Clinical Trial Teport - Interventional Cardiology (2012) Volume 4, Issue 6
Coronary and endovascular applications of the Absorb bioresorbable vascular scaffold
- Corresponding Author:
- Marc Bosiers
Department of Vascular Surgery, AZ St-Blasius
Kroonveldaan 50, 9200
Dendermonde, Belgium
Tel: +32 0 5225 28 22
Fax: +32 0 5225 22 89
E-mail: marc.bosiers@telenet.be
Abstract
Keywords
absorbable stent, bioresorbable scaffold, coronary artery disease, everolimus, peripheral vascular disease
Over the past 50 years, magnificent strides have been made in the understanding and primary prevention of atherosclerosis. Nonetheless, as patients age and arteries become brittle, early atherosclerotic plaques inexorably progress to their occlusive end-stage and induce the clinical syndromes of angina pectoris, transient ischemic attack and claudication and, all too frequently, their sinister counterparts myocardial infarction, stroke and amputation.
In the current era, revascularization of endstage atherosclerotic plaques is most commonly afforded through catheter-based balloon dilation with or without the implantation of permanent metal stents. Although successful recanalization can be achieved in almost all cases, the chronic presence of an indwelling foreign body forever creates the potential for ongoing neointimal hyperplasia and neoatherosclerosis, culminating in ‘late catch-up’ restenosis. Indeed, a recent study of the long-term outcome following percutaneous coronary intervention (PCI) suggested that, during 5-year follow-up, a full 26% of patients sustain clinical events, including 10% mortality, 8.4% myocardial infarction and 17% repeat revascularization [1]. Symptomatic recurrence following percutaneous peripheral intervention is even more frequent. In the superficial femoral artery, for example, restenosis still complicates approximately 40% of all procedures in the first year [2–19]. Indwelling metal stents in the superficial femoral artery are particularly problematic, given their unsettling tendency toward fracture. Although only occasionally observed in the coronary [20,21], carotid [22,23], renal [24,25], iliac [26] and venous systems [27], stent fracture following femoropopliteal implantation is disturbingly common [10,28–36], as high as 65% in one clinical report [37].
Although bothersome and costly, fracture and restenosis are complications of stenting that are generally treatable. More disquieting is the ongoing risk of frank coronary thrombosis which, even using the most conservative estimates, can be expected to generate immediate mortality in 20% of cases [38]. The risk of stent thrombosis within the first year following PCI with drugeluting stents (DES) is thankfully low, between 1 and 2% when using older generation devices and <1% with newer entrants [39]. However, stent thrombosis beyond the first year continues to be observed. In fact, it’s estimated that so-called ‘very late stent thrombosis’ occurs at a persistent rate of about 0.6%/year [40,41] such that, after 10 years of treatment, the patient’s cumulative risk of stent thrombosis has accrued to an alarming 6%. Patients in certain subgroups are exposed to even higher risk – for example, patients undergoing PCI for acute myocardial infarction may sustain thrombosis in as many as 10% of cases [42].
Stent occlusion in the peripheral vasculature is less well-studied but no less important. In the setting of critical limb ischemia (CLI), failure to maintain patency in stented segments of ‘flowdependent’ limbs will result in amputation. Also, because lesions tends to be longer and more complex, stent occlusion in the periphery tends to be more common than in the coronary arteries; incidences of up to 6–25% have been reported depending on the clinical scenario and the length and veracity of follow-up [43–49].
Critical limb ischemia
CLI represents the end-stage of chronic peripheral arterial disease. It arises when lowerextremity blood flow and oxygen delivery have decreased to such an extent as to be inadequate to maintain tissue viability, giving rise to ischemic rest pain, ulceration and/or gangrene. Without prompt intervention, CLI will result in limb loss.
Endovascular revascularization is an attractive therapeutic option for patients with CLI given its minimally invasive nature and the potential for recanalization of multiple affected arteries. Successful endovascular recanalization can now be achieved in the majority of cases, even in tibial arteries with complex disease. However, arterial patency following endovascular recanalization tends to be short-lived, as sluggish blood flow in the long and diminutive conduits generates elastic recoil, neointimal hyperplasia, restenosis and therapeutic failure. In general, only approximately 50% of tibial lesions treated with percutaneous transluminal angioplasty (PTA) will remain patent and free from restenosis after the first year [50–53]. The use of intravascular stents following tibial angioplasty certainly enhances acute results, but whether primary bare metal stenting improves long-term patency remains controversial [53–55].
In the coronary arteries, the risk of restenosis has been profoundly attenuated by DES, and some have theorized that coronary DES might also be useful in the similarly sized infrapopliteal arteries. Several nonrandomized clinical registries support such a notion [53,56–62], and to date, three randomized trials addressing the hypothesis that DES would enhance tibial artery patency have been conducted: YUKON BTK [63], DESTINY [64] and ACHILLES [65].
The purpose of the prospective, randomized, multicenter, double-blind YUKON BTK trial was to compare a polymer-free sirolimus-eluting stent (Yukon®; Translumina, Hechingen, Germany) with a placebo-coated bare metal stent in patients with either intermittent claudication or CLI with a de novo occlusive lesion in an infrapopliteal artery [63]. The main study end point was 1-year primary patency defined as freedom from in-stent-restenosis (luminal narrowing of ≥50%) detected with duplex ultrasound or angiography. 161 patients with a mean lesion length of 31 ± 9 mm were included in the trial (25 patients died during follow-up, leaving 125 for analysis). After 1-year, the primary patency rate was significantly higher in the sirolimus-eluting stent group (80.6%) than in the bare-metal stent group (55.6%; p = 0.004) and, clinically, the median (interquartile range) change in Rutherford–Becker classification was -2 (-3 to -1) in the sirolimus-eluting stent group compared with only -1 (-2 to 0) in the bare-metal stent group (p = 0.004).
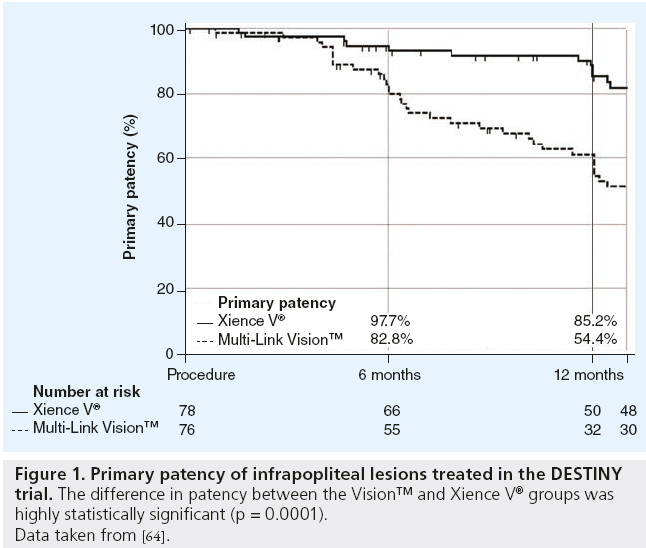
The purpose of the prospective, randomized, controlled DESTINY trial was to test the hypothesis that treatment of infrapopliteal arterial occlusive lesions with an everolimus-eluting stent (Xience V®; Abbott Laboratories, Abbott Park, IL, USA) would provide superior patency to treatment with a bare metal stent (Multi-Link Vision™; Abbott) [64]. The primary end point was arterial patency at 12 months defined as the absence of ≥50% restenosis based on quantitative analysis of contrast angiography. Between March of 2008 and September of 2009, 74 patients were treated with Xience V and 66 patients were treated with Vision. After 12 months, the primary patency rate following treatment with Xience V was 85% compared with 54% after treatment with Vision (p = 0.0001; Figure 1). Treatment with Xience V significantly reduced mean in-stent diameter stenosis (21 ± 21% vs 47 ± 27%; p < 0.0001) and mean in-stent late lumen loss (0.78 ± 0.63 mm vs 1.41 ± 0.89 mm; p = 0.001), and the use of the Xience V stent significantly reduced the need for repeat intervention (freedom from target lesion revascularization 95% for Xience V vs 65% for Vision; p = 0.005).
Figure 1: Primary patency of infrapopliteal lesions treated in the DESTINY trial. The difference in patency between the Vision™ and Xience V® groups was
highly statistically significant (p = 0.0001).
Data taken from [64].
Lastly, the purpose of the prospective, randomized, multicenter ACHILLES trial was to compare PTA to sirolimus-eluting stent implantation (Cypher® Select Plus; Cordis Corporation, Miami Lakes, FL, USA) for the treatment of infrapopliteal arterial disease. As of the time of writing, the results of ACHILLES have not been formally published, although the data have been presented publicly [65]. The study enrolled 99 patients that received the sirolimuseluting (Cypher) stent, and 101 that were treated with PTA alone. Mean lesion lengths were 26.9 ± 20.9 mm and 26.8 ± 21.3 mm in the Cypher and PTA groups, respectively. After 1 year, the use of the Cypher stent resulted in a highly statistically significant decrease in binary restenosis (45.5 vs 21.3%; p = 0.004), as well as a significant improvement in clinical status (change in Rutherford–Becker Clinical Category -2.2 ± 1.6 vs -1.6 ± 1.8; p = 0.044). Unfortunately, although the results were strongly positive, the Cypher device has been removed from the market by the manufacturer. Nonetheless, these three clinical trials suggest that DES implantation in the tibial arteries yields superior patency to either PTA or bare metal stenting, and form the basis of the rationale for the use of drug-eluting bioresorbable vascular scaffolds (BVS) for the treatment of CLI.
Bioresorbable vascular scaffolds
Stack and colleagues, from Duke University (NC, USA), are generally credited with the first descriptions of intravascular bioresorbable scaffolds [66]. In 1990, a technique was described wherein monofilaments of poly(l-lactic acid) (PLLA) were braided into an open tubular mesh and implanted in dogs for up to 12 weeks [67]. The stented arteries maintained patency without significant inflammation or thrombosis. More extensive preclinical studies of bioresorbable scaffolds were conducted by Keiji Igaki and Hideo Tamai in the mid- and late-1990s. They fashioned balloon-expandable knitted stents from polyglycolic acid or PLLA filaments and implanted the devices in both canine and porcine models [68,69]. Although significant diameter reductions were observed in the early postprocedure period, the devices were welltolerated without evidence for thrombosis or undue neointimal reaction. Interestingly, this same group more recently explored the feasibility of coating the device with a tyrosine kinase inhibitor, which had the effect of attenuating experimental neointimal hyperplasia [70].
The first bioresorbable vascular scaffold developed for clinical use was the Igaki-Tamai® device. The nondrug-coated scaffold was designed as a coil made of PLLA monofilament with a zigzag helical pattern [69–71]. Deployment of the device was facilitated with a balloonexpandable covered sheath system and included two radiopaque gold markers to confirm placement. Due to the thermal properties of the scaffold, balloon inflation was performed with a heated dye at 80°C to ensure adequate expansion. Following deployment, it was designed to fully resorb within 6–12 months.
The Igaki-Tamai coronary scaffold was first clinically tested in 1998 at the Shiga Medical Center for Adults in Shiga (Japan) [72]. A total of 25 scaffolds were successfully implanted in 19 lesions. After 6 months, both the restenosis and target lesion revascularization rates were only 10.5%, representing a major advance in the field at that time. The authors of this initial clinical experience suggested that the device was “feasible, safe and effective in humans”. 1-year clinical and angiographic results from a larger cohort were subsequently reported by Tsuji et al. in 2001 in abstract form [73]. Serial quantitative angiography at 3, 6 and 12 months demonstrated somewhat disappointing percent diameter stenoses of 12 ± 8%, 38 ± 23% and 33 ± 23%, respectively, including ≥50% binary restenosis in 21% of patients at 6 months. Given the superior results of drug-eluting stents, further development of these nondrug scaffolds was halted. Nonetheless, the original cohort of patients treated with the Igaki-Tamai device were continuously followed, and their 4‑year results were reported in abstract form in 2004 [74]. In total, 50 patients had been followed for 40–61 months, with 4‑year overall survival and MACE-free survival rates of 98% and 82%, respectively. More recently, the long-term results (>10 years) of this 50‑patient original cohort have been published [75]. There was only a single cardiac death, six noncardiac deaths and four myocardial infarctions. The cumulative rates of target lesion revascularization were 16% at 1 year, 18% at 5 years and 28% at 10 years, with only two definite scaffold thromboses reported (one subacute and one very late). Although these rates of target lesion revascularization are probably unacceptable in the current era of coronary drug-eluting stents, the long-term experience using the Igaki-Tamai device demonstrates the safety and feasibility of resorbable coronary scaffolds.
The Absorb™ bioresorbable vascular scaffold
The Absorb™ Bioresorbable Vascular Scaffold (Abbott Laboratories) is the first resorbable device to incorporate antiproliferative drug elution into its design [76]. It is composed of three basic components: a PLLA polymer scaffold, a poly-d,l-lactide coating and the antiproliferative drug everolimus [77,78].
The backbone structure of the Absorb scaffold is shown in Figure 2 [77]. Absorb is made of PLLA, a semicrystalline polymer with a microstructure that can be tuned by varying the mechanical and thermal conditions of processing. In this application, a polymer scaffold was created, having similar radial force as a balloonexpandable metal stent, as shown by standard in vitro force measurements in comparison with multiple approved bare-metal and drug-eluting stents [76,77]. The Absorb scaffold is coated with a 1:1 mixture of poly-d,l-lactide to incorporate and elute everolimus. The equimolar mixture of polylactide d- and l-stereoisomers is fully amorphous, making it ideal as a drug carrier. The scaffold’s strut thickness of 158 μm [76,79,80] is similar to traditional sirolimus-eluting stainless steel stents (Cypher, 154 μm) [81] but somewhat larger than newer generation everolimus-eluting cobalt–chromium stents (Xience, 81 μm) [82]. The antiproliferative drug, everolimus (40-O-[2- hydroxyethyl]-rapamycin), is a macrolide immunosuppressant that, in conjunction with cyclosporine, has been shown to prevent chronic rejection episodes of solid organ transplants [83–85] and, more recently, has been suggested as an oncologic adjuvant in patients with certain solid malignant tumors [86–89]. In vascular tissue, everolimus effectively inhibits neointimal hyperplasia, enhances remodeling [90–93], and has been shown to be safe and effective as the drug component of coronary and peripheral drug-eluting stents [94–99]. Similar to the Xience everolimus-eluting cobalt chromium stent, everolimus is loaded onto the Absorb device at a dose of 100 μg everolimus per cm² device area. Via a nonenzymatic hydrolytic conversion to lactic acid, the Absorb PLA scaffold resorbs after approximately 24 months, after which the structure of the device cannot be readily discerned radiographically or histologically [100].
Figure 2: The ABSORB COHOR T B 3.0 x 18 mm everolimus-eluting bioresorbable vascular scaffold. Data taken from [77].
Clinial experience in percutaneous coronary intervention: the ABSORB trials
Clinical and morphological outcomes of patients treated with Absorb during PCI have been documented in the ABSORB serial of trials. Two different devices have been tested: the BVS 1.0 device tested in the ABSORB COHORT A trial and the BVS 1.1 device tested in the COHORT B trial. Early experience with BVS 1.0 in the COHORT A trial informed several important alterations in the evolution of the device, and also allows for examination of its noteworthy effects in the long-term.
The ABSORB COHORT A trial, begun in 2006, was initially reported in Lancet in 2008 [101]. In this prospective open-label study, 30 patients having either stable, unstable, or silent coronary ischemia from a single de novo occlusive lesion were treated with either a 3 × 12 mm or 3 × 18 mm Absorb scaffold. Procedural success was 100%, and the only subacute complication was a non-Q-wave myocardial infarction in a patient that required nonischemia-driven target lesion revascularization 46 days after the index procedure. After 6 months, mean in-scaffold late lumen loss was 0.44 ± 0.35 mm, mean in-scaffold percent diameter stenosis was 27% ± 14% and ≥50% binary restenosis was observed in only three of 26 evaluable lesions (12%) [102]. After 5 years of follow-up, all patients were well, without further episodes of myocardial infarction or target lesion revascularization [103–105].
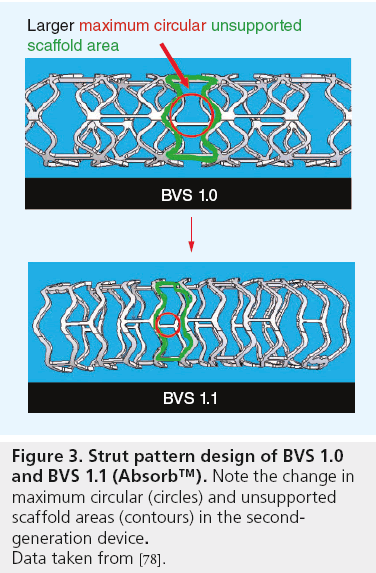
Careful review of the imaging results of the ABSORB COHORT A study suggested that the scaffolding properties of the device required enhancement. The device was therefore redesigned with a more uniform strut distribution and reduced maximum circular unsupported scaffold area (Figure 3), which theoretically enhances radial strength, reduces recoil and provides more even support to the arterial wall [78,106]. The new device, called BVS 1.1 or, simply, Absorb, employs the same materials, markers, drug and elution rate as the original device.
Figure 3: Strut pattern design of BVS 1.0
and BVS 1.1 (Absorb™). Note the change in
maximum circular (circles) and unsupported
scaffold areas (contours) in the secondgeneration
device.
Data taken from [78].
The newly designed scaffold was tested clinically in the ABSORB COHORT B trial which was designed to angiographically examine scaffolded arteries at multiple time points ranging from 6 months to 3 years. The trial was designed to enroll 101 patients with de novo occlusive coronary lesions and either stable angina, unstable angina or silent ischemia. Lesions were required to have a diameter range of 2.5–3.3 mm, length ≤14 mm, percent diameter stenosis ≥50% and <100%, and thrombolysis in myocardial infarction flow grade ≥1 [78,107]. Implanting physicians were advised to utilize quantitative coronary angiography to carefully size target arteries, as overdilatation of the polymer device was not allowed and ill-advised [108–110]. In order to avoid multiple serial radiographic examinations in the same patient, two distinct patient groups were enrolled: the first group of 45 patients was subjected to repeat angiographic examinations at 6 months and 2 years, while the second group of 56 patients was subjected to repeat examinations at 1 and 3 years. The currently available 2-year results show that in-scaffold late lumen loss was significantly improved over the first-generation device, and remained consistently low over time (6 months: 0.19 ± 0.18 mm; 1 year: 0.27 ± 0.32 mm; 2 years: 0.27 ± 0.19 mm) [78,107,111,112]. The binary restenosis rate remained low as well, <4% throughout the 2-year study (6 months: 2.4%; 1 year: 3.5%; 2 years: 0%). The average 2-year in-scaffold late lumen loss of only 0.27 ± 0.19 mm is particularly noteworthy, being the lowest reported amount of lost lumen in any trial of PCI [113–115].
The ABSORB series of trials also generated several additional observations that were unexpected and heretofore unimagined in the field of vascular intervention. Included among these novel findings were:
▪ That the Absorb device, although made of PLLA polymer, maintains its overall circular shape and area within the artery for at least 1 year [78,107];
▪ That dissolution of the device can be followed qualitatively using standard imaging techniques [76,100,116];
▪ That, compared with metal platforms, the resorbable device causes significantly less straightening of tortuous arterial segments [117], reducing untoward mechanical phenomena such as over-stretch, hinging and edge deformation;
▪ That many scaffolded arteries regain their ability to respond to exogenous vasoactive agents over time [103,107];
▪ That orifices of side branches that are ‘jailed’ by the device return to full patency after dissolution [118,119].
Without question, however, the most intriguing observation from the ABSORB series of trials is the finding that some coronary arteries, unencumbered by a permanent metal cage, can actually adapt and increase their lumen size over time. For instance, in the ABSORB COHORT A study, Intravascular ultrasound (IVUS)-measured mean lumen area, which decreased from 6.04 ± 1.12 mm2 postprocedure to 5.19 ± 1.33 mm2 after 6 months, had subsequently rebounded to 5.47 ± 2.11 mm2 in the ensuing 18 months [107]. Although focal angiographic assessment suggested a slight decrease in minimum lumen diameter during this same interval (from 1.89 ± 0.31 mm to 1.76 ± 0.35 mm), the IVUS results suggested an overall increase in total lumen area and volume over time. Even more impressive was that this same observation was repeated in the ABSORB COHORT B trial although the secondgeneration device produced little in the way of initial neointima. The 2-year value for IVUSmeasured mean lumen area of 6.85 ± 1.78 mm2 was statistically significantly greater than the result at 6 months (6.36 ± 1.18 mm2; p = 0.01), and remarkably similar to the original postprocedure result (6.53 ± 1.24 mm2) [112]. Although the mechanism is speculative, late luminal enlargement after intervention with Absorb could conceivably arise from:
▪ The slow maturation, solidification and fibroelastic remodeling of neointimal hyperplasia as extracellular matrix is absorbed [120–126];
▪ Continued adaptation to the new environment of physiologically normal mean and oscillatory shear stress as the scaffold slowly dissolves;
▪ Physical reduction in lesion volume as the scaffold is resorbed;
▪ And/or a pharmacological effect of everolimus which has been shown to favorably affect human coronary artery remodeling even in the absence of a stent [84].
The ABSOR B BTK clinical trial
Given the success of the Absorb everolimuseluting bioresorbable scaffold in the coronary arteries, some have suggested that this approach might also be feasible for the peripheral arteries [127]. Such rationale is based upon: the favorable clinical results of the redesigned Absorb scaffold outlined above; the favorable histologic findings in experimental peripheral arteries treated with Absorb [128]; and the recent observation that everolimus-eluting metal stents significantly enhance patency in recalcitrant tibial artery occlusive disease [99].
The purpose of the ABSORB BTK Clinical Investigation is to evaluate the safety and efficacy of the Abbott Vascular Bioresorbable Vascular Scaffold System for the treatment of subjects with CLI from occlusive vascular disease of the tibial arteries. The trial is a prospective, single-arm, open-labeled, multicenter clinical investigation that will enroll approximately 90 patients in Europe and Australasia. The primary end point of the trial, as suggested by the Society for Vascular Surgery, is freedom from major adverse limb events occurring within 1 year or peri-procedural death occurring within 30 days [129].
Future perspective
The future of intravascular intervention is bright. From its humble beginnings of battlefield amputations and digitalis leaves, has emerged a field wherein most occluded arteries in humans can be reopened without surgical incision. Percutaneous intervention has progressed rapidly from rudimentary balloon dilatation to streamlined, small-caliber instrumentation that maintains patency via the world’s first true application of localized intracorporeal drug delivery.
Despite these advances, the majority of patients with occlusive arterial syndromes are still treated by insertion of rigid metal stents within their fragile intravascular systems. It is hoped that the development of transient, resorbable, drug-eluting scaffolds will usher in a new interventional era wherein vascular disease can be treated organically and enduringly without the need for permanent metal implants.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Executive summary
Critical limb ischemia
▪ Critical limb ischemia is the most severe form of peripheral arterial disease.
▪ Only 50% of patients remain alive and free from major amputation 1 year after the diagnosis of critical limb ischemia is made.
▪ The YUKON BTK trial showed significantly higher patency in tibial arteries treated with sirolimus-eluting stents (81%) compared with bare-metal stents (56%; p = 0.004).
▪ The DESTINY trial showed significantly higher patency in tibial arteries treated with everolimus-eluting stents (85%) compared with bare-metal stents (54%; p = 0.00014).
▪ The ACHILLES trial showed significantly decreased incidence of restenosis in tibial arteries treated with sirolimus-eluting stents (21%) compared with balloon angioplasty (46%; p = 0.004).
Bioresorbable vascular scaffolds
▪ The first bioresorbable scaffold that was tested clinically was the Igaki-Tamai™ PLA (nondrug) coronary device.
▪ The cumulative rates of coronary target lesion revascularization in the original Igaki-Tamai cohort were 16% at 1 year, 18% at 5 years and 28% at 10 years.
The Absorb™ bioresorbable vascular scaffold
▪ Absorb™ is the first resorbable device to incorporate antiproliferative drug elution into its design.
▪ Absorb is composed of three basic components: a poly-l-lactide polymer scaffold, a poly-d,l-lactide coating and the antiproliferative drug everolimus.
Clinial experience in percutaneous coronary intervention: the ABSORB trials
▪ The ABSORB COHORT B trial has shown the lowest 2‑year late loss of any trial of percutaneous coronary intervention to date (6 months 0.19 ± 0.18 mm, 1 year 0.27 ± 0.32 mm, 2 years 0.27 ± 0.19 mm).
▪ The binary restenosis rate in the 2‑year cohort was 0%.
▪ The Absorb device maintains its overall circular shape and area within the artery for at least 1 year.
▪ Dissolution of the Absorb device occurs within 24 months and can be followed qualitatively using standard imaging techniques.
▪ Compared with metal platforms, the Absorb device causes significantly less straightening of tortuous arterial segments.
▪ Many scaffolded arteries regain their ability to respond to exogenous vasoactive agents over time.
▪ Some scaffolded coronary arteries, unencumbered by a permanent metal cage, can adapt and increase their lumen size over time.
The ABSORB BTK clinical trial
▪ The rationale for the ABSORB BTK trial is based upon:
– Favorable clinical results in coronary arteries treated with the redesigned Absorb scaffold;
– Favorable histologic findings in experimental peripheral arteries treated with Absorb;
– The recent observation that everolimus-eluting metal stents significantly enhance patency in recalcitrant tibial artery occlusive disease.
▪ The purpose of the ABSORB BTK trial is to evaluate the safety and efficacy of the Absorb device for the treatment of subjects with critical limb ischemia from occlusive vascular disease of the tibial arteries.
▪ The primary end point of the ABSORB BTK trial is freedom from major adverse limb events occurring within 1 year or peri-procedural death occurring within 30 days.
References
- Zellweger MJ, Kaiser C, Jeger R et al. Coronary artery disease progression late after successful stent implantation. J. Am. Coll. Cardiol. 59, 793–799 (2012).
- Pentecost MJ, Criqui MH, Dorros G et al. Guidelines for peripheral percutaneous transluminal angioplasty of the abdominal aorta and lower extremity vessels: a statement for health professionals from a special writing group of the Councils on cardiovascular radiology, arteriosclerosis, cardio-thoracic and vascular surgery, clinical cardiology, and epidemiology and prevention, the American Heart Association. J. Vasc. Interv. Radiol. 14, S495–S515 (2003).
- Costanza MJ, Queral LA, Lilly MP, Finn WR. Hemodynamic outcome of endovascular therapy for TransAtlantic Inter Society Consensus type B femoropopliteal arterial occlusive lesions. J. Vasc. Surg. 39, 343–350 (2004).
- BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 366, 1925–1934 (2005).
- Ouriel K. Peripheral arterial disease. Lancet 358, 1257–1264 (2001).
- Gray BH, Sullivan TM, Childs MB, Young JR, Olin JW. High incidence of restenosis/ reocclusion of intravascular stents in the percutaneous treatment of long-segment superficial femoral artery disease after suboptimal angioplasty. J. Vasc. Surg. 25, 74–83 (1997).
- Gray B, Olin J. Limitations of percutaneous transluminal angioplasty with stenting for femoropopliteal arterial occlusive disease. Sem. Vasc. Surg. 10(1), 8–16 (1997).
- Schillinger M, Sabeti S, Loewe C et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N. Engl. J. Med. 354, 1879–1888 (2006).
- Hirsch AT, Haskal ZJ, Hertzer NR et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. A collaborative report from the American Association for Vascular Surgery/ Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease). J. Am. Coll. Cardiol. 1239–1312 (2006).
- Sabeti S, Schillinger M, Amighi J et al. Primary patency of femoropopliteal arteries treated with Nitinol versus stainless steel selfexpanding stents: propensity score-adjusted analysis. Radiology 232, 516–521 (2004).
- Sabeti S, Mlekusch W, Amighi J, Minar E,Schillinger M. Primary patency of longsegment self-expanding Nitinol stents in the femoropopliteal arteries. J. Endovasc. Ther. 12, 6–12 (2005).
- Morgan JH 3rd, Wall CE Jr, Christie DB 3rd, Harvey RL, Solis MM. The results of superficial femoral, popliteal, and tibial artery stenting for peripheral vascular occlusive disease. Am. Surg. 71, 905–910 (2005).
- Conroy RM, Gordon IL, Tobis JM et al. Angioplasty and stent placement in chronic occlusion of the superficial femoral artery: technique and results. J. Vasc. Interv. Radiol. 11, 1009–1020 (2000).
- Mucelli FP, Fisicaro M, Calderan L et al. Percutaneous revascularization of femoropopliteal artery disease: PTA and PTA plus stent. Results after six years’ follow-up. La Radiologia Medica 105, 339–349 (2003).
- Ruef J, Hofmann M, Haase J. Endovascular intervention in iliac and infrainguinal occlusive artery disease. J. Interven. Cardiol. 17, 427–435 (2004).
- Treiman GS, Treiman R, Whiting J. Results of percutaneous subintimal angioplasty using routine stenting. J. Vasc. Surg. 43, 513–519 (2006).
- Ascher E, Marks NA, Hingorani AP, Schutzer RW, Mutyala M. Duplex-guided endovascular treatment for occlusive and stenotic lesions of the femoral-popliteal arterial segment: a comparative study in the first 253 cases. J. Vasc. Surg. 44, 1230–1238 (2006).
- Bui TD, Gordon IL, Nguyen T, Fujitani RM, Wilson SE, Conroy RC. Transluminal stenting for femoropopliteal occlusive disease: analysis of restenosis by serial arteriography. Ann. Vasc. Surg. 20, 200–208 (2006).
- Cheng SWK, Ting ACW, Ho P. Angioplasty and primary stenting of high-grade, longsegment superficial femoral artery disease: is it worthwhile? Ann. Vasc. Surg. 17, 430–437 (2003).
- Sianos G, Hofma S, Ligthart JMR et al. Stent fracture and restenosis in the drug-eluting stent era. Catheter. Cardiovasc. Interv. 61, 111–116 (2004).
- Min PK, Yoon YW, Kwon HM. Delayed strut fracture of sirolimus-eluting stent: a significant problem or an occasional observation? Int. J. Cardiol. 106, 404–406 (2006).
- Valibhoy AR, Mwipatayi BP, Sieunarine K. Fracture of a carotid stent: an unexpected complication. J. Vasc. Surg. 45, 603–606 (2007).
- Chakravarty T, White AJ, Buch M et al. Metaanalysis of incidence, clinical characteristics and implications of stent fracture. Am. J. Cardiol. 106, 1075–1080 (2010).
- Bessias N, Sfyroeras G, Moulakakis KG, Karakasis F, Ferentinou E, Andrikopoulos V. Renal artery thrombosis caused by stent fracture in a single kidney patient. J. Endovasc. Ther. 12, 516–520 (2005).
- Sahin S, Memis A, Parildar M, Oran I. Fracture of a renal artery stent due to mobile kidney. Cardiovasc. Intervent. Radiol. 28, 683–685 (2005).
- Sacks BA, Miller A, Gottlieb MM. Fracture of an iliac artery Palmaz stent. J. Vasc. Inter. Radiol. 7, 53–55 (1996).
- Kapoor B, Lockhart M, Sharma D, Maya ID. Brachiocephalic vein stent fracture: case series and literature review. Semin. Dial. 23, 110–113 (2010).
- Scheinert D, Scheinert S, Sax J et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J. Am. Coll. Cardiol. 45, 312–315 (2005).
- Schlager O, Petra D, Schila S et al. Longsegment SFA stenting – the dark sides: instent restenosis, clinical deterioration, and stent fractures. J. Endovasc. Ther. 12, 676–684 (2005).
- Peck R, Wattam J. Fracture of Memotherm metallic stents in the biliary tract. Cardiovasc. Intervent. Radiol. 23, 55–56 (2000).
- Iida O, Nanto S, Uematsu M et al. Effect of exercise on frequency of stent fracture in the superficial femoral artery. Am. J. Cardiol. 98, 272–274 (2006).
- Arena FJ. Arterial kink and damage in normal segments of the superficial femoral and popliteal arteries abutting nitinol stents – a common cause of late occlusion and restenosis? A single-center experience. J. Invasive Cardiol. 17, 482–486 (2005).
- Babalik E, Gülbaran M, Gürmen T, Oztürk S. Fracture of popliteal artery stents. Circ. J. 67, 643–645 (2003).
- Rits J, Van Herwaarden JA, Jahrone AK, Krievins D, Moll FL. The incidence of arterial stent fractures with exclusion of coronary, aortic, and non-arterial settings. Eur. J. Vasc. Endovasc. Surg. 36, 339–345 (2008).
- Adlakha S, Sheikh M, Wu J et al. Stent fracture in the coronary and peripheral arteries. J. Interv. Cardiol. 23, 411–419 (2010).
- Chang IS, Chee HK, Park SW et al. The primary patency and fracture rates of selfexpandable nitinol stents placed in the popliteal arteries, especially in the P2 and P3 segments, in Korean patients. Korean J. Radiol. 12, 203–209 (2011).
- Allie DE, Hebert CJ, Walker CM. Nitinol stent fractures in the SFA. Endovasc. Today 7, 22–34 (2004).
- Holmes DR Jr, Kereiakes DJ, Garg S et al. Stent thrombosis. J. Am. Coll. Cardiol. 56, 1357–1365 (2010).
- Räber L, Magro M, Stefanini GG et al. Very late coronary stent thrombosis of a newergeneration everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation 125, 1110–1121 (2012).
- Stettler C, Wandel S, Allemann S et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370, 937–948 (2007).
- Weisz G, Leon MB, Holmes DR Jr et al. Fiveyear follow-up after sirolimus-eluting stent implantation: results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) trial. J. Am. Coll. Cardiol. 53, 1488–1497 (2009).
- Pfisterer M. Late stent thrombosis after drugeluting stent implantation for acute myocardial infarction: a new red flag is raised. Circulation 118, 1117–1119 (2008).
- Ihnat DM, Duong ST, Taylor ZC et al. Contemporary outcomes after superficial femoral artery angioplasty and stenting: the influence of TASC classification and runoff score. J. Vasc. Surg. 47(5), 967 (2008).
- Trabattoni D, Agrifoglio M, Cappai A, Bartorelli AL. Incidence of stent fractures and patency after femoropopliteal stenting with the nitinol self-expandable SMART stent: a single-center study. J. Cardiovasc. Med. 11, 678–682 (2010).
- Baril DT, Chaer RA, Rhee RY, Makaroun MS, Marone LK. Endovascular interventions for TASC II D femoropopliteal lesions. J. Vasc. Surg. 51, 1406–1412 (2010).
- Gary T, Rief P, Stojakovic T et al. Lipoproteins and the development of restenosis after stent implantation in the superficial femoral artery in patients with peripheral artery disease. Cardiovasc. Intervent. Radiol. 34, 739–743 (2011).
- Scheinert D, Grummt L, Piorkowski M et al. A novel self-expanding interwoven nitinol stent for complex femoropopliteal lesions: 24-month results of the SUPERA SFA Registry. J. Endovasc. Ther. 18, 745–752 (2011).
- Iida O, Uematsu M, Soga Y et al. Timing of the restenosis following nitinol stenting in the superficial femoral artery and the factors associated with early and late restenoses. Cath. Cardiovasc. Inter. 78, 611–617 (2011).
- Bui TD, Mills JL Sr, Ihnat DM, Gruessner AC, Goshima KR, Hughes JD. The natural history of duplex-detected stenosis after femoropopliteal endovascular therapy suggests questionable clinical utility of routine duplex surveillance. J. Vasc. Surg. 55, 346–352 (2012).
- Kudo T, Chandra FA, Ahn SS. The effectiveness of percutaneous transluminal angioplasty for the treatment of critical limb ischemia: a 10-year experience. J. Vasc. Surg. 41, 423–435 (2005).
- Romiti M, Albers M, Brochado-Neto FC, Durazzo AES, Pereira CaB, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J. Vasc. Surg. 47, 975–981 (2008).
- Nair V, Chaisson G, Abben R. Strategies in infrapopliteal intervention: improving outcomes in challenging patients. J. Interv. Cardiol. 22, 27–36 (2009).
- Biondi-Zoccai GGL, Sangiorgi G, Lotrionte M et al. Infragenicular stent implantation for below-the-knee atherosclerotic disease: clinical evidence from an international collaborative meta-analysis on 640 patients. J. Endovasc. Ther. 16, 251–260 (2009).
- Rand T, Basile A, Cejna M et al. PTA versus carbofilm-coated stents in infrapopliteal arteries: pilot study. Cardiovasc. Interv. Radiol. 29, 29–39 (2006).
- Randon C, Jacobs B, De Ryck F, Vermassen F. Angioplasty or primary stenting for infrapopliteal lesions: results of a prospective randomized trial. Cardiovasc. Interv. Radiol. 33, 260–269 (2010).
- Scheinert D, Ulrich M, Scheinert S et al. Comparison of sirolimus-eluting vs. baremetal stents for the treatment of infrapopliteal obstructions. EuroIntervention 2, 169–174 (2006).
- Rosales OR, Mathewkutty S, Gnaim C. Drug-eluting stent for below-the-knee lesions in patients with critical limb ischemia: longterm follow-up. Catheter. Cardiovasc. Interv. 72, 112–115 (2008).
- Siablis D, Karnabatidis K, Katsanos K et al. Sirolimus-eluting versus bare stents after suboptimal infrapopliteal angioplasty for critical limb ischemia: Enduring 1-year angiographic and clinical benefit. J. Endovasc. Ther. 14, 241–250 (2007).
- Siablis D, Karnabatidis D, Katsanos K et al. Infrapopliteal application of sirolimus-eluting versus bare metal stents for critical limb ischemia: Analysis of long-term angiographic and clinical outcome. J. Vasc. Inter. Radiol. 20, 11141–11150 (2009).
- Karnabatidis D, Katsanos K, Spiliopoulos S, Diamantopoulos A, Kagadis GC, Siablis D. Incidence, anatomical location, and clinical significance of compressions and fractures in infrapopliteal balloon-expandable metal stents. J. Endovasc. Ther. 16, 15–22 (2009).
- Feiring AJ, Krahn M, Nelson L, Wesolowski A, Eastwood D, Szabo A. Preventing leg amputations in critical limb ischemia with below-the-knee drug-eluting stents: the PaRADISE (PReventing Amputations using Drug eluting StEnts) Trial. J. Am. Coll. Cardiol. 55, 1580–1590 (2010).
- Rastan A, Schwarzwalder U, Noory E et al. Primary use of sirolimus-eluting stents in the infrapopliteal arteries. J. Endovasc. Ther. 17, 480–487 (2010).
- Rastan A, Tepe G, Krankenberg H et al. Sirolimus-eluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur. Heart J. 32, 2274–2281 (2011).
- Bosiers M, Scheinert D, Peeters P et al. Randomized comparison of everolimuseluting vs. bare metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J. Vasc. Surg. 55, 390–399 (2012).
- Schmidt A. ACHILLES-Study (DES for BTK). Presented at: Leipzig Interventional Course. Leipzig, Germany, 19–22 January 2011.
- Stack RS, Califf RM, Phillips HR et al. Interventional cardiac catheterization at Duke Medical Center. Am. J. Cardiol. 62, 3F–24F (1988).
- Agrawal CM, Haas KF, Leopold DA, Clark HG. Evaluation of poly(l-lactic acid) as a material for intravascular stents. Biomaterial 13, 176–182 (1992).
- Tamai H, Doi T, Hsu YS et al. Initial and long-term results of biodegradable polymer stent in canine coronary artery (abstract). J. Invas. Cardiol. 7, 9A (1995).
- Tamai H, Igaki K, Tsuji T et al. A biodegradable poly-l-lactic acid coronary stent in the porcine coronary artery. J. Interv. Cardiol. 12, 443–450 (1999).
- Yamawaki T, Shimokawa H, Kozai T et al. Intramural delivery of a specific tyrosine kinase inhibitor with biodegradable stent suppresses the restenotic changes of the coronary artery in pigs in vivo. J. Am. Coll. Cardiol. 32, 780–786 (1998).
- Tsuji T, Tamai H, Igaki K et al. Biodegradable polymer stents. Curr. Interv. Cardiol. Rep. 3, 10–17 (2001).
- Tamai H, Igaki K, Kyo E et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 102(4), 399–404 (2000).
- Tsuji T, Tamai H, Igaki K et al. One year follow-up of biodegradable self-expanding stent implantation in humans (abstract). J. Am. Coll. Cardiol. 37, A47 (2001).
- Tsuji T, Tamai H, Igaki K et al. Four-year follow-up of the biodegradable stent (IGAKITAMAI) (abstract). Circ. J. 68(Suppl. I), 135 (2004).
- Nishio S, Kosuga K, Igaki K et al. Long-term (>10 years) clinical outcomes of first-in-man biodegradable poly-l-lactic acid coronary stents: Igaki-Tamai Stents. Circulation 125, 2343–2353 (2012).
- Onuma Y, Piazza N, Ormison JA, Serruys PW. Everolimus-eluting bioarbsorbable stent – Abbott vascular programme. EuroIntervention 5(Suppl. F), F98–F102 (2009).
- Oberhauser JP, Hosseiny S, Rapoza RJ. Design principles and performance of bioresorbable polymeric coronary scaffolds. EuroIntervention 5(Suppl. F), F15–F22 (2009).
- Serruys PW, Onuma Y, Ormiston JA et al. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation 122(22), 2301–2312 (2010).
- Bokov P, Flaud P. [Metal and biodegradable coronary stents]. Sang. Thrombose Vasiieaux 23, 465–476 (2011).
- Sheehy A, GutiéRrez-Chico JL, Diletti R et al. In vivo characterisation of bioresorbable vascular scaffold strut interfaces using optimal coherence tomography with Gaussian line spread function analysis. EuroIntervention 7, 1227–1235 (2012).
- Tanigawa J, Barlis P, Dimopoulos K, Dalby M, Moore P, Dimario C. The influence of strut thickness and cell design on immediate apposition of drug-eluting stents assessed by optical coherence tomorgaphy Int. J. Cardiol. 134, 180–188 (2009).
- Ding ND, Pacetti SD, Tang FW, Gada M, Roorda W. XIENCE V stent design and rationale. J. Interv. Cardiol. 22, S18–S27 (2009).
- Dunn C, Croom KF. Everolimus: a review of its use in renal and cardiac transplantation. Drugs 66, 547–570 (2006).
- Eisen HJ, Tuzcu EM, Dorent R et al. Everolimus for the prevention of allograft rejection and vasculopathy in cardiactransplant recipients. N. Engl. J. Med. 349, 847–858 (2003).
- Lorber MI, Mulgaonkar S, Butt KMH et al. Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: A 3-year randomized, multicenter, Phase III study. Transplantation 80, 244–252 (2005).
- Milton DT, Riely GJ, Azzoli CG et al. Phase 1 trial of everolimus and gefitinib in patients with advanced nonsmall-cell lung cancer. Cancer 110, 599–605 (2007).
- Awada A, Cardoso F, Fontaine C et al. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: results of a phase I study with pharmacokinetics. Eur. J. Cancer 44, 84–91 (2008).
- Yao JC, Shah MH, Ito T et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 514–523 (2011).
- Oudard S, Medioni J, Ayllon J et al. Everolimus (RAD001): an mTOR inhibitor for the treatment of metastatic renal cell carcinoma. Expert Rev. Anticancer Ther. 9, 705–717 (2009).
- Farb A, John M, Acampado E, Kolodgie FD, Prescott MF, Virmani R. Oral everolimus inhibits in-stent neointimal growth. Circulation 106, 2379–2384 (2002).
- Carter AJ, Brodeur A, Collingwood R et al. Experimental efficacy of an everolimus eluting cobalt chromium stent. Catheter Cardiovasc. Interv. 68, 97–103 (2006).
- Waksman R, Pakala R, Baffour R et al. Optimal dosing and duration of oral everolimus to inhibit in-stent neointimal growth in rabbit iliac arteries. Cardiovasc. Revasc. Med. 7, 179–184 (2006).
- Semsroth S, Stigler RG, Bernecker OY et al. Everolimus attenuates neointimal hyperplasia in cultured human saphenous vein grafts. Eur. J. Cardiothorc. Surg. 35, 515–520 (2009).
- Grube E, Sonoda S, Ikeno F et al. FUTURE I: six- and twelve-month results fro first human experience using everolimus-eluting stents with bioabsorbable polymer. Circulation 109, 2168–2171 (2004).
- Serruys PW, Ong ATL, Piek JJ et al. A randomized comparison of a durable polymer everolimus-eluting stent with a bare metal coronary stent: The SPIRIT FIRST trial. EuroIntervention 1, 58–65 (2005).
- Serruys PW, Ruygrok P, Neuzner J et al. A randomised comparison of an everolimuseluting coronary stent with a paclitaxeleluting coronary stent: the SPIRIT II trial. EuroIntervention 2, 286–294 (2006).
- Tsuchiya Y, Lansky AJ, Costa RA et al. Effect of everolimus-eluting stents in different vessel sizes (from the Pooled FUTURE I and II Trials). Am. J. Cardiol. 98, 464–469 (2006).
- Stone GW. Clinical, angiographic and IVUS results from the pivotal U.S. randomized SPIRIT III Trial of the XIENCE V everolimus-eluting coronary stent system. Presented at: American College of Cardiology 56th Annual Scientific Session. New Orleans, LA, 24–27 March 2007.
- Karnabatidis D, Spiliopoulos S, Diamantopoulos A et al. Primary everolimuseluting stenting versus balloon angioplasty with bailout bare metal stenting of long infrapopliteal lesions for treatment of critical limb lschemia. J. Endovasc. Ther. 18, 1–12 (2011).
- Onuma Y, Serruys PW, Perkins LEL et al. Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimuseluting bioresorbable vascular scaffolds in a porcine coronary artery model. An attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation 122, 1912–1924 (2010).
- Ormiston JA, Serruys PW, Regar E et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet 371(9616), 899 (2008).
- Stone GW, Midei M, Newman W et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA 299(16), 1903–1913 (2008).
- Serruys PW, Ormiston JA, Onuma Y et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 373, 897–910 (2009).
- Onuma Y, Serruys PW, Ormiston JA et al. Three-year results of clinical follow-up after a bioresorbable everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB trial. EuroIntervention 6, 447–453 (2010).
- Rapoza R. Absorb BVS program: long-term experimental data angiography, IVUS, OCT, histology and micro CT. Presented at: Local Drug Delivery and Cardiovascular Course on Revascularisation. Geneva, Switzerland, 2–4 February 2012.
- Okamura T, Gard S, Gutierrez-Chico JL et al. In vivo evaluation of stent strut distribution patterns in the bioabsorbable everolimuseluting device: an OCT ad hoc analysis of the revision 1.0 and revision 1.1 stent design in the ABSORB clinical trial. EuroIntervention 5, 932–938 (2010).
- Serruys PW, Onuma Y, Dudek D et al. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12-month clinical and imaging outcomes. J. Am. Coll. Cardiol. 58, 1578–1588 (2011).
- Belardi JA, Alberal M. Objective versus subjective guidance of (bioabsorbable) stent implantation: the saga continues. Catheter Cardiovasc. Interv. 79, 889 (2012).
- Farooq V, Gomez-Lara J, Brugaletta S et al. Proximal and distal maximal luminal diameters as a guide to appropriate deployment of the ABSORB everolimus-eluting bioresorbable vascular scaffold: a sub-study of the ABSORB Cohort B and the ongoing ABSORB EXTEND single arm study. Catheter Cardiovasc. Interv. 79, 880–888 (2012).
- Ormiston J, De Vroey F, Serruys PW, Webster MWI. Bioresorbable polymeric vascular scaffolds: a cautionary tale. Circ. Cardiovasc. Interv. 4, 535–538 (2011).
- Ormiston J, Serruys PW. ABSORB cohort B trial – two year clinical and angiographic results of the ABSORB everolimus eluting bioresorbable vascular scaffold (poster). Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 7–11 November 2011.
- Serruys PW, Onuma Y. ABSORB cohort B: 6M, 12M, 18M and 24M FUP. Presented at: Bioresorbable Vascular Scaffolds. Rotterdam, The Netherlands, 9 March 2012.
- Byrne RA, Kastrati A, Kufner S et al. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur. Heart J. 30, 2441–2449 (2009).
- Byrne RA, Iijima R, Mehilli J et al. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. J. Am. Coll. Cardiol. Cardiovasc. Interv. 2, 291–299 (2009).
- Park KW, Kim C-H, Lee H-Y et al. Does ‘late catch-up’ exist in drug-eluting stents: insights from a serial quantitative coronary angiography analysis of sirolimus versus paclitaxel-eluting stents. Am. Heart J. 159, 446–453 (2010).
- Bruining N, De Winter S, Roelandt JRTC et al. Monitoring in vivo absorption of a drugeluting bioabsorbable stent with intravascular ultrasound-derived parameters: a feasibility study. J. Am. Coll. Cardiol. Interv. 3, 449–456 (2010).
- Gomez-Lara J, Brugaletta S, Farooq V et al. Angiographic geometric changes of the lumen arterial wall after bioresorbable vascular scaffolds and metallic platform stents at 1-year follow-up. J. Am. Coll. Cardiol. Interv. 4, 789–799 (2011).
- Okamura T, Serruys PW, Regar E. The fate of bioresorbable struts located at a side branch ostium: serial three-dimensional optical coherence tomography assessment. Eur. Heart J. 31(17), 2179 (2010).
- Okamura T, Onuma Y, Garcia-Garcia H et al. 3-dimensional optical coherence tomography assessment of jailed side branches by bioresorbable vascular scaffolds: a proposal for classification. J. Am. Coll. Cardiol. Cardiovasc. Interv. 3, 836–844 (2010).
- Dubé H, Clifford AG, Barry CM, Schwarten DE, Schwartz LB. Comparison of the vascular responses to balloon-expandable stenting in the coronary and peripheral circulations: long-term results in an animal model using the TriMaxx stent. J. Vasc. Surg. 45, 821–827 (2007).
- Kimura T, Yokoi H, Nakagawa Y et al. Threeyear follow-up after implantation of metallic coronary-artery stents. N. Engl. J. Med. 334, 561–566 (1996).
- Shofti R, Tio F, Beyar R. Neointimal vascularization and intimal thickening in response to self-expanding stents: a swine model. Int. J. Cardiovasc. Interv. 2, 61–67 (2004).
- Radeleff B, Grenacher L, Christoph P et al. Comparison of a microporous thermoplastic polyurethane-covered stent with a selfexpanding bare nitinol stent in a porcine Iliac artery model. J. Vasc. Interv. Radiol. 20, 927–935 (2009).
- Kimura T, Abe K, Shizuta S et al. Long-term clinical and angiographic follow-up after coronary stent placement in native coronary arteries. Circulation 105, 2986–2991 (2002).
- Vorwerk D, Radha F, Neuerburg J, Clerc C, Gunther RW. Neointima formation following arterial placement of self-expanding stents of different radial force: experimental results. Cardiovasc. Interv. Radiol. 17, 27–32 (1994).
- Prunotto M, Isaia C, Gatti MA, Monari E, Pasquino E, Galloni M. Nitinol carbofilm coated stents for peripheral applications: Study in the porcine model. J. Mater. Sci. Mater. Med. 16, 1231–1238 (2005).
- Peeters P, Keirse K, Verbist J, Deloose K, Bosiers M. Are bio-absorbable stents the future of SFA treatment? J. Cardiovasc. Surg. 51, 121–124 (2010).
- Vorpahl M, Finn AV, Nakano M, Virmani R. The bioabsorption process: tissue and cellular mechanisms and outcomes. EuroIntervention 5(Suppl. F), F28–F35 (2009).
- Conte MS, Geraghty PJ, Bradbury AW et al. Suggested objective performance goals and clinical trial design for evaluating catheterbased treatment of critical limb ischemia. J. Vasc. Surg. 50, 1462–1473 (2009).