcinoma. Hepatology 42, 1208–1236 (2005).
- Kudo M, Okanoue T; Japan Society of Hepatology: Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology 72(Suppl. 1), 2–15 (2007).
- Kim CK, Lim JH, Lee WJ: Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: accuracy of ultrasonography in transplant patients. J. Ultrasound Med. 20, 99–104 (2001)
- Jang HJ, Kim TK, Wilson SR: Imaging of malignant liver masses: characterization and detection. Ultrasound Q. 22, 19–29 (2006).
- Jang HJ, Lim JH, Lee SJ, Park CK, Park HS, Do YS: Hepatocellular carcinoma: are combined CT during arterial portography and CT hepatic arteriography in addition to triple-phase helical CT all necessary for preoperative evaluation? Radiology 215, 373–380 (2000).
- Semelka RC, Armao DM, Elias J Jr, Huda W: Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J. Magn. Reson. Imaging 25, 900–909 (2007).
- Martin DR, Danrad R, Hussain SM: MR imaging of the liver. Radiol. Clin. N. Am. 43, 861–886 (2005).
- Zech CJ, Reiser MF, Herrmann KA: Imaging of hepatocellular carcinoma by computed tomography and magnetic resonance imaging: state of the art. Dig. Dis. 27, 114–124 (2009).
- Kanematsu M, Kondo H, Goshima S, Tsuge Y, Watanabe H: Magnetic resonance imaging of hepatocellular carcinoma. Oncology 75(Suppl. 1), 65–71 (2008)
- Kim MJ, Choi JY, Chung YE, Choi SY: Magnetic resonance imaging of hepatocellular carcinoma using contrast media. Oncology 75(Suppl. 1), 72–82 (2008).
- Hirohashi S, Marugami N, Kitano S et al.: The effect of Gd-EOB-DTPA on T2-weighted images in the diagnosis of malignant tumours [abstract no. 1422]. Proc. Int. Soc. Magn. Reson. Med. 11, 286 (2003).
- Ni Y, Marchal G, Yu J, Mühler A, Lukito G, Baert AL: Prolonged positive contrast enhancement with Gd-EOB-DTPA in experimental liver tumors: potential value in tissue characterization. J. Magn. Reson. Imaging 4, 355–363 (1994).
- Hong H-S, Choi J-Y, Kim M-J, Lim JS, Yoo H, Kim KW: Gadoxetic acid-enhanced hepatobiliary phase MR imaging of hepatocellular carcinoma (HCC): correlation with histological grade. Proceeding of the 94th Scientific Assembly and Annual Meeting of the Radiological Society of North America, Chicago, USA, 30 November–5 December 2008 (Abstract E450A).
- Zech CJ, Grazioli L, Breuer J, Reiser MF, Schoenberg SO: Diagnostic performance and description of morphological features of focal nodular hyperplasia in Gd-EOB-DTPAenhanced liver magnetic resonance imaging: results of a multicenter trial. Invest. Radiol. 43, 504–511 (2008).
- Zech CJ, Herrmann KA, Reiser MF, Schoenberg SO: MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOBDTPA. Magn. Reson. Med. Sci. 6, 43–52 (2007).
- Reimer P, Schneider G, Schima G: Hepatobiliary contrast agents for contrastenhanced MRI of the liver: properties, clinical development and applications. Eur. Radiol. 14, 559–578 (2004).
- Vander Elst L, Maton F, Laurent S, Seghi F, Muller RN: Multinuclear MR characterization of a new hepatobiliary contrast agent. Preliminary results. Acta Radiol. Suppl. 412, 135–138 (1997).
- Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ: Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest. Radiol. 40, 715–724 (2005).
- Vogl TJ, Kümmel S, Hammerstingl R et al.: Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology 200, 59–67 (1996).
- Frenzel T, Lengsfeld P, Schirmer H, Hütter J, Weinmann HJ: Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37°C. Invest. Radiol. 43, 817–828 (2008).
- van Montfoort JE, Stieger B, Meijer DK, Weinmann HJ, Meier PJ, Fattinger KE: Hepatic uptake of the magnetic resonance imaging contrast agent gadoxetate by the organic anion transporting polypeptide OATP1. J. Pharmacol. Exp. Ther. 290, 153–157 (1999).
- Hamm B, Staks T, Mühler A et al.: Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology 195, 785–792 (1995).
- Benness G, Khangure M, Morris I et al.: Hepatic kinetics and magnetic resonance imaging of gadolinium-EOB-DTPA in dogs. Invest. Radiol. 31, 211–217 (1996).
- Weinmann HJ, Schuhmann-Giampieri G, Schmitt-Willich H, Vogler H, Frenzel T, Gries H: A new lipophilic gadolinium chelate as a tissue-specific contrast medium for MRI. Magn. Reson. Med. 22, 233–237 (1991).
- Clément O, Mühler A, Vexler V, Berthezene Y, Brasch RC: Gadoliniumethoxybenzyl- DTPA, a new liver-specific magnetic resonance contrast agent. Kinetic and enhancement patterns in normal and cholestatic rats. Invest. Radiol. 27, 612–619 (1992).
- Schuhmann-Giampieri G, Schmitt-Willich H, Press WR, Negishi C, Weinmann HJ, Speck U: Preclinical evaluation of Gd-EOB-DTPA as a contrast agent in MR imaging of the hepatobiliary system. Radiology 183, 59–64 (1992).
- Mühler A, Clément O, Saeed M et al.: Evaluation of radiation-induced liver injury with MR imaging: comparison of hepatocellular and reticuloendothelial contrast agents. Radiology 185, 163–168 (1993).
- Mühler A, Freise CE, Kuwatsuru R et al.: Acute liver rejection: evaluation with cell-directed MR contrast agents in a rat transplantation model. Radiology 186, 139–146 (1993).
- Mühler A, Weinmann HJ: Biodistribution and excretion of 153Gd-labeled gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid following repeated intravenous administration to rats. Acad. Radiol. 2, 313–318 (1995).
- Giovagnoni A, Paci E: Liver. III: Gadolinium-based hepatobiliary contrast agents (Gd-EOB-DTPA and Gd-BOPTA/ Dimeg). Magn. Reson. Imaging Clin. N. Am. 4, 61–72 (1996).
- Schmitz SA, Mühler A, Wagner S, Wolf KJ: Functional hepatobiliary imaging with gadolinium-EOB-DTPA. A comparison of magnetic resonance imaging and 153gadolinium-EOB-DTPA scintigraphy in rats. Invest. Radiol. 31, 154–160 (1996).
- Goudemant JF, Van Beers BE, Demeure R, Grandin C, Delos M, Pringot J: Comparison of unenhanced and gadoxetate disodiumenhanced spin-echo magnetic resonance imaging for the detection of experimental hepatocellular carcinoma in the rat. Invest. Radiol. 33, 80–84 (1998).
- Tsuda N, Kato N, Murayama C, Narazaki M, Yokawa T: Potential for differential diagnosis with gadolinium-ethoxybenzyldiethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging in experimental hepatic tumors. Invest. Radiol. 39, 80–88 (2004).
- Reimer P, Rummeny EJ, Shamsi K et al.: Phase II clinical evaluation of Gd-EOBDTPA: dose, safety aspects, and pulse sequence. Radiology 199, 177–183 (1996).
- Reimer P, Rummeny EJ, Daldrup HE et al.: Enhancement characteristics of liver metastases, hepatocellular carcinomas, and hemangiomas with Gd-EOB-DTPA: preliminary results with dynamic MR imaging. Eur. Radiol. 7, 275–280 (1997).
- Reimer P, Tombach B, Dalrup H et al.: New MR contrast media in liver diagnosis. Initial clinical results with hepatobiliary Eovist (gadolinium-EOB-DTPA) and RES-specific Resovist (SH U 555 A). Radiologe 36, 124–133 (1996).
- Stern W, Schick F, Kopp AF et al.: Dynamic MR imaging of liver metastases with Gd-EOB-DTPA. Acta Radiol. 41, 255–262 (2000).
- Hammerstingl R, Zangos S, Schwarz W et al.: Contrast-enhanced MRI of focal liver tumors using a hepatobiliary MR contrast agent: detection and differential diagnosis using Gd-EOB-DTPA-enhanced versus Gd-DTPAenhanced MRI in the same patient. Acad. Radiol. 9(Suppl. 1), S119–S120 (2002).
- Huppertz A, Balzer T, Blakeborough A et al.: Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 230, 266–275 (2004).
- Bluemke DA, Sahani D, Amendola M et al.: Efficacy and safety of MR imaging with liver-specific contrast agent: U.S. multicenter Phase III study. Radiology 237, 89–98 (2005).
- Huppertz A, Haraida S, Kraus A et al.: Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT – initial observations. Radiology 234, 468–478 (2005).
- Halavaara J, Breuer J, Ayuso C et al.: Liver tumor characterization: comparison between liver-specific gadoxetic acid disodiumenhanced MRI and biphasic CT – a multicenter trial. J. Comput. Assist. Tomogr. 30, 345–354 (2006).
- Hammerstingl R, Huppertz A, Breuer J et al.: Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur. Radiol. 18, 457–467 (2008).
- Raman S, Leary C, Bluemke DA et al.: Improved characterization of focal liver lesions with liver specific Gd-EOB-DTPAenhanced magnetic resonance imaging: multicentre Phase III clinical trial. J. Comput. Assist. Tomogr. (2009) (In Press).
- Hammerstingl RM, Vogl TJ: Abdominal MDCT: protocols and contrast considerations. Eur. Radiol. 15(Suppl. 5), E78–E90 (2005).
- Kim SH, Kim SH, Lee J et al.: Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am. J. Roentgenol. 192, 1675–161 (2009).
- di Martino M, Marin D, Guerrisi A, Geiger D, Catalano C, Passariello R: Intraindividual comparison of gadoxetic acid (Gd-EOB-DTPA) enhanced MR imaging and multiphasic 64-slice CT for the detection of hepatocellular carcinoma (HCC) in patients with cirrhosis. Proceedings of the European Congress of Radiology. Vienna, Austria, 6–10 March 2009.
- Luca A, Grazioli L, Caruso S, Tinti R, Milazzo M, Bondioni M: A two-centre study for the comparison of Gd-EOB-DTPA (Primovist®)-enhanced MRI versus triple-phase MDCT for the detection of hepatocellular carcinoma in cirrhosis. Proceedings of the European Congress of Radiology. Vienna, Austria, 6–10 March 2009.
- Zech CJ, Vos B, Nordell A et al.: Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses Gd-EOB-DTPA (gadoxetic acid) with standard Gd-DTPA. Invest. Radiol. 44, 305–310 (2009).
- Asbach P, Warmuth C, Stemmer A et al.: High spatial resolution T1-weighted MR imaging of liver and biliary tract during uptake phase of a hepatocyte-specific contrast medium. Invest. Radiol. 43, 809–815 (2008).
- Motosugi U, Ichikawa T, Tominaga L et al.: Delay before the hepatocyte phase of Gd-EOB-DTPA-enhanced MR imaging: is it possible to shorten the examination time? Eur. Radiol. (2009) (Epub ahead of print).
- Clément O, Mühler A, Vexler VS et al.: Gadolinium-ethoxybenzyl-DTPA, a new liver-directed magnetic resonance contrast agent. Absence of acute hepatotoxic, cardiovascular, or immunogenic effects. Invest. Radiol. 28, 26–32 (1993).
- Kato N, Yokawa T, Tamura A, Heshiki A, Ebert W, Weinmann HJ: Gadoliniumethoxybenzyl- diethylenetriamine-pentaacetic acid interaction with clinical drugs in rats. Invest. Radiol. 37, 680–684 (2002).
- Döhr O, Hofmeister R, Trecher M, Schweinfurth H: Preclinical safety evaluation of Gd-EOB-DTPA (Primovist). Invest. Radiol. 42, 830–841 (2007).
- Mühler A, Heinzelmann I, Weinmann HJ: Elimination of gadolinium-ethoxybenzyl- DTPA in a rat model of severely impaired liver and kidney excretory function. An experimental study in rats. Invest. Radiol. 29, 213–216 (1994).
- Kühnen J, Shamsi K, Carter R et al.: Clinical safety experience from Phase III studies of Gd-EOB-DTPA, a new liver specific MR contrast medium. Proceedings of the 10th Annual Meeting of International Society for Magnetic Resonance in Medicine. Honolulu, HI, USA, 18–24 May 2002 (Abstract 675).
- Agarwal R, Brunelli SM, Williams K, Mitchell MD, Feldman HI, Umscheid CA: Gadolinium-based contrast agents and nephrogenic systemic fibrosis: a systematic review and meta-analysis. Nephrol. Dial. Transplant. 24, 856–863 (2009).
- Dahlström N, Persson A, Albiin N, Smedby Ö, Brismar TB: Contrast-enhanced magnetic resonance cholangiography with Gd-BOPTA and Gd-EOB-DTPA in healthy subjects. Acta Radiol. 48, 362–368 (2007).
- Narita M, Hatano E, Arizono S et al.: Expression of OATP1BA determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J. Gastroentrol. 44(7), 793–798 (2009).
- Abe T, Unno M, Onogawa T et al.: LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology 120, 89–99 (2001).
- Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A: Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol. Ther. 4, 815–818 (2005).
- Nilsson H, Nordell A, Vargas R, Douglas L, Jonas E, Blomqvist L: Assessment of hepatic extraction fraction and input relative blood flow using dynamic hepatocyte-specific contrast-enhanced MRI. J. Magn. Reson. Imaging 29, 1323–1331 (2009).
- Zech CJ, Grazioli L, Jonas E et al.: Healtheconomic evaluation of three imaging strategies in patients with suspected colorectal liver metastases: Gd-EOB-DTPA-enhanced MRI vs. extracellular contrast mediaenhanced MRI and 3-phase MDCT in Germany, Italy and Sweden. Eur. Radiol. 19(Suppl. 3), S753–S763 (2009).
- American Cancer Society. Liver Cancer Overview. American Cancer Institute, Atlanta, GA, USA (2009). www.cancer.org/docroot/CRI/content/ CRI_2_2_1X_How_many_people_get_ liver_cancer (Accessed 17 June 2009)
- National Cancer Institute. Liver (Hepatocellular) Cancer Prevention (PDQ®). National Cancer Institute, Bethesda, MD, USA (2008). www.cancer.gov/cancertopics/pdq/ prevention/hepatocellular (Accessed 4 June 2009)
- WHO. Mortality Database. WHO, Geneva (2005). www.who.int/whosis/en (Accessed 4 June 2009)
• • Comprehensive overview on imaging modalities for evaluation of hepatocellular carcinoma.
• Describes the special pharmacokinetics and pharmacodynamics of Gd-EOB-DTPA, including dual elimination pathway and imaging window.
• • Describes specific Gd-EOB-DTPA enhancement patterns in different lesion types.
• • Publication of data derived from the European Phase III study on lesion detection using Gd-EOB-DTPA-enhanced MRI demonstrating superior lesion detection ability compared with computed tomography.
• Study comparing Gd-EOB-DTPA-enhanced MRI and multidetector computed tomography for detection of hepatocellular carcinoma in cirrhosis.
• Study on the use of Gd-EOB-DTPA to assess liver function by the measurement of hepatic extraction fraction and input relative blood flow.
Websites