Research Article - Neuropsychiatry (2019) Volume 9, Issue 2
Contribution of Sodium-Calcium Exchanger Isoform-3 in Aβ1-42 Induced Cell Death
- *Corresponding Authors:
- Henok Kessete Afewerky
Department of Neurobiology, School of Basic Medicine, Tongji Medical College
Huazhong University of Science and Technology, Wuhan, 430030, China
Tel: 0086-13260654649 - Youming Lu
Department of Physiology, School of Basic Medicine, Tongji Medical College
Huazhong University of Science and Technology, Wuhan, 430030, China
Tel: 0086-18171337550
Abstract
Aβ1-42 is a peptide which can alter intracellular calcium (Ca2+) homeostasis and subsequently cause cell death. The molecular mechanisms underlying Aβ1-42 induced Ca2+ dyshomeostasis and neurodegeneration are not fully elucidated. The third isoform of the sodium-calcium exchanger (NCX) family, NCX3, plays a vital role in adjusting excitable cells Ca2+ homeostasis. In this study, using NCX3 stably transfected baby hamster kidney (BHK) cells, we aim to study the role of NCX3 against Aβ1-42 induced cell death. Our result demonstrates that NCX3 stably transfected cells (BHK-NCX3) are more resistant to Aβ1-42 aggregation compared to their wild types (BHK-WT). The increased cellular loss in BHK-WT was correlated to increased cytoplasmic Ca2+ concentration ([Ca2+]i) induced by Aβ1-42. These results present that NCX3 protects against Aβ1-42 induced cell death and proclaim NCX3 and its downstream molecular pathways as critical components that take part in Aβ1-42 neurotoxicity. Our study substantiates molecular events through which Aβ1-42 can induce neurodegeneration such as Alzheimers disease and marks potential targets to slow down neuropathogenesis.
Keywords
Amyloid-beta, Sodium–calcium exchanger, Baby Hamster Kidney Cells, Alzheimer’s disease
Abbreviations
Aβ: Amyloid-beta; NCX: Sodium-calcium exchanger; BHK: Baby Hamster Kidney cells; AD: Alzheimer’s disease
Introduction
Amyloid-beta (Aβ) peptides are metabolic products consisting of 38 to 43 amino acids, which exists in two prevalent isoforms: soluble Aβ1-40 (∼80-90%) and insoluble Aβ1-42 (∼5- 10%) [1,2]. Monomers of Aβ1-40 are much more ubiquitous than the aggregation-prone and tosxic Aβ1-42 species. Aβ peptides originate from sequential cleavage of the Aβ precursor protein (APP) by the enzymatic actions of β-secretase and γ-secretase [3,4]. Its primacy has been evidenced in the ‘amyloid cascade hypothesis’, which postulates that the accumulation of Aβ (resulting from excessive production, distorted processing or unbalance between production and clearance) is the potential mechanism that leads neurodegeneration in Alzheimer’s disease (AD) [5]. However, the mechanism of pathogenicity for Aβ has not been fully elucidated. In the last decade, several studies have reported that Aβ is implicated in several pathological conditions of the central nervous system, such as frontotemporal dementia (FTLD) [6,7] and AD [5,8,9]. These findings consistently pioneered the contribution of Ca2+ dishomeostasis in Aβ induced cell death [5,9].
The sodium–calcium exchanger (NCX) is a plasma membrane protein that plays a major role in the maintenance of Ca2+ and Na+ homeostasis in most excitable and non-excitable cells. NCX plays a significant role in neurons, where an alteration in cytosolic Ca2+ concentration represents a vital event in several physiological (e.g. synaptic transmission) and pathological phenomenon (e.g. AD) [5,10]. NCX can function in two modes of action which are referred to as the ‘‘Ca2+ outflow mode’’ and ‘‘Ca2+ inflow mode’’ [5,11]. The functioning mode, effectively the direction of exchange, depends on the Ca2+ and Na+ electrochemical gradients across the plasma membrane and on the membrane potential. The Ca2+ outflow mode of exchange (forward mode) is defined functionally as an outside Na+-dependent Ca2+ outflow and an inside Ca2+-dependent Na+-inflow. The Ca2+ inflow mode of exchange (reverse mode) is usually defined as an inside Na+-dependent Ca2+- inflow and outside Ca2+-dependent Na+-outflow. Because of the lack of specific inhibitors, whether NCX contributes to both net Ca2+ inflow and net Ca2+ outflow under normal physiological conditions is not thoroughly elucidated. However, there is a common understanding that the exchanger plays a significant role in removing Ca2+ in various cell types [12-14]. Three isoforms of the exchanger (NCX1, NCX2, and NCX3) encoded by different genes have been cloned [5,15]. NCX1 is predominantly expressed in the heart and is common in excitable cells [16,17]. The NCX2 expression is limited to the brain and spinal cord [16,18], while NCX3 expression is confined to the central nervous system and skeletal muscle [16,19]. In situ hybridization studies using rodent brain depicted that NCX1, NCX2, and NCX3 mRNA are found dispersed in all brain regions including the hippocampus, a region mostly provoked in AD [5,20,21]. The operational roles of each isoform in neurons yet have to be examined. Recently, Henok et al. [5] presented the protective role of NCX against ischemic damage and its role for cognitive function. Moreover, this study specifically implied that NCX3 is a potential molecular target in AD therapy.
In the current study, using NCX3 stably transfected baby hamster kidney (BHK) cells, we aimed to study whether NCX3 contributes against cell death induced by the Aβ1-42. We determined that BHK-NCX3 are more resistant to Aβ1-42 aggregation compared to wild-type (BHK-WT) cells which do not constitutively express NCX3 [22,23], as revealed by the staining with calcein-AM (a marker of living cells) and propidium iodide (a marker of nonviable cells) (Figure 1A). This increase in Aβ1-42-induced death in BHK-WT cells correlate with a significant increase in intracellular Ca2+ concentration ([Ca2+]i) which subsequently contributes toward mitochondrial dysfunction and then ATP depletion [24,25]. In conclusion, our determinations contemplate that NCX3 might offer a therapeutic target to decelerate neurodegenerations such as AD.
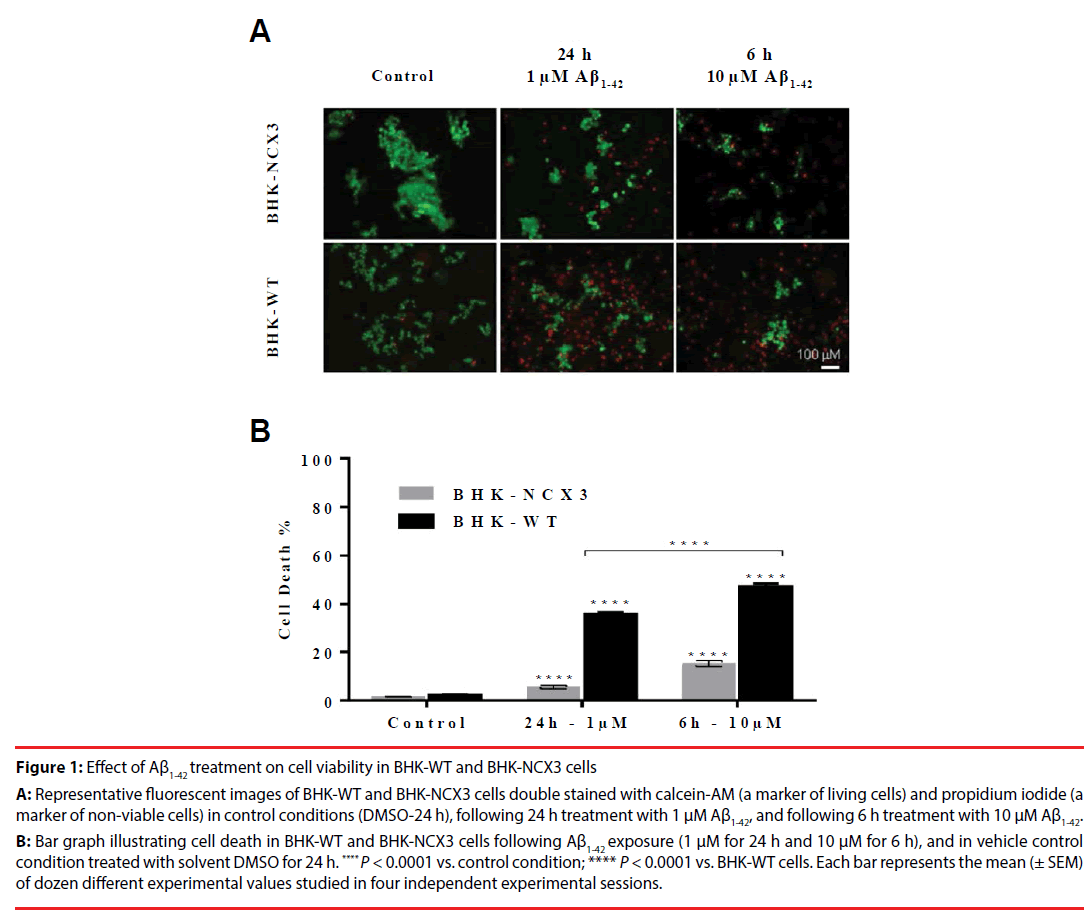
Figure 1: Effect of Aß1-42 treatment on cell viability in BHK-WT and BHK-NCX3 cells
A: Representative fluorescent images of BHK-WT and BHK-NCX3 cells double stained with calcein-AM (a marker of living cells) and propidium iodide (a
marker of non-viable cells) in control conditions (DMSO-24 h), following 24 h treatment with 1 µM Aß1-42, and following 6 h treatment with 10 µM Aß1-42.
B: Bar graph illustrating cell death in BHK-WT and BHK-NCX3 cells following Aß1-42 exposure (1 µM for 24 h and 10 µM for 6 h), and in vehicle control
condition treated with solvent DMSO for 24 h. **** P < 0.0001 vs. control condition; **** P < 0.0001 vs. BHK-WT cells. Each bar represents the mean (± SEM)
of dozen different experimental values studied in four independent experimental sessions.
Materials and Methods
▪Materials
Baby Hamster Kidney (BHK) Cells were obtained from BioVector NTCC Inc. (GNHa10; Beijing, China). Aβ1-42 (ab120301), Rabbit polyclonal anti-NCX3 antibody (1:2000) (ab84708) and mouse monoclonal anti-tubulin (1:1000) (ab7751) were purchased from Abcam (Wuhan, Hubei, China). Nutrient Mixture F-12 (Hams’ F-12) (11320082), Dulbecco’s Modified Eagle’s Medium (DMEM) (12491-015), Trypsin-EDTA 0.25% (25200056), Penicillin-streptomycin (15140122), Heat-inactivated fetal bovine serum (FBS) (16000044), fluorescent dyes (i.e. Trypan Blue (15250061), and DNA binding dye (Hoechst-33258)), Poly-L-lysine (132706), and Protease inhibitor cocktail II (78437) as well as all other unmentioned materials, were all from Thermo Fisher Scientific (Wuhan, Hubei, China).
▪ Cell culture
The baby hamster kidney (BHK) cells were grown on plastic dishes in a mix of DMEM and Ham’s F12 media (1:1) supplemented with 5% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were cultured in a humidified 5% CO2 atmosphere; the culture medium was changed every 2 days. For microfluorimetric studies, cells were plated on glass coverslips (102260, Thermo Fisher Scientific) coated with poly-L-lysine (30 μg/ml) and used at least 12 hours after plating [26].
▪ Cell transfection
In the next day following the seeding of the cells to 70-90% confluence in a flask, complete medium (DMEM solution) was replaced with Opti-MEMTM medium. Cells were transfected by adding complex mix of NCX3 plasmid (5 μl) diluted in a solution of LipofectamineTM as described in the manufacturer’s protocol.
▪ Aβ1-42 peptide treatment
Aβ1-42 was prepared as 0.1 mg/0.1 ml stock solution in sterile phosphate buffer solution (PBS) incubated at 37°C for 24 hours to enhance aggregation, and stored at -20°C. Stock solution was then directly diluted in cell culture media to give the desired experimental concentrations (1 μM/10 μM).
▪ Cell viability count
Following 24 h treatment with 1 μM Aβ1-42 and following 6 h treatment with 10 μM Aβ1-42, at various time points, cells were incubated in an aliquot containing 0.4% Trypan Blue at room temperature (23°C) for 15 min and then blue cells were counted on a haemocytometer. The percentage of Trypan Blue positive cells was averaged over five fields’ total number of cells.
▪ Assessment of nuclear morphology
Nuclear morphology was evaluated by using the fluorescent DNA-binding dye Hoechst-33258. To this aim, cells were fixed in 4% paraformaldehyde and incubated for 5 minutes in PBS containing 1 μg/ml Hoechst-33258 at 37°C. Coverslips were mounted on glass slides and observed with the Olympus fluorescence microscope 1X71S1F-3 (Tokyo, Japan). Digital images were taken with a DP72 camera (Olympus, Tokyo, Japan), stored on the hard-disk of a Pentium III computer, and analyzed with the Image-Pro Plus 4.5 software (Media Cybernetics Inc, Silver Spring, MD, USA). Pathological nuclei were characterized by chromatin condensation (pyknosis) and fragmentation, or by decreases and increases in size [27,28].
▪ Immunoblotting
After treatment, cells were lysed with a buffer containing 20 mM Tris–HCl (pH 7.5), 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1% NONIDET P-40, 1 mM Na3VO4, 0.1% aprotinin, 0.7 mg/ml pepstatin and 1 μg/ml leupeptin. Samples were cleared by centrifugation and supernatants were used for Western blot. Protein concentration in supernatants was determined by Bradford method [29]. Protein samples (50 μg) were analyzed on 8% sodium dodecyl sulfate polyacrilamide gel with 5% sodium dodecyl sulfate stacking gel (SDS-PAGE) and electrotransferred onto Hybond ECL nitrocellulose paper (LC2006, Thermo Fisher Scientific). Membranes were blocked with 5% nonfat dry milk in 0.1% Tween 20 (TBS-T; 2 mmol/l Tris–HCl, 50 mmol/l NaCl, pH 7.5) for 1 h at room temperature and subsequently incubated overnight at 4°C in the blocked buffer with rabbit polyclonal NCX3 antibody (1:2000) [17,30].
The membranes were washed with 0.1% Tween 20 and incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (1:1000) (ab21058, Abcam) for 1 h. Immunoreactive bands were detected with the ECL. The optical density of the bands (normalized with those of actin) was determined by Chemi Doc Imaging System (Biorad).
▪ Statistical analysis
Statistical analysis was performed on actual cell counts using Prism software (GraphPad 6 software, San Diego, CA). All experiments were performed at least three times on separate days. On individual days, experiments were performed in triplicate and averaged to form a single experiment unit. Analyses were performed using one-way analysis of variance (ANOVA) followed by Student t test, and differences were considered to be statistically significant when P values were <.05. Data are presented as means ± standard error of the mean (SEM).
Results
To investigate the contribution of NCX3 against Aβ1-42-induced cell death, NCX3 cDNA was stably transfected in BHK cells, which do not constitutively express NCX isoforms [16]. In both cell lines, BHK-WT and BHK-NCX3, toxicological damages caused by a 24 h exposure of 1 μM Aβ1-42 was compared to an acute exposure of 6 h with a 10 times higher dose of Aβ1-42 [31]. The results of this study, demonstrated that the NCX3 gene product may play a relevant role in the cells resistance to Aβ1-42-induced death. Whereby, BHK-WT cells were more susceptible to Aβ1-42 cytotoxicity than BHK-NCX3 exposed to 1 μM Aβ1-42 for 24 h (36.1 ± 0.7% of cell death in BHK-WT vs. 5.6 ± 0.8% of cell death in BHK-NCX3; P<0.0001) or 10 μM Aβ1-42 for 6 h (47.4 ± 1.1% of cell death in BHK-WT vs. 15.2 ± 1.3% of cell death in BHK-NCX3; P<0.0001) (Figure 1B). In controlled conditions, cell viability was similar in both the transfected and wild type BHK cells as revealed by the haemocytometer cell count.
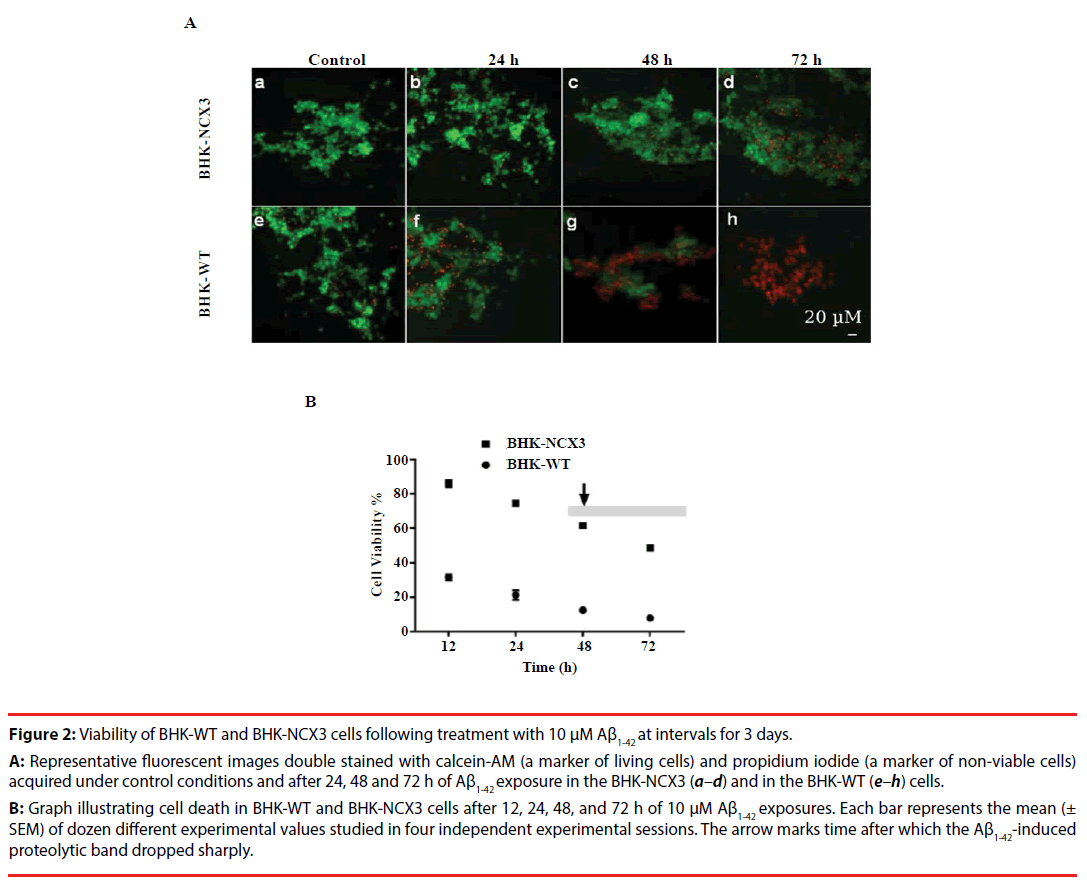
To evaluate BHK-WT and BHK-NCX3 cells resistance to Aβ1-42-induced death at 72 h, these cells were exposed to 10 μM Aβ1-42 following the seeding of the cells to 70-90% confluence. At this period of time, BHK-NCX3 cells were more resistant to the insult as compared to BHK-WT cells as revealed by the staining with calcein-AM and propidium iodide microscopic fluorescence (Figure 2A); and Trypan Blue cell viability count (Figure 2B). In addition, BHK-WT cells showed hastened Aβ1-42-induced abnormal nuclear morphology at 48 h, as detected by Hoechst 33258 (data not shown). Here we present substantiative evidence that BHK cells stably expressing NCX3 isoform are more resistant to Aβ1-42-induced cell death.
Figure 2: Viability of BHK-WT and BHK-NCX3 cells following treatment with 10 µM Aß1-42 at intervals for 3 days.
A: Representative fluorescent images double stained with calcein-AM (a marker of living cells) and propidium iodide (a marker of non-viable cells)
acquired under control conditions and after 24, 48 and 72 h of Aß1-42 exposure in the BHK-NCX3 (a–d) and in the BHK-WT (e–h) cells.
B: Graph illustrating cell death in BHK-WT and BHK-NCX3 cells after 12, 24, 48, and 72 h of 10 µM Aß1-42 exposures. Each bar represents the mean (±
SEM) of dozen different experimental values studied in four independent experimental sessions. The arrow marks time after which the Aß1-42-induced
proteolytic band dropped sharply.
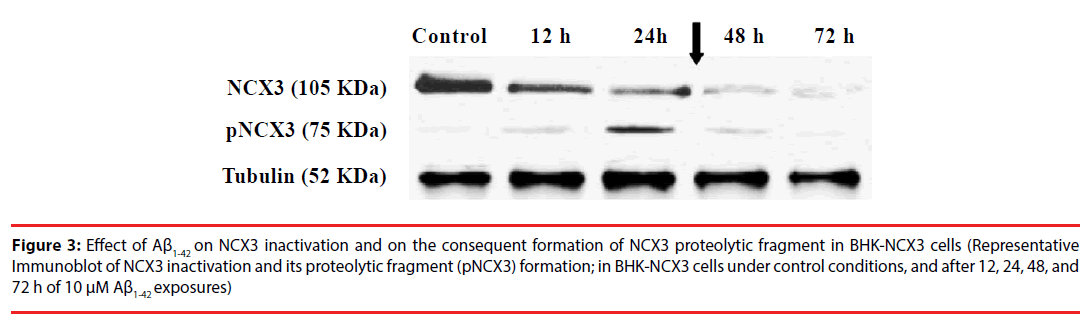
Most importantly, immunoblot analysis revealed that following 10 μM Aβ1-42 peptide treatment, the NCX3 native band, detected at ~105 kDa, decreased at 12, 24, 48, and 72 h, whereas the proteolytic band, migrating at ~75 kDa, increased progressively from 12 to 48 h (Figure 3). Moreover, coincident with the rapid rise in cell death, interestingly the Aβ1-42-induced proteolytic band abruptly dropped after 48 h of the peptide treatment. These results demonstrate the effects of Aβ1-42 on NCX3.
Figure 3: Effect of Aß1-42 on NCX3 inactivation and on the consequent formation of NCX3 proteolytic fragment in BHK-NCX3 cells (Representative Immunoblot of NCX3 inactivation and its proteolytic fragment (pNCX3) formation; in BHK-NCX3 cells under control conditions, and after 12, 24, 48, and 72 h of 10 µM Aß1-42 exposures)
Discussion
Conceptually, physiological Aβ concentrations are necessary for normal synaptic plasticity and memory to occur [32,33]. But, previous lines of research using primary neurons have shown that abnormal Aβ concentration can induce behavioral changes and loss of neurons through the apoptosis program [34,35]. However, the molecular mechanism(s) by which extracellular Aβ peptide damages neurons is still mostly unknown.
One of the main mechanisms responsible for the neurotoxicity by Aβ may be that Aβ interacts with cell membrane components which further directly damages and/or enhances the vulnerability of neurons [36]. To address this, NCX3 stably transfected BHK cells which otherwise do not constitutively express NCX isoforms were cultured.
Using BHK cells expressing brain specific NCX3 isoform, we demonstrate that NCX3 plays an important role in Aβ1-42-induced cell death. Our findings reveal that NCX3 stably transfected BHK cells show significant resistance to Aβ1-42- induced plaque formation and death compared to BHK-WT. These results are consistent to previous reports were NCX3+/+ primary brainstem cultures exposed to Aβ1-42 showed resistance compared to neurons obtained from wild-type mice [37,38]. Furthermore, in most studies, Ca2+ dysregulation following to Aβ1-42 aggregation was the leading cause of neuronal cell death [39,40]. In the current study, results present two important findings. First, NCX3 expression unprovoked Aβ1-42 induced toxicity in BHK cells; whereby NCX3 genetic transfection in to BHK cells was found protective against Aβ1-42-induced cell death. BHK-NCX3 cells treated with Aβ1-42 showed significant resistance to cell death compared to BHK-WT.
Second, we showed that following Aβ1-42 treatment, inhibition of NCX3 expression and ultimately cell death (Figures 2 and 3) over a period of time. We can speculate that the increase of cell death observed in BHK-NCX3 cells in after 48 h of treatment with Aβ1-42 peptide was caused by the peptide fair specificity for the NCX3 functional modes of action (Ca2+ exchange) and its ability to inhibit the normal Krebs cycle complex I in the mitochondrial respiratory chain [30,41]. This speculation is supported by our observation in BHK-NCX3 cells, where NCX3 expression is totally abolished following to 48-72 h Aβ1-42 treatment.
It may be premature to call at this point, but in all probability, mitochondrial dysfunction and accordingly limited ATP production were the contributing factors to the compromised BHK-NCX3 cells survival following Aβ1-42 exposure. The extent to which the mitochondrial dysfunction and the decreased ATP production are a consequence of NCX3 inhibition by Aβ1- 42 is not yet fully elucidated. However, previous studies showed that, mitochondria play a major role in sequestration of Ca2+ [42,43] and since Ca2+ is a positive effector of mitochondrial function [44], any perturbation in mitochondrial and/or cytosolic Ca2+ homeostasis have major consequences to cell function. It is plausible that Aβ1-42 induces a positive-feedback loop between mitochondrial dysfunction and cytoplasmic Ca2+ dysregulation. Overall, these findings present NCX3 protein as a substantial molecular target that may have a potential therapeutic value in modulating Aβ1-42 induced pathogenesis. Further studies are needed to elucidate the exact sequence of events between mitochondrial and Ca2+ pathways of Aβ1-42 cytotoxicity.
Conclusion
Indeed, BHK-NCX3 cells, unlike BHK-WT cells are able to better survive Aβ1-42 aggregation possibly by virtue of the NCX3 Ca2+-buffering properties in conditions of ATP depletion [5,15]. Therefore, our findings assert the protective role of NCX3 against Aβ1-42-induced cell death, and suggest reduced NCX3 and its downstream molecular pathways as key factors involved in Aβ1-42-induced neuropathogenesis such as Alzheimer’s disease. Further studies, including in-vivo animal experiments, are necessary for the determination of the actual contribution of NCX3 in Aβ1-42-induced human neuronal death.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Chinese Government Scholarship–Chinese University Program (No.2018GXZ024192) and National Natural Science Foundation of China (No.31500832).
Author’s Contributions
Henok Kessete Afewerky conceived and designed the study, performed the experiments, analyzed the data and wrote the manuscript. Tongmei Zhang helped to design the experiments. Hao Li and Pei Pang helped to analyze the data. Youming Lu supervised the study.
References
- Gandy S. APP processing, A beta-amyloidogenesis, and the pathogenesis of Alzheimer's disease. Neurobiol. Aging 15(2), 253-256 (1994).
- Zhang YW, Xu H. Molecular and cellular mechanisms for Alzheimer's disease: understanding APP metabolism. Curr. Mol. Med 7(7), 687-696 (2007).
- Serrano-Pozo A, Frosch MP, Masliah E, et al. Neuropathological alterations in Alzheimer disease. Cold. Spring. Harb. Perspect. Med 1(1), a006189 (2011).
- Xu X. Gamma-secretase catalyzes sequential cleavages of the AbetaPP transmembrane domain. J. Alzheimers. Dis 16(2), 211-224 (2009).
- Henok KA, Zhang T, Hao Li, et al. Roles of sodium-calcium exchanger isoform-3 toward calcium ion regulation in Alzheimers disease. J. Alzheimers. Dis. Parkinsonism 6(7), 1-4 (2016).
- Alcolea D, Vilaplana E, Suárez-Calvet M, et al. CSF sAPPbeta, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology 89(2), 178-188 (2017).
- Kim BJ, Irwin DJ, Song D, et al. Optical coherence tomography identifies outer retina thinning in frontotemporal degeneration. Neurology 89(15), 1604-1611(2017).
- Czeczor JK, McGee SL. Emerging roles for the amyloid precursor protein and derived peptides in the regulation of cellular and systemic metabolism. J. Neuroendocrinol 29(5) (2017).
- Garcia-Ribas G, García-Ribas G, Carrió I, et al. PET biomarkers: Use of imaging techniques in Alzheimer disease and neurodegeneration clinical diagnosis. Neurologia 32(5), 275-277 (2017).
- Bano D, Young KW, Guerin CJ, et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 120(2), 275-285 (2005).
- Hilge M. Ca2+ regulation of ion transport in the Na+/Ca2+ exchanger. J. Biol. Chem 287(38), 31641-31649 (2012).
- Michel LY, Hoenderop JG, Bindels RJ. Towards understanding the role of the Na(2)(+)-Ca(2)(+) exchanger isoform 3. Rev. Physiol. Biochem. Pharmacol 168(1), 31-57 (2015).
- Pelzl L, Hosseinzadeh Z, Alzoubi K, et al. Impact of Na+/Ca2+ exchangers on therapy resistance of ovary carcinoma cells. Cell. Physiol. Biochem 37(5), 1857-1868 (2015).
- Sirabella R, Secondo A, Pannaccione A, et al. ERK1/2, p38, and JNK regulate the expression and the activity of the three isoforms of the Na+ /Ca2+ exchanger, NCX1, NCX2, and NCX3, in neuronal PC12 cells. J. Neurochem 122(5), 911-922 (2012).
- Linck B, Zhiyong Q, Zhaoping H, et al. Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3). Am. J. Physiol 274(2), 415-423 (1998).
- Minelli A, Castaldo P, Gobbi P, et al. Cellular and subcellular localization of Na+-Ca2+ exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell. Calcium 41(3), 221-234 (2007).
- Thurneysen T, Nicoll DA, Philipson KD, et al. Sodium/calcium exchanger subtypes NCX1, NCX2 and NCX3 show cell-specific expression in rat hippocampus cultures. Brain. Res. Mol. Brain. Res 107(2), 145-156 (2002).
- Jeon D, Yang YM, Jeong MJ, et al. Enhanced learning and memory in mice lacking Na+/Ca2+ exchanger 2. Neuron 38(6), 965-76(2003).
- Sokolow S, Manto M, Gailly P, et al. Impaired neuromuscular transmission and skeletal muscle fiber necrosis in mice lacking Na/Ca exchanger 3. J. Clin. Invest 113(2), 265-273 (2004).
- Annunziato L, Pignataro G, Boscia F, et al. ncx1, ncx2, and ncx3 gene product expression and function in neuronal anoxia and brain ischemia. Ann. N. Y. Acad. Sci 1099(1), 413-426 (2007).
- Canitano A, Papa M, Boscia F, et al. Brain distribution of the Na+/Ca2+ exchanger-encoding genes NCX1, NCX2, and NCX3 and their related proteins in the central nervous system. Ann. N. Y. Acad. Sci 976(1), 394-404 (2002).
- Jin J, Lao AJ, Katsura M, et al. Involvement of the sodium-calcium exchanger 3 (NCX3) in ziram-induced calcium dysregulation and toxicity. Neurotoxicology 45(1), 56-66 (2014).
- Secondo A, Staiano IR, Scorziello A, et al., The Na+/Ca2+ exchanger isoform 3 (NCX3) but not isoform 2 (NCX2) and 1 (NCX1) singly transfected in BHK cells plays a protective role in a model of in vitro hypoxia. Ann. N. Y. Acad. Sci 1099(1), 481-485 (2007).
- Dodson M, Darley-Usmar V, Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free. Radic. Biol. Med 63(1), 207-221 (2013).
- Wang M, Li Y, Ni C, et al. Honokiol attenuates oligomeric amyloid beta1-42-induced Alzheimer's disease in mice through attenuating mitochondrial apoptosis and inhibiting the nuclear factor kappa-b signaling pathway. Cell. Physiol. Biochem 43(1), 69-81 (2017).
- Secondo A, Staiano RI, Scorziello A, et al. BHK cells transfected with NCX3 are more resistant to hypoxia followed by reoxygenation than those transfected with NCX1 and NCX2: Possible relationship with mitochondrial membrane potential. Cell. Calcium 42(6), 521-535 (2007).
- Ghavami S, Shojaei S, Yeganeh B, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol 112(1), 24-49 (2014).
- Salminen A, Kaarniranta K, Kauppinen A, et al. Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome. Prog. Neurobiol 106-107(1), 33-54 (2013).
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 72(1), 248-254 (1976).
- Brustovetsky T, Brittain MK, Sheets PL, et al. KB-R7943, an inhibitor of the reverse Na+ /Ca2+ exchanger, blocks N-methyl-D-aspartate receptor and inhibits mitochondrial complex I. Br. J. Pharmacol 162(1), 255-270 (2011).
- Lanz TA, Wood KM, Richter KE, et al. Pharmacodynamics and pharmacokinetics of the gamma-secretase inhibitor PF-3084014. J. Pharmacol. Exp. Ther 334(1), 269-277 (2010).
- Puzzo D, Arancio O. Amyloid-beta peptide: Dr. Jekyll or Mr. Hyde?. J. Alzheimers. Dis 33(1), 111-120 (2013).
- Wen J, Fang F, Guo SH, et al. Amyloid beta-Derived Diffusible Ligands (ADDLs) Induce Abnormal Autophagy Associated with Abeta Aggregation Degree. J. Mol. Neurosci 64(2), 162-174 (2017).
- Du YF, Yan P, Guo SG, et al. Effects of fibrillar Abeta(1-40) on the viability of primary cultures of cholinergic neurons and the expression of insulin signaling-related proteins. Anat. Rec. (Hoboken) 294(2), 287-294 (2011).
- Zheng WH, Bastianetto S, Mennicken F, et al. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neurosci 115(1), 201-211 (2002).
- Perini G, Della-Bianca V, Politi V, et al. Role of p75 neurotrophin receptor in the neurotoxicity by beta-amyloid peptides and synergistic effect of inflammatory cytokines. J. Exp. Med 195(7), 907-918 (2002).
- Wu A, Colvin RA. Characterization of exchange inhibitory peptide effects on Na+/Ca2+ exchange in rat and human brain plasma membrane vesicles. J. Neurochem 63(6), 2136-2143 (1994).
- Wu A, Derrico CA, Hatem L, et al. Alzheimer's amyloid-beta peptide inhibits sodium/calcium exchange measured in rat and human brain plasma membrane vesicles. Neurosci 80(3), 675-684 (1997).
- Lee WS, Tsai WJ, Yeh PH, et al. Divergent role of calcium on Abeta- and MPTP-induced cell death in SK-N-SH neuroblastoma. Life. Sci 78(11), 1268-1275 (2006) .
- Piacentini R, Ripoli C, Leone L, et al. Role of methionine 35 in the intracellular Ca2+ homeostasis dysregulation and Ca2+-dependent apoptosis induced by amyloid beta-peptide in human neuroblastoma IMR32 cells. J. Neurochem 107(4),1070-1082 (2008).
- Roberts DE, Matsuda T, Bose R. Molecular and functional characterization of the human platelet Na(+) /Ca(2+) exchangers. Br. J. Pharmacol 165(4), 922-936(2012).
- Einat H, Yuan P, Manji HK. Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: further support for the involvement of mitochondrial function in anxiety disorders. Behav. Brain. Res 165(2), 172-180 (2005).
- Holmes A, Lit Q, Murphy DL, et al. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes. Brain. Behav 2(6), 365-380 (2003).
- Feissner RF, Skalska J, Gaum WE, et al. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front. Biosci. (Landmark Ed) 14(1), 1197-1218 (2009).