Review Article - Interventional Cardiology (2010) Volume 2, Issue 2
Contrast-induced nephropathy: a contemporary and simplified review
- Corresponding Author:
- Huseyin Uyarel
Department of Cardiology
Balikesir University School of Medicine, Balikesir, Turkey
Tel: +90 505 648 1590
Fax: +90 266 612 1459
E-mail:uyarel@yahoo.com; uyarel@balikesir.edu.tr
Abstract
Keywords
contrast agent, contrast-induced nephropathy, interventional cardiology
Definition, incidence & clinical significance
Although there is no widely accepted definition for contrast-induced nephropathy (CIN), it is generally defined as an absolute (≥0.5 mg/dl) or relative (≥25%) increase in serum creatinine (SCr) with respect to baseline within 48–72 h of contrast media administration in the absence of alternative causes.
The incidence of CIN varies, ranging from almost zero to over 50% [1–4]. The reasons for this high variance result from the CIN definition not being standardized in studies (i.e., if 1 mg/dl is used instead of 0.5 mg/dl for SCr, the incidence obviously decreases), as well as risk factors and CM type and quantity.
Contrast-induced nephropathy is the third most common reason for hospital-acquired acute kidney injury. In-hospital and 1- and 5‑year mortality and morbidity (e.g., stroke, myocardial infarction and vessel re-occlusion) incidences are higher in patients who develop CIN as opposed to patients without CIN [5–9]. It is not known whether this can be attributed to CIN that directly causes the increased mortality or to the fact that patients who develop CIN have more serious and mortal comorbidities.
Clinical features & risk factors
Contrast-induced nephropathy can be detected from an increase in SCr level beginning within the 12–24 h after intake of CM; however, the increased SCr level may not be detected until 72–96 h post-intake. In many of the cases, oliguria is not detected. Renal dysfunction is frequently mild and transient, but sometimes may be as severe as to require hemodialysis.
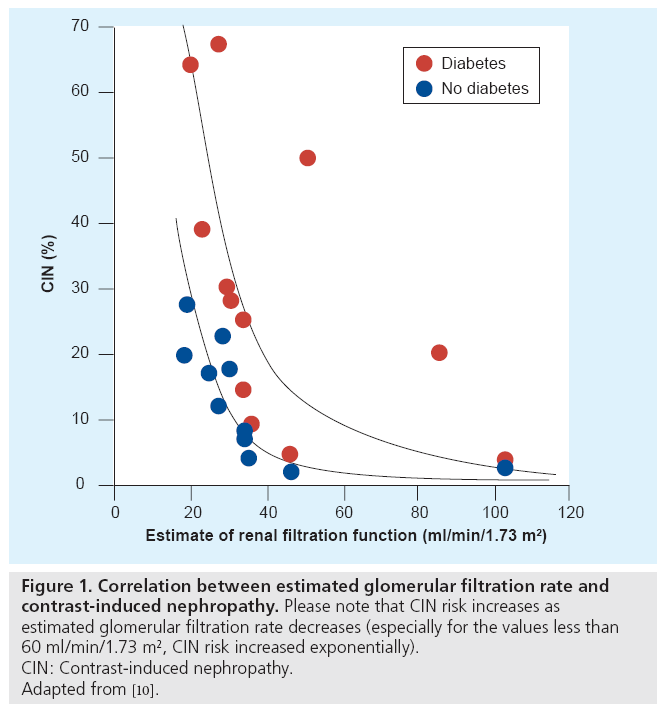
For the development of CIN, chronic kidney disease (CKD) is the most important risk factor (Figure 1) [10] (CKD is defined as SCr ≥1.5 mg/dl or estimated glomerular filtration rate <60 ml/ min/1.73 m2). For those who have cardiovascular disease (especially for elderly or obese patients), the modification of diet in renal disease (MDRD) formula for estimated glomerular filtration rate gives a more precise result than the Cockcroft–Gault formula [11,12]. For the MDRD formula, the SCr, age, gender and race (being African or not) are required whereas weight is not. The alternative MDRD formula that also includes blood urea and albumin values can be used, but the result will be similar. Current Personal Digital Assistants have built‑in programs for this complex formula.
Figure 1: Correlation between estimated glomerular filtration rate and contrast-induced nephropathy. Please note that CIN risk increases as estimated glomerular filtration rate decreases (especially for the values less than 60 ml/min/1.73 m2, CIN risk increased exponentially). CIN: Contrast-induced nephropathy. Adapted from [10].
Other important risk factors are diabetes mellitus, hypovolemia, hypotension, advanced age (>70 years), chronic heart failure (CHF), anemia, nephrotoxic drug use (e.g., gentamycin and nonsteroidal anti-inflammatory drugs), hyperuricaemia and recent (within 10 days, especially the first 3 days) and overuse of CM. The greater number of risk factors, especially elevation of baseline SCr value, the greater the probability of CIN.
In oliguric hemodialysis patients, CM may reduce glomerular filtration rate and lead to reduced urine output and accordingly quality of life may be disturbed. In these cases, CM should be avoided as much as possible. However, according to some authors, if the chronic hemodialysis patient is anuric, CIN is not a matter of concern since there is nothing to lose for residual kidney function, and any type of CM can be used without apprehension [13]. However, whether anuria itself has an effect on quality of life is somewhat doubtful [14].
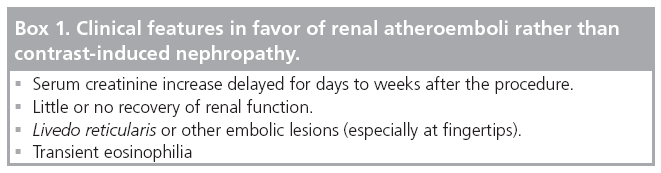
In order to diagnose CIN accurately, other causes of renal dysfunction such as sepsis and renal atheroemboli should also be considered (Box 1) [15]. During coronary angiography (CAG) or percutanous coronary intervention (PCI), microatheroemboli may detach from the atherosclerotic aortic wall while catheterizing, and travel to the kidney.
The route of CM administration is also important regarding CIN risk [16]. It is thought that CIN risk is higher when CM is administered via a suprarenal arterial route (e.g., coronary angiography [CAG] or PCI) than via an intravenous route (e.g., computerized tomography or intravenous pyelography). This may be caused by a higher concentration of CM within kidney after the intra-arterial rather than intravenous injection. However, renal atheroemboli in the intra-arterial route may be the other cause of increased SCr levels.
Pathophysiologic mechanisms
The pathophysiology of CIN is not clearly known owing to the fact that it is generally transient, and histopathologic changes caused by underlying renal dysfunction complicate biopsy assessment. For this reason, current data are mostly obtained from animal experiments.
The most important mechanisms are renal vasoconstriction (e.g., reduced medullary blood flow) and acute tubular injury. Reduced medullary blood flow is caused, in part, by contrast- induced release of vasoconstrictor agents such as endothelin, by blockage of vasodilator agents such as nitric oxide and prostaglandins, and by increased viscosity of the vascular bed by high osmolar CM (HOCM). The other mechanism, acute tubular injury, is possibly caused either by a direct toxic effect of CM, triggering of oxygen free radical formation by CM, or both. Acute tubuler injury may also be exacerbated by renal vasoconstriction. High oxygen demands due to increased workload make the residual functioning tubuli in CKD more susceptible to injury caused by all these effects.
Contrast media
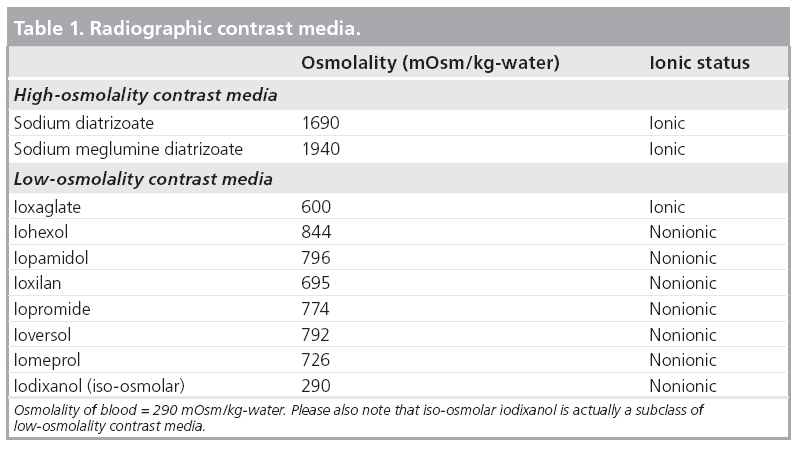
The first manufactured CM were ionic and had high osmolality (>1500 mOsm/kg-water); however, because this increased the osmotic load of the kidney the risk of nephropathy was quite high. For this reason, nonionic CM were developed. As these also had relatively lower osmolality with respect to older HOCM versions, these CM were named as low osmolality CM (LOCM). Despite their name being LOCM, they still have higher osmolality than blood (blood: 290, LOCM: <850 mOsm/kg/water) (Table 1). After discovering that a reduction of osmolality was less nephrotoxic; iodixanols having the same osmolality as blood were developed, and classified as iso-osmolar CM.
Prevention strategies
Once CIN develops, the therapy is conservative. Fluid and electrolyte balance should be maintained and the patient should be followed-up with serial blood tests, whether or not he or she is proceeding to dialysis. However, the best treatment is to prevent CIN. To date, many studies have been conducted about prevention of CIN. Owing to numerous contradicting studies, and for clarity in order to simplify the true approach to CIN, we are going to review only randomized controlled studies’ results from 2000 to 2010.
▪ Type & quantity of contrast media
High osmolar CM began to be substituted with LOCM in practice owing to CIN risk, allergy, bradycardia, asystole and myocardial depressive effects. The lowering of LOCM prices down close to HOCM prices has had an important role in this alternation.
Iodixanol is equivalent to other LOCMs in terms of reduction of CIN risk in 11 studies [17–27], superior in four [28–31] and inferior in two [32,33]. In a meta-analysis, iodixanol, although having a partially lower risk of CIN, hemodialysis and death compared with iohexol and ioxaglate, was similar regarding risks when other LOCMs (iopamidol, ioversol, iopromide and iomeprol) were taken altogether [34].
Whether the CM is a monomer or dimer does not affect our selection. Another key point is that HOCMs are possibly not more nephrotoxic when compared with LOCMs in patients with no risk factors [35–37].
It can be estimated that if the amount of CM has increased, the CIN risk would also have increased [38,39]. A retrospective study on this subject suggests that for diagnostic procedures, 30 ml of CM should not be exceeded, and for interventional procedures the maximum level is 100 ml [40], whereas some others suggest definite amounts of CM to be given on the basis of weight [41]. However, owing to the complexity and unpredictability of PCI, and since it would be impractical in the real-world to interrupt the procedure, these values have often been exceeded, the most practical method is to complete the procedure with the least amount of CM possible. This can be assured by utilization of biplane or rotational devices, working with small-sized catheters, avoidance of ventriculography (despite the intense pressure by some surgeons before coronary artery bypass grafting) for the cases whose left ventricular and valvular functions are clearly assessed by echocardiography or MRI, and administration of the least amount of CM – just enough for a complete wash of coronary vessels. At this point, even though gadolinium-enhanced MRI was considered to be an interesting alternative to prevent CIN, newly defined nephrogenic systemic fibrosis, which is irreversible and a more serious problem than CIN, revealed that gadolinium is not so harmless [42–44].
In fact, the best way to administer the least amount of CM is not to use it at all; that is, not to proceed with an unnecessary procedure. If it is expected to produce the same results or unlikely to change the therapeutical approach, a preference for echocardiography use instead of catheterization, MRI without gadolinium, CT without contrast, myocardial perfusion scintigraphy, and avoiding unnecessary CAG or PCI are important procedures to consider. As an alternative contrast agent, carbon dioxide can be considered in digital subtraction angiography to be applied in subdiaphragmatic vessels. Carbon dioxide, however, is not to be nephrotoxic; its disadvantage is the probability of being neurotoxic (in the presence of right‑to-left shunt or in supradiaphragm imaging) [45–48].
In the case of stable angina pectoris in particular, as the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) study stressed, keeping in mind that there is no long-term mortality difference between medical therapy and PCI; trying medical therapy before deciding upon CAG will probably prevent much more CIN in the real-world compared with application of all the preventive strategies mentioned in this article. On the other hand, certain procedures should not be avoided whenever necessary, even if there is a CIN risk. For example; in obstructive coronary artery disease patients who have CKD, the PCI procedure has been shown to provide a better prognosis than no PCI in a registy [49]. The same situation may also apply to patients with heart failure. As a result, the physician should consider the case globally, and carefully assess the necessary therapeutic options by weighting profit and loss, even if the therapy is risky.
▪ Hydration
The most effective prophylactic strategy in reducing the risk of CIN is hydration. It is a low-cost and low-risk strategy for most patients. It dilutes CM and decreases contact time within kidney, increases diuresis, and suppresses production of endogen vasoconstrictor agents. There are several studies investigating different fluids. However, because alkalinization is known to reduce free radical production, studies regarding hydration with fluids that have alkali properties, such as sodium bicarbonate, have been performed, and fluids with sodium bicarbonate were compared with isotonic sodium chloride. Five of six prospective studies showed that isotonic sodium bicarbonate caused fewer incidences of CIN than isotonic sodium chloride [50–54], where in one study there was no difference [55]. However, in a retrospective cohort study consisting of 7977 patients, sodium bicarbonate caused more CIN than sodium chloride [56]. Furthermore, there are conflicting results between meta-analyses [57–61]. For this reason, well-designed studies are awaited to decide which one should be the first choice (sodium bicarbonate solution is prepared by adding three 50 ml doses of 8.4% mEq/l sodium bicarbonate to either 850 ml of 5% dextrose or sterile water).
Administration of volume replacement in the form of infusion pre- or post-procedure was seen to be more effective than oral fluid replacement or if given in the form of bolus during procedure [62–64]. In addition, in one study the combination of 0.45% sodium chloride and 5% dextrose prevented less CIN in comparison to isotonic sodium chloride [65]. However, it must be considered that this study did not control for the amount of sodium administered: the difference between the isotonic sodium chloride and 0.45% sodium chloride is that you have to give twice as much of the latter to give the same amount of sodium as the former.
Hydration is problematic for patients with CHF. It is rational to appraise the clinical signs for volume status, and hydrate only the hypovolemic patients with CHF meticulously. Patients with CHF should not be hydrated routinely.
It is suggested that patients are given sodium bicarbonate 1 h before the procedure as 3 ml/kg/h, or isotonic sodium chloride 6–12 h before the procedure as 1 ml/kg/h. After the procedure, hydration (whichever you have chosen) should continue with 1 ml/kg/h dose for 6–12 h (with a longer time in hot weather or for advanced CKD cases).
▪ N-acetylcysteine
Owing to antioxidant and vasodilatation effects, there have been many studies conducted considering the possible potential of N-acetylcysteine (NAC) to prevent CIN. Results of 31 studies are as follows: in nine studies, administering NAC is superior to not administering [66–73], more harmful in one [74] and indifferent in others [75–95]. Several meta-analyses and even overanalysis have been made in this area and the results have differed [96–99]. To dispel this confusion, a welldesigned (multicenter, prospective, randomized, controlled and large scale) aceylcysteine for contrast-induced nephropathy (ACT) trial is still ongoing [100]. Until the release of the results, we suggest applying this prophylaxy, which is at least known to be inexpensive and harmless. Despite the controversies regarding the administration dose and route, the most widely accepted mode is to give NAC 1200 mg orally, 1 day before and 1 day after the procedure, and twice-daily from then on [96]. Superiority of the intravenous route to oral is controversial owing to lack of evidence and risk of anaphylaxis. It should be emphasized that all NAC studies were conducted in patients whose hydration is maintained, that is, NAC is not an alternative to hydration.
▪ Hemodialysis/hemofiltration
Owing to the capability of eliminating more than half of the volume of CM by hemodialysis, three studies were conducted. In one of them, hemodialysis prevented CIN [101], and in the other two it unexpectedly increased CIN risk [81,102]. Here it is necessary to distinguish prophylactic hemodialysis (just after CM administration, yet before CIN development), of which benefit was not clearly demonstrated from therapeutic hemodialysis implemented for acute kidney injury, the most advanced state of CIN.
In two studies on hemofiltration, CIN risk was reduced provided hemofiltration was initiated before CM administration [103,104]. However, the obtained benefit may have been a pseudo-effect resulting from bicarbonate administered simultaneously or direct clearance of SCr by hemofiltration. Accordingly, although it may be useful for very high-risk patients (e.g., stage 5 CKD), it is difficult to justify hemofiltration in view of its cost, invasiveness and aforementioned reasons.
With all these data, expensive, invasive and ineffective prophylactic hemodialysis cannot be recommended for prevention of CIN. However, hemofiltration may be used for very high-risk patients, but there is a need to conduct further investigations in order to provide more supported recommendations.
▪ Angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers & metformin
In the results of some studies, angiotensinconverting enzyme inhibitors and angiotensin- receptor blockers increased the risk of CIN [105,106], in other studies they decreased the risk of CIN [107,108]. Accordingly, at present, the issue of whether to discontinue before the procedure is not clear.
Metformin is important not for being nephrotoxic but for the risk of lactic acidosis if CIN develops. There are five guidelines regarding the use of metformin in diabetic patients receiving CM, and these guidelines have inconsistent recommendations owing to the low level of evidence for ceasing metformin therapy [109]. It seems reasonable to discontinue metformin before 2 days of CM administiration and remain off for 2 days after CM if renal dysfunction presents (SCr ≥ 1.5 mg/dl or estimated glomerular filtration rate <60 ml/dk/1.73 m2) [43]. If renal function is normal, it may not be necessary to stop taking metformin, owing to the low risk of developing lactic acidosis.
▪ Other methods
Endothelin-receptor antagonists and phenoldopam, a renal vasodilator, increased CIN incidence, contrary to expectations [75,110–113]. Dopamine, furosemide, mannitole, calciumchannel blockers and theophylline showed no benefit [40,45,114–117]. The effect of statins, ascorbic acid, erythropoietin, prostacyclin analogs and trimetazidine should be assessed after large-scale studies [118–126]. In brief, according to our present knowledge, the agents mentioned in this paragraph cannot be recommended to prevent CIN.
Future perspective
The incidence of CIN has been increasing gradually. An increase in incidence of diabetes mellitus, hypertension, advanced age and CKD in the population play an important role. Awareness about the presence and severity of nephrogenic systemic fibrosis caused by gadolinium- enhanced MRI appears to have led to an increase in the number procedures utilizing CM. This makes us believe that CIN will be a more serious problem in the future. Therefore, the requirement of well-designed studies that do not bear the drawbacks of most of the present studies will increase (Box 2).
Box 2: What a well-designed prevention study regarding contrast-induced nephropathy should entail.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Definition & importance
▪ Contrast-induced nephropathy is generally defined as an absolute (≥0.5 mg/dl) or relative (≥25%) increase in serum creatinine with respect to baseline within 48 to 72 h of contrast media administration in the absence of alternative causes.
▪ It is related to short- and long-term mortality and morbidity, and is the third most common reason for hospital-acquired acute kidney injury.
Risk factors
▪ The most important one is chronic kidney disease (CKD; serum creatinine ≥1.5 mg/dl or estimated glomerular filtration rate <60 ml/dl/1.73 m2; for estimated glomerular filtration rate, the ‘modification of diet in renal disease’ formula is preferred to the Cockcroft–Gault formula).
▪ Other risk factors include diabetes mellitus, hypovolemia, hypotension, advanced age, chronic heart failure, anemia, nephrotoxic drug use, hyperuricemia and high quantity or recent (within 10 days) use of contrast media.
▪ The greater the number of risk factors a person has, the greater their risk for CIN.
Prevention strategies
▪ For people with no risk factors it would probably be enough to prevent only volume depletion and make a note of contrast media amount.
▪ Our suggestions for people with any risk factors are:
- Prefer echocardiography, computerized tomography without contrast, magnetic resonance imaging without gadolinium or myocardial perfusion scintigraphy to the procedure using contrast media, if appropriate.
- Do not give contrast media redundantly; always strive to finish the procedure with the least amount of contrast media. Avoid ventriculography if it is unlikely to provide extra information.
- Consider if coronary angiography or percutanous coronary intervention are really necessary scientifically; in some situations (especially heart failure or refractory angina) do not abandon the procedure despite contrast-induced nephropathy risk. If possible avoid a second procedure (e.g., percutanous coronary intervention) within 10 days of the first procedure (e.g., coronary angiography). If this is not practical for your institute, wait at least 3 days.
- Discontinue nonsteroidal antiinflammatory drugs and other nephrotoxic drugs. Discontinue metformin for 2 days before and after the procedure if renal dysfunction exists (angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers are controversial and to be used at the physician’s discretion).
- Do not use high osmolar contrast media; use iso- or low-osmolar contrast media (except ioxaglate and iohexol).
- Avoid volume depletion; give fluid infusion (except in chronic heart failure). Prefer isotonic solutions (isotonic sodium chloride or isotonic sodium bicarbonate) to hypotonic ones (5% dextrose and 0.45% sodium chloride).
- Administer N-acetylcysteine 1200 mg pre- and post-24 h, twice-daily. The oral route is preferable to intravenous.
- Do not use prophylactic hemodialysis. Hemofiltration should only be considered for stage 5 chronic kidney disease patients.
- Check postprocedural 48-h serum creatinine value.
References
Papers of special note have been highlighted as:
▪ of interest
- McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW: Acute renal failure after coronary intervention:incidence, risk factors, and relationship to mortality. Am. J. Med. 103, 368–375 (1997).
- Uyarel H, Cam N, Ergelen M et al.: Contrastinduced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction: incidence, a simple risk score, and prognosis. Arch. Med. Sci. 5, 550–558 (2009).
- Rihal CS, Textor SC, Grill DE et al.: Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 105, 2259–2264 (2002).
- Barrett BJ, Parfrey PS: Clinical practice: preventing nephropathy induced by contrast medium. N. Engl. J. Med. 354, 379–386 (2006).
- Gruberg L, Mintz GS, Mehran R et al.: The prognostic implications of further renal function deterioration within 48 hrs of interventional coronary procedures in patients with pre-existent chronic renal insufficiency J. Am. Coll. Cardiol. 36(5), 1542–1548 (2000).
- Rudnick M, Feldman H: Contrast-induced nephropathy: what are the true clinical consequences? Clin. J. Am. Soc. Nephrol. 3, 263–272 (2008).
- Kingman JG Jr, Chantal V, Rouleau JR, Kingma I: Influence of acute renal failure on coronary vasoregulation in dogs. J. Am. Soc. Nephrol. 17, 1316–1324 (2006).
- Hölscher B, Heitmeyer C, Fobker M, Breithardt G, Schaefer RM, Reinecke H: Predictors for contrast media-induced nephropathy and long-term survival: prospectively assessed data from the randomized controlled Dialysis-Versus- Diuresis (DVD) trial. Can. J. Cardiol. 24(11), 845–850 (2008).
- Lindsay J, Apple S, Pinnow EE et al.: Percutaneous coronary interventionassociated nephropathy foreshadows increased risk of late adverse events in patients with normal baseline serum creatinine. Catheter Cardiovasc. Interv. 59, 338–343 (2003).
- McCullough PA: Contrast-induced acute kidney injury. J. Am. Coll. Cardiol. 51, 1419–1428 (2008).
- Brosius FC, Hostetter TH, Kelepouris A et al.: AHA science advisory. Detection of chronic kidney disease in patients with or at increased risk of cardiovascular disease. Circulation 114, 1–6 (2006).
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130, 461–470 (1999).
- Ellis JH, Cohan RH: Prevention of contrast-induced nephropathy: an overview. Radiol. Clin. N. Am. 47, 801–811 (2009).
- Perlman RL, Finkelstein FO, Liu L et al.: Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute – CKD study. Am. J. Kidney Dis. 45(4), 658–666 (2005).
- Rudnick MR, Berns JS, Cohen RM, Goldfarb S: Nephrotoxic risks of renal angiography: contrast-media associated nephrotoxicity and atheroembolism. A critical review. Am. J. Kidney Dis. 24, 713 (1994).
- Byrd L, Sherman RL: Radiocontrast-induced acute renal failure: a clinical and pathophysiologic review. Medicine 58, 270–279 (1979).
- Rudnick M, Laskey W, Aspelin P et al.: Nephrotoxicity of iodixanol versus iopamidol in patients with chronic kidney disease and diabetes mellitus undergoing coronary angiographic procedures. Am. Heart J. 158, 822–828 (2009).
- Solomon RJ, Natarajan MK, Doucet S et al.: Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation 115, 3189–3196 (2007).
- Feldkamp T, Baumgart D, Elsner M et al.: Nephrotoxicity of iso-osmolar versus low-osmolar contrast media is equal in low risk patients. Clin. Nephrol. 66, 322–330 (2006).
- Barrett BJ, Katzberg RW, Thomsen HS et al.: Contrast-induced nephropathy in patients with chronic kidney disease undergoing computed tomography: a double-blind comparison of iodixanol and iopamidol. Invest. Radiol. 41, 815–821 (2006).
- Kuhn MJ, Chen N, Sahani DV et al.: The PREDICT study: a randomized double-blind comparison of contrast-induced nephropathy after low- or isoosmolar contrast agent exposure. Am. J. Roentgenol. 191(1), 151–157 (2008).
- Chuang FR, Chen TC, Wang IK et al.: Comparison of iodixanol and iohexol in patients undergoing intravenous pyelography: a prospective controlled study. Ren. Fail. 31(3), 181–188 (2009).
- Hardiek KJ, Katholi RE, Robbs RS, Katholi CE: Renal effects of contrast media in diabetic patients undergoing diagnostic or interventional coronary angiography. J. Diabetes Complications 22(3), 171–177 (2008).
- Mehran R, Nikolsky E, Kirtane AJ et al.: Ionic low-osmolar versus nonionic isoosmolar contrast media to obviate worsening nephropathy after angioplasty in chronic renal failure patients: the ICON (Ionic Versus Non-ionic Contrast to Obviate Worsening Nephropathy after Angioplasty in Chronic Renal Failure Patients) study. Cardiovasc. Interv. 2(5), 415–421 (2009).
- Juergens CP, Winter JP, Nguyen-Do P et al.: Nephrotoxic effects of iodixanol and iopromide in patients with abnormal renal function receiving N-acetylcysteine and hydration before coronary angiography and intervention: a randomized trial. Intern. Med. J. 39(1), 25–31 (2009).
- Laskey W, Aspelin P, Davidson C et al.; DXV405 Study Group: Nephrotoxicity of iodixanol versus iopamidol in patients with chronic kidney disease and diabetes mellitus undergoing coronary angiographic procedures. Am. Heart J. 158(5), 822–828 (2009).
- Wessely R, Koppara T, Bradaric C et al.: Contrast media and nephrotoxicity following coronary revascularization by angioplasty trial investigators. Choice of contrast medium in patients with impaired renal function undergoing percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2(5), 430–437 (2009).
- Jo SH, Youn TJ, Koo BK et al.: Renal toxicity evaluation and comparison between Visipaque™ (iodixanol) and Hexabrix™ (ioxaglate) in patients with renal insufficiency undergoing coronary angiography: the RECOVER study: a randomized controlled trial. J. Am. Coll. Cardiol. 48, 924–930 (2006).
- Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ: Nephrotoxic effects in high-risk patients undergoing angiography. N. Engl. J. Med. 348, 491–499 (2003).
- Nguyen SA, Suranyi P, Ravenel JG et al.: Iso-osmolality versus low-osmolality iodinated contrast medium at intravenous contrast-enhanced CT: effect on kidney function. Radiology 248(1), 97–105 (2008).
- Nie B, Cheng WJ, Li YF et al.: A prospective, double-blind, randomized, controlled trial on the efficacy and cardiorenal safety of iodixanol vs. iopromide in patients with chronic kidney disease undergoing coronary angiography with or without percutaneous coronary intervention. Catheter Cardiovasc. Interv. 72(7), 958–965 (2008).
- Thomsen HS, Morcos SK, Erley CM et al.; Investigators in the Abdominal Computed Tomography: IOMERON 400 Versus VISIPAQUE 320 Enhancement (ACTIVE) Study: Comparison of the effects on renal function of iomeprol-400 and iodixanol-320 in patients with chronic kidney disease undergoing abdominal computed tomography. Invest. Radiol. 43(3), 170–178 (2008).
- Thomsen HS, Morcos SK: Risk of contrastmedium- induced nephropathy in high-risk patients undergoing MDCT-A pooled analysis of two randomized trials. Eur. Radiol. 19, 891–897 (2008).
- Reed M, Meier P, Tamhane UU, Welch KB, Moscucci M, Gurm HS: The relative renal safety of iodixanol compared with lowosmolar contrast media: a meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2, 645–654 (2009).
- Rudnick MR, Goldfarb S, Wexler L et al.: Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. Kidney Int. 47, 254 (1995).
- Schwab SJ, Hlatky MA, Pieper KS et al.: Contrast nephrotoxicity: a randomized controlled trial of a nonionic and an ionic radiographic contrast agent. N. Engl. J. Med. 320, 149 (1989).
- Moore RD, Steinberg EP, Rowe NR et al.: Nephrotoxicity of high-osmolality versus low-osmolality contrast media: randomized clinical trial. Radiology 182, 649 (1992).
- Laskey WK, Jenkins C, Selzer F et al.: Volume-to-creatinine clearance ratio. J. Am. Coll. Cardiol. 50, 584–590 (2007).
- Cigarroa RG, Lange RA, Williams RH, Hillis LD: Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am. J. Med. 86, 649 (1989).
- Mehran R, Aymong ED, Nikolsky E et al.: A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J. Am. Coll. Cardiol. 44, 1393–1399 (2004).
- Freeman RV, O’Donnell M, Share D et al.: Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am. J. Cardiol. 90, 1068–1073 (2002).
- Kane GC, Stanson AW, Kalnicka D et al.: Comparison between gadolinium and iodine contrast for percutaneous intervention in atherosclerotic renal artery stenosis: clinical outcomes. Nephrol. Dial. Transplant 23, 1233–1240 (2008).
- Thomsen HS: European Society of Urogenital Radiology (ESUR) guidelines on the safe use of iodinated contrast media. Eur. J. Radiol. 60(3), 307–313 (2006).
- Schieren G, Wirtz N, Altmeyer P, Rump LC, Weiner SM, Kreuter A: Nephrogenic systemic fibrosis a rapidly progressive disabling disease with limited therapeutic options. J. Am. Acad. Dermatol. 61, 868–874 (2009).
- Liss P, Eklof H, Hellberg O et al.: Renal effects of CO2 and iodinated contrast media in patients undergoing renovascular intervention: a prospective, randomized study. J. Vasc. Interv. Radiol. 16, 57 (2005).
- Coffey R, Quisling RG, Mickle JP et al.: The cerebrovascular effects of intraarterial CO2 in quantities required for diagnostic imaging. Radiology 151, 405 (1984).
- Wilson AJ, Boxer MM: Neurotoxicity of angiographic carbon dioxide in the cerebral vasculature. Invest. Radiol. 37, 542 (2002).
- Hawkins IF, Cho KJ, Caridi JG: Carbon dioxide in angiography to reduce the risk of contrast-induced nephropathy. Radiol. Clin. N. Am. 47, 813–825 (2009).
- Reddan DN, Klassen PS: Chronic kidney disease and cardiovascular risk: Time to focus on therapy. J. Am. Soc. Nephrol. 13, 2415–2416 (2002).
- Ozcan EE, Guneri S, Akdeniz B et al.: Sodium bicarbonate, N-acetylcysteine, and saline for prevention of radiocontrastinduced nephropathy: a comparison of 3 regimens for protecting contrast-induced nephropathy in patients undergoing coronary procedures: a single-center prospective controlled trial. Am. Heart J. 154, 539–544 (2007).
- Recio-Mayoral A, Chaparro M, Prado B et al.: The renoprotective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the RENO Study. J. Am. Coll. Cardiol. 49, 1283–1288 (2007).
- Briguori C, Airoldi F, D’Andrea D et al.: Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation 115, 1211–1217 (2007).
- Masuda M, Yamada T, Mine T et al.: Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast-induced nephropathy in patients undergoing an emergent coronary procedure. Am. J. Cardiol. 100, 781–786 (2007).
- Merten GJ, Burgess WP, Gray LV et al.: Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA 291, 2328–2334 (2004).
- Vasheghani-Farahani A, Sadigh G, Kassaian SE et al.: Sodium bicarbonate plus isotonic saline versus saline for prevention of contrast-induced nephropathy in patients undergoing coronary angiography: a randomized controlled trial. Am. J. Kidney Dis. 54, 610–618 (2009).
- Bartholmai BJ, Williams AW, Cha SS, Pflueger A, McDonald FS: Sodium bicarbonate is associated with an increased incidence of contrast nephropathy: a retrospective cohort study of 7977 patients at Mayo Clinic. Clin. J. Am. Soc. Nephrol. 3, 10–18 (2008).
- Zoungas S, Ninomiya T, Huxley R et al.: Systematic review: sodium bicarbonate treatment regimens for the prevention of contrast-induced nephropathy. Ann. Intern. Med. 151(9), 631–638 (2009).
- Meier P, Ko DT, Tamura A, Tamhane U, Gurm HS: Sodium bicarbonate-based hydration prevents contrast-induced nephropathy: a meta-analysis. BMC Med. 7, 23 (2009).
- Kanbay M, Covic A, Coca SG, Turgut F, Akcay A, Parikh CR: Sodium bicarbonate for the prevention of contrastinduced nephropathy: a meta-analysis of 17 randomized trials. Int. Urol. Nephrol. 41(3), 617–627 (2009).
- Joannidis M, Schmid M, Wiedermann CJ: Prevention of contrast media-induced nephropathy by isotonic sodium bicarbonate: a meta-analysis. Wien Klin. Wochenschr. 120(23–24), 742–748 (2008).
- Navaneethan SD, Singh S,Appasamy S, Wing RE, Sehgal AR: Sodium bicarbonate therapy for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Am. J. Kidney Dis. 53(4), 617–627 (2009).
- Dussol B, Morange S, Loundoun A, Auquier P, Berland Y: A randomized trial of saline hydration to prevent contrast nephropathy in chronic renal disease patients. Nephrol. Dial. Transplant. 21, 2120–2126 (2006).
- Krasuski RA, Beard BM, Geoghagan JD, Thompson CM, Guidera SA: Optimal timing of hydration to erase contrast-associated nephropathy: the OTHER CAN study. J. Invasive Cardiol. 15, 699–702 (2003).
- Trivedi HS, Moore H, Nasr S et al.: A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin. Pract. 93, 29–34 (2003).
- Mueller C, Buerkle G, Buettner HJ et al.: Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch. Intern. Med. 162, 329–336 (2002).
- Marenzi G, Assanelli E, Marana I et al.: N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N. Engl. J. Med. 354, 2773–2782 (2006).
- Ochoa A, Pellizzon G, Addala S et al.: Abbreviated dosing of N-acetylcysteine prevents contrast-induced nephropathy after elective and urgent coronary angiography and intervention. J. Interv. Cardiol. 17, 159–165 (2004).
- Baker CS, Wragg A, Kumar S, De Palma R, Baker LR, Knight CJ: A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J. Am. Coll. Cardiol. 41, 2114–2118 (2003).
- Kay J, Chow WH, Chan TM et al.: Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: a randomized controlled trial. JAMA 289, 553–558 (2003).
- Shyu KG, Cheng JJ, Kuan P: Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J. Am. Coll. Cardiol. 40, 1383–1388 (2002).
- Diaz-Sandoval LJ, Kosowsky BD, Losordo DW: Acetylcysteine to prevent angiography-related renal tissue injury (the APART trial). Am. J. Cardiol. 89, 356–358 (2002).
- Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W: Prevention of radiographic-contrast agent-induced reductions in renal function by acetylcysteine. N. Engl. J. Med. 343, 180–184 (2000).
- Carbonell N, Sanjuán R, Blasco M, Jordá A, Miguel A: N-acetylcysteine: short-term clinical benefits after coronary angiography in high-risk renal patients. Rev. Esp. Cardiol. 63(1), 12–19 (2010).
- Coyle LC, Rodriguez A, Jeschke RE, Simon-Lee A, Abbott KC, Taylor AJ: Acetylcysteine In Diabetes (AID): a randomized study of acetylcysteine for the prevention of contrast nephropathy in diabetics. Am. Heart J. 151, 1032 (2006).
- Ferrario F, Barone MT, Landoni G et al.: Acetylcysteine and non-ionic isosmolar contrast-induced nephropathy – a randomized controlled study. Nephrol. Dial. Transplant 24, 3103–3107 (2009).
- Amini M, Salarifar M, Amirbaigloo A, Masoudkabir F, Esfahani F: N-acetylcysteine does not prevent contrast-induced nephropathy after cardiac catheterization in patients with diabetes mellitus and chronic kidney disease: a randomized clinical trial. Trials 10, 45–51 (2009).
- Poletti PA, Saudan P, Platon A et al.: IV N-acetylcysteine and emergency CT: use of serum creatinine and cystatin C as markers of radiocontrast nephrotoxicity. Am. J. Roentgenol. 189, 687–692 (2007).
- Seyon RA, Jensen LA, Ferguson IA, Williams RG: Efficacy of N-acetylcysteine and hydration versus placebo and hydration in decreasing contrast-induced renal dysfunction in patients undergoing coronary angiography with or without concomitant percutaneous coronary intervention. Heart Lung 36, 195–204 (2007).
- Carbonell N, Blasco M, Sanjuan R et al.: Intravenous N-acetylcysteine for preventing contrast-induced nephropathy: a randomized trial. Int. J. Cardiol. 115, 57–62 (2007).
- Staniloae CS, Doucet S, Sharma SK et al.: N-acetylcysteine added to volume expansion with sodium bicarbonate does not further prevent contrast-induced nephropathy: results from the cardiac angiography in renally impaired patients study. J. Interv. Cardiol. 22, 261–265 (2009).
- Reinecke H, Fobker M, Wellmann J et al.: A randomized controlled trial comparing hydration therapy to additional hemodialysis or N-acetylcysteine for the prevention of contrast medium-induced nephropathy: the Dialysis-Versus-Diuresis (DVD) Trial. Clin. Res. Cardiol. 96, 130–139 (2007).
- Briguori C, Colombo A, Violante A et al.: Standard vs double dose of N-acetylcysteine to prevent contrast agent associated nephrotoxicity. Eur. Heart J. 25, 206–211 (2004).
- Rashid ST, Salman M, Myint F et al.: Prevention of contrast-induced nephropathy in vascular patients undergoing angiography: a randomized controlled trial of intravenous N-acetylcysteine. J. Vasc. Surg. 40, 1136–1141 (2004).
- Fung JW, Szeto CC, Chan WW et al.: Effect of N-acetylcysteinefor prevention of contrast nephropathy in patients with moderate to severe renal insufficiency: a randomized trial. Am. J. Kidney Dis. 43, 801–808 (2004).
- Boccalandro F, Amhad M, Smalling RW, Sdringola S: Oral N-acetylcysteine does not protect renal function from moderate to high doses of intravenous radiographic contrast. Catheter Cardiovasc. Interv. 58, 336–341 (2003).
- Goldenberg I, Schecter M, Matetzky S et al.: Oral N-acetylcysteine as an adjunct to saline hydration for the prevention of contrastinduced nephropathy following coronary angiography: a randomized controlled trial and review of the current literature. Eur. Heart J. 25, 212–218 (2004).
- Kefer JM, Hanet CE, Boitte S, Wilmotte L, De Kock M: N-acetylcysteine, coronary procedure and prevention of contrast-induced worsening of renal function: which benefit for which patient? Acta Cardiol. 58, 555–560 (2003).
- El Mahmoud R, Le Feuvre C, Le Quan Sang KH et al.: Absence of nephro-protective effect of N-acetylcysteine in patients with chronic renal disease investigated by coronary angiography. (Article in French). Arch. Mal. Coeur. Vaiss. 96, 1157–1161 (2003).
- Oldemeyer JB, Biddle WP, Wurdeman RL, Mooss AN, Cichowski E, Hilleman DE: N-acetylcysteine in the prevention of contrast-induced nephropathy after coronary angiography. Am. Heart J. 146, 23 (2003).
- Durham JD, Caputo C, Dokko J et al.: A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int. 62, 2202–2207 (2002).
- Vallero A, Cesano G, Pozzato M et al.: Contrast nephropathy in cardiac procedures: no advantages with prophylactic use of N-acetylcysteine (NAC) (Article in Italian). G. Ital. Nefrol. 19, 529–533 (2002).
- Briguori C, Manganelli F, Scarpato P et al.: Acetylcysteine and contrast agent-associated nephrotoxicity. J. Am. Coll. Cardiol. 40, 298–303 (2002).
- Allaqaband S, Tumuluri R, Malik AM et al.: Prospective randomized study of N-acetylcysteine, fenoldopam, and saline for prevention of radiocontrast-induced nephropathy. Catheter Cardiovasc. Interv. 57, 279–283 (2002).
- Amini M, Salarifar M, Amirbaigloo A, Masoudkabir F, Esfahani F: N-acetylcysteine does not prevent contrast-induced nephropathy after cardiac catheterization in patients with diabetes mellitus and chronic kidney disease: a randomized clinical trial. Trials 29, 10–45 (2009).
- Ferrario F, Barone MT, Landoni G et al.: Acetylcysteine and non-ionic isosmolar contrast-induced nephropathy – a randomized controlled study. Nephrol. Dial. Transplant 24(10), 3103 (2009).
- Trivedi H, Daram S, Szabo A, Bartorelli AL, Marenzi G: High-dose N-acetylcysteine for the prevention of contrast-induced nephropathy. Am. J. Med. 122, 874 (2009).
- Brown JR, Block CA, Malenka DJ, O’Connor GT, Schoolwerth AC, Thompson CA: Sodium bicarbonate plus N-acetylcysteine prophylaxis: a meta-analysis. Cardiovasc. Interv. 2(11), 1116–1124 (2009).
- Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC: Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann. Intern. Med. 148(4), 284–294 (2008).
- H-Gonzales DA, Norsworthy KJ, Kern SJ et al.: A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med. 5, 32 (2007).
- ACT Trial Investigators: rationale, design, and baseline characteristics of the Acetylcystein for Contrast-Induced Nephropathy (ACT) Trial: a pragmatic randomized controlled trial to evaluate the efficacy of acetylcysteine for the prevention of contrast-induced nephropathy. Trials 10, 38 (2009).
- Lee PT, Chou KJ, Liu CP et al.: Renal protection for coronary angiography in advanced renal disease patients by prophylactic hemodialysis: a randomized controlled trial. J. Am. Coll. Cardiol. 50, 1015–1020 (2007).
- Vogt B, Ferrari P, Schonholzer C et al.: Prophylactic hemodialysis after radiocontrast media in patients with renal insufficiency is potentially harmful. Am. J. Med. 111, 692–698 (2001).
- Marenzi G, Lauri G, Campodonico J et al.: Comparison of two hemofiltration protocols for prevention of contrast-induced nephropathy in high-risk patients. Am. J. Med. 119, 155–162 (2006).
- Marenzi G, Marana I, Lauri G et al.: The prevention of radiocontrast- agentinduced nephropathy by hemofiltration. N. Engl. J. Med. 349, 1333–1340 (2003).
- Cirit M, Toprak O, Yesil M et al.: Angiotensin-converting enzyme inhibitors as a risk factor for contrast-induced nephropathy. Nephron Clin. Pract. 104, 20–27 (2006).
- Kiski D, Stepper W, Brand E, Breithardt G, Reinecke H: Impact of rennin–angiotensin– aldosterone blockade by angiotensinconverting enzyme inhibitors or AT1 blockers on frequency of contrast medium-induced nephropathy: a post-hoc analysis from the Dialysis-versus-Diuresis (DVD) trial. Nephrol. Dial. Transplant DOI:10.1093/ndt/ gfp582 (2009) (Epub ahead of print).
- Rosenstock JL, Bruno R, Kim JK et al.: The effect of withdrawal of ACE inhibitors or angiotensin receptor blockers prior to coronary anjiography on the incidence of contrast-induced nephropathy. Int. Urol. Nephrol. 40, 749–755 (2008).
- Saudan P, Muller H, Feraille E, Martin PY, Mach F: Renin-angiotensin system blockade and contrast-induced renal toxicity. J. Nephrol. 21(5), 681–885 (2008).
- Goergen SK, Rumbold G, Compton G, Harris C: Systematic review of current guidelines, and their evidence base, on risk of lactic acidosis after administration of contrast medium for patients receiving metformin. Radiology 254, 261–269 (2010).
- Ng TM, Shurmur SW, Silver M et al.: Comparison of N-acetylcysteine and fenoldopam for preventing contrast-induced nephropathy (CAFCIN). Int. J. Cardiol. 109, 322–328 (2006).
- Briguori C, Colombo A, Airoldi F et al.: N-Acetylcysteine versus fenoldopam mesylate to prevent contrast media-associated nephrotoxicity. J. Am. Coll. Cardiol. 44, 762–765 (2004).
- Stone GW, McCullough PA, Tumlin JA et al.: Fenoldopam mesylate for the prevention of contrast-induced nephropathy: a randomized controlled trial. JAMA 290, 2284–2291 (2003).
- Wang A, Holcslaw T, Bashore TM et al.: Exacerbation of radiocontrast nephrotoxicity by endothelin receptor antagonism. Kidney Int. 57, 1675–1680 (2000).
- Gare M, Haviv YS, BeN-Yehuda A et al.: The renal effect oflow-dose dopamine in high-risk patients undergoing coronary angiography. J. Am. Coll. Cardiol. 34, 1682–1688 (1999).
- Majumdar SR, Kjellstrand CM, Tymchak WJ, Hervas-Malo M, Taylor DA, Teo KK: Forced euvolemic diuresis with mannitol and furosemide for prevention of contrast-induced nephropathy in patients with CKD undergoing coronary angiography: a randomized controlled trial. Am. J. Kidney Dis. 54, 602–609 (2009).
- Bagshaw SM, Ghali WA: Theophylline for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Arch. Intern. Med. 165, 1087 (2005).
- Anto HR, Chou SY, Porush JG, Shapiro WB: Infusion intravenous pyelography and renal function. Effects of hypertonic mannitol in patients with chronic renal insufficiency. Arch. Intern. Med. 141, 1652 (1981).
- Xinwei J, Xianghua F, Jing Z et al.: Comparison of usefulness of simvastatin 20 mg versus 80 mg in preventing contrastinduced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am. J. Cardiol. 104, 519–524 (2009).
- Patti G, Nusca A, Chello M et al.: Usefulness of statin pretreatment to prevent contrastinduced nephropathy and to improve long-term outcome in patients undergoing percutaneous coronary intervention. Am. J. Cardiol. 101, 279–285 (2008).
- Khanal S, Attallah N, Smith DE et al.: Statin therapy reduces contrast-induced nephropathy: an analysis of contemporary percutaneous interventions. Am. J. Med. 118, 843–849 (2005).
- Bouzas-Mosquera A, Vazquez-Rodriguez JM, Calvino-Santos R et al.: Statin therapy and contrast-induced nephropathy after primary angioplasty. Int. J. Cardiol. 134, 430–431 (2009).
- O’Driscoll G, Green D, Taylor RR: Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation 95, 1126 (1997).
- Koch JA, Plum J, Grabensee B et al.: Prostaglandin E1: a new media for the prevention of renal dysfunction in high risk patients caused by radiocontrast media? Nephrol. Dial. Transplant 15, 43–49 (2000).
- Yokomaku Y, Sugimoto T, Kume S et al.: Asial-erythropoietin prevents contrastinduced nephropathy. J. Am. Soc. Nephrol. 19, 321–328 (2008).
- Spargias K, Alexopoulos E, Kyrzopoulos S et al.: Ascorbic acid prevents contrastmediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 110, 2837 (2004).
- Boscheri A, Weinbrenner C, Botzek B, Reynen K, Kuhlisch E, Strasser RH: Failure of ascorbic acid to prevent contrast media induced nephropathy in patients with renal dysfunction. Clin. Nephrol. 68, 279–286 (2007).
- Toprak O: What is the best definition of contrast-induced nephropathy? Ren. Fail. 29, 387–388 (2007).
▪ Meta-analysis of important studies comparing iso-osmolar iodixanol with other low-osmolar contrast media.
▪ A study to prevent contrast-induced nephropathy with sodium bicarbonate. Despite the low number of patients it was quite well designed in other aspects.
▪ Retrospective study that demonstrated nonsuperiority of sodium bicarbonate, contrary to all prospective studies that showed the superiority of sodium bicarbonate to sodium chloride. However, the high number of patients is remarkable.
▪ The best planned (large-scale, multicenter, prospective, randomized and double-blind) study regarding N-acetylcysteine so far. The results will be essential to eliminate controversies.
▪ This article questioned different contrastinduced nephropathy definitions that have hindered the comparison of to-date contrast-induced nephropathy studies.