Device Evaluations - Interventional Cardiology (2014) Volume 6, Issue 6
Coronary artery disease evolution in bare-metal stent implantation: safety and efficacy of REBEL platinum ̉̉chromium coronary stent system
- Corresponding Author:
- Didier Carrié
Service de Cardiologie B, CHU Toulouse Rangueil, France
Tel: +33 05 61 32 33 11
Fax: +33 05 61 32 33 25
E-mail: carrie.didier@chu-toulouse.fr
Abstract
Coronary artery disease affects over 5,000,000 people worldwide. Despite technology evolution and extension of indications of drug-eluting and bioresorbable stents, bare-metal stents play an important role to treat single lesions less than 15 mm of length and/or greater than 2.5 mm of diameter. The REBEL™ stent is made from a new platinum–chromium alloy combined with a customized stent architecture and an enhanced delivery system that allows improved performances of this stent such as high radial and axial strength and lower stent recoil. The feasibility and efficacy of percutaneous implantation has been studied worldwide with a prospective, single-arm, multicenter, clinical trial with a de novo atherosclerotic coronary artery lesion currently underway.
Keywords
bare-metal stent, coronary artery disease, platinum–chromium coronary stent, REBEL™ stent system
Clinical trials have demonstrated that drugeluting stents (DES) have pronounced ability to reduce restenosis and target lesion revascularization (TLR) [1,2]. These findings fueled the exponential increase of DES usage in up to 70% of percutaneous coronary interventions (PCI) procedures [3–5], even in the setting in which they have not shown exceptional benefit [6]. As implantation of DES mandates longer dual antiplatelet therapy this has become a major problem for many patients, particularly those with comorbidities that are worsened due to aggressive antiplatelet therapy. The selection of an appropriate stent for each individual patient is still an unresolved question, which is further affected by varying DES penetration rates across the different regions. Although the efficacy of DES is indisputable in the prevention of restenosis, it is still believed that many patients will do well with a bare-metal stents (BMS) and that this technology requires further refinements to improve outcomes. Third-generation BMS have emerged, with advanced design characteristics leading to improved performance compared with prior generations. Furthermore, most of the new BMS are considered as platforms for next-generation DES and therefore during their development several important mechanical characteristics to enhance procedures are taken into consideration.
Current status & stent design
The newly developed coronary BMS, REBEL™ stent system, (Boston Scientific, USA), is a balloon expandable platinum– chromium (PtCr) stent, premounted onto a high pressure, semi-compliant balloon on a rapid exchange delivery catheter.
The REBEL stent has three key components (PtCr alloy, customized stent architecture and an enhanced delivery system), which offers advantages over earlier generations of BMS.
PtCr alloy
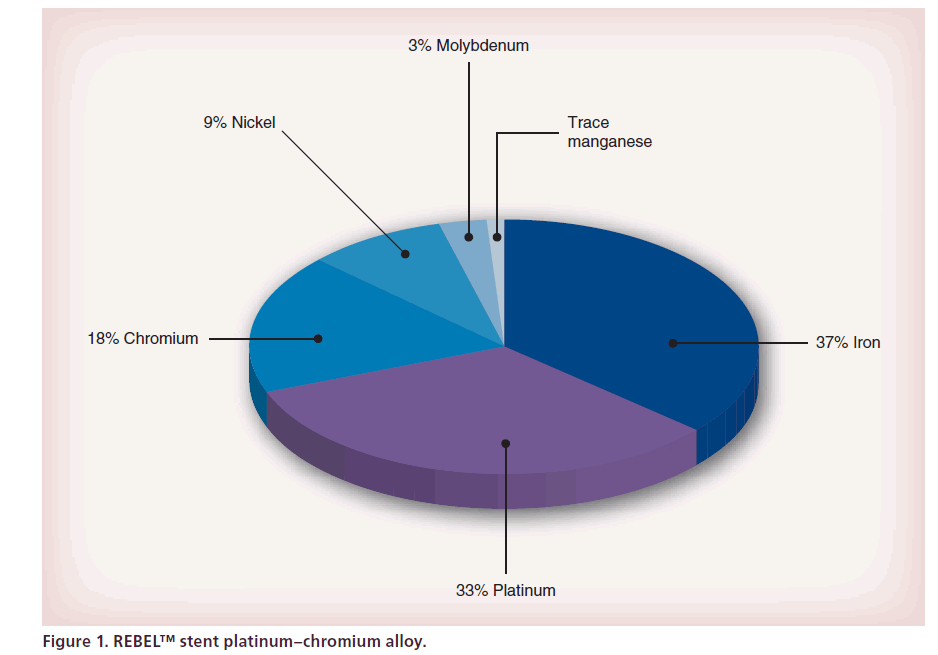
PtCr alloy (Figure 1) has over twice the density of iron or cobalt, resulting in enhanced visibility under fluoroscopy during coronary stenting.
For outstanding visibility and stent placement (Figure 2), the radiopacity of PtCr alloy is accurate and a visibility bench test comparison with other BMS is excellent. So, the stent placement is facilitated and can avoid a gap between two contiguous stents.
Customized stent architecture
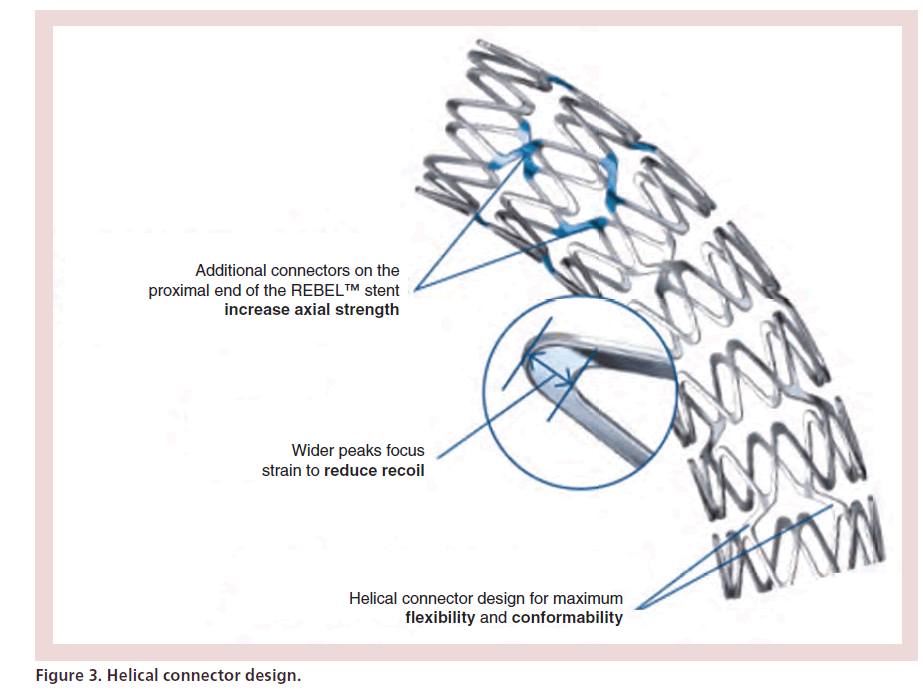
Due to complex lesions with angulation and calcifications, it is important to find a balance between strength and flexibility. The helical connector design (Figure 3) with short and increased segments improves flexibility, conformability and minimizes gaps on a bend [7]. Moreover, additional connectors on the proximal end of REBEL stent were added to increase axial strength. Finally wider peaks focus strain to reduce recoil.
Radial strength
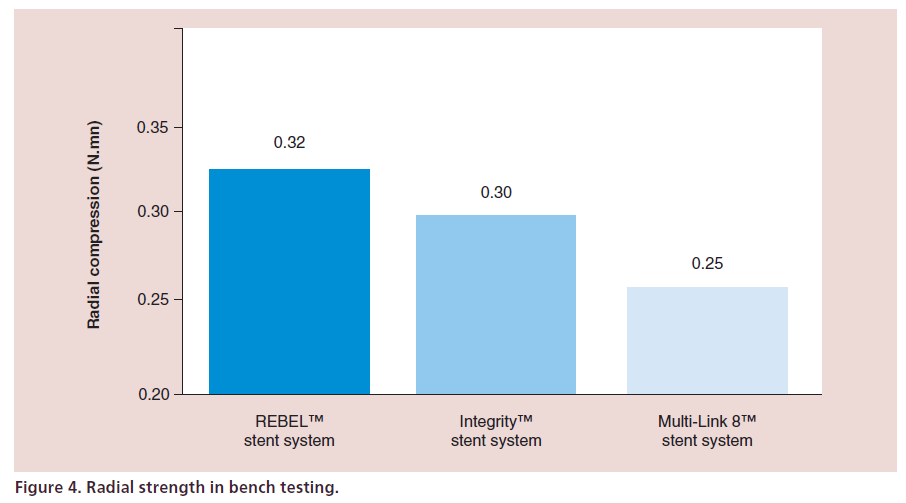
Radial strength and low recoil are particularly important in BMS as TLR rates are generally higher than DES. Radial strength is the amount of force needed to compress the stent 1 mm.
The PtCr alloy combined with the REBEL customized architecture stent design also allows for high radial and axial strength (Figure 4) and the lowest stent recoil.
Enhanced delivery system
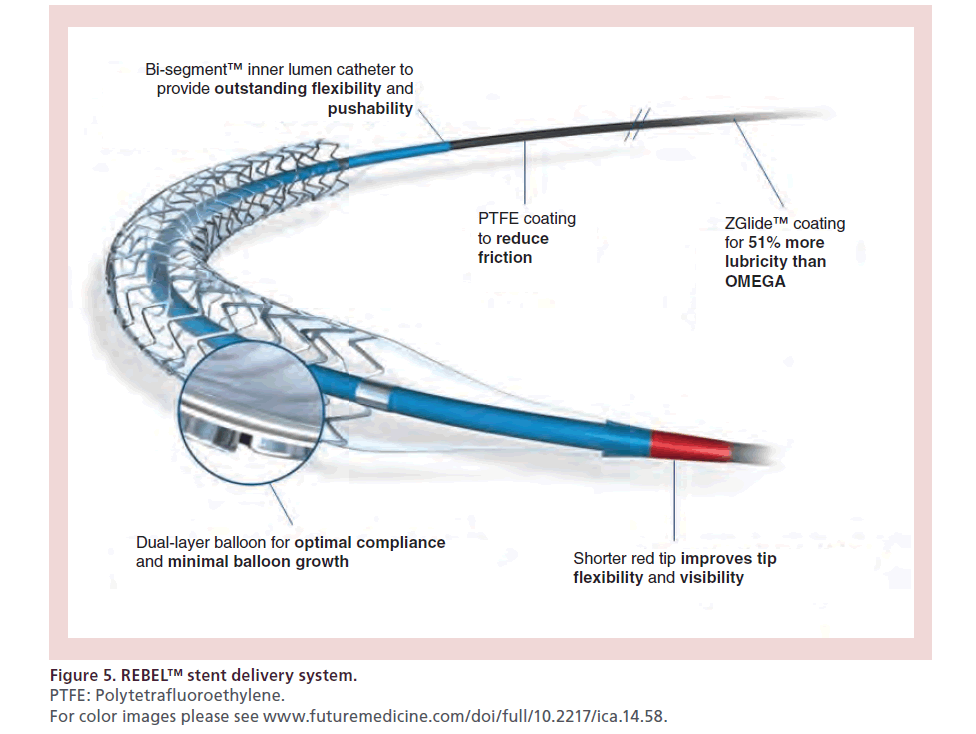
Different components enhance the delivery system of the REBEL stent (Figure 5).
Figure 5: REBEL™ stent delivery system.
PTFE: Polytetrafluoroethylene.
For color images please see www.futuremedicine.com/doi/full/10.2217/ica.14.58.
The dual-layer balloon, the bisegment inner lumen catheter and the Z-Glide coating on the distal catheter, according to manufacturer statement, were improved to achieve a better deliverability.
Finally, the shorter tip and red tip colorant improve tip flexibility and tip visibility when loading onto a guidewire.
BMS clinical data
Not every patient is a candidate for DES. For those who need a bare metal option, the REBEL stent seems a good choice. Optimal vessel coverage, low metal:artery ratio, good flexibility and visibility, low crossing profile and easy access to a side branch for bifurcation stenting have also been considered as top priority design specifications, to respond to the most critical needs of interventional practice. The current clinical Omega study was specifically designed to evaluate the safety and effectiveness of the newly developed REBEL stent in PCIs.
Omega trial
Study design
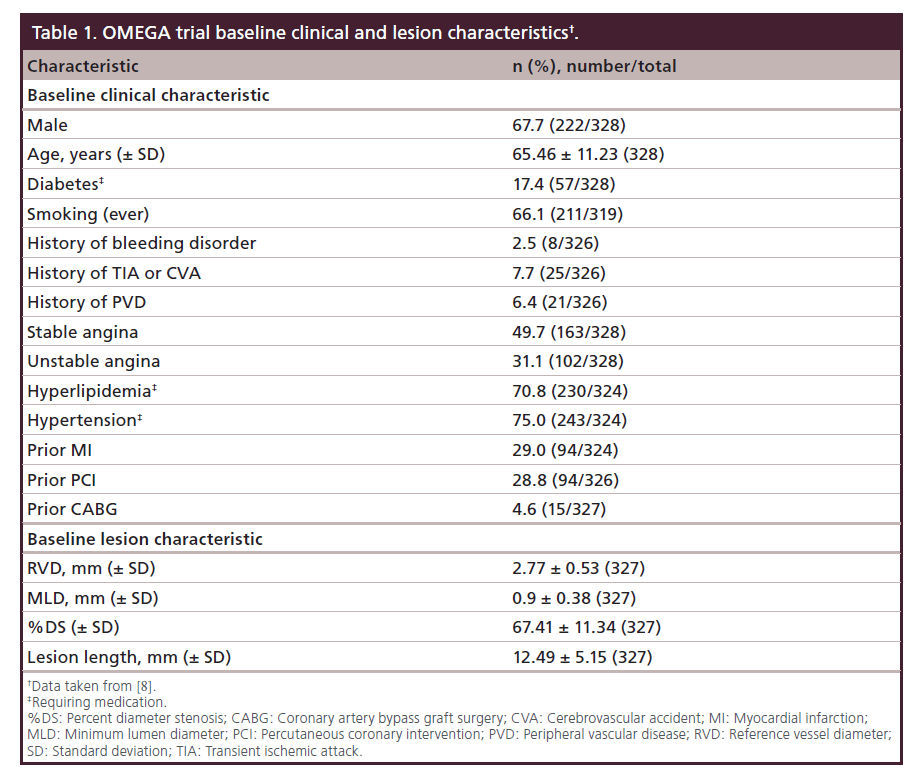
It’s a prospective, single-arm, multicenter trial which enrolled 328 subjects with a de novo atherosclerotic coronary artery lesion less than 28 mm in length (by visual estimate) in a native coronary artery greater than or equal to 2.25 mm to less than or equal to 4.50 mm in diameter (by visual estimate). Baseline clinical and lesion characteristics are listed in Table 1. These 9-month results were presented by John Wang at the CRT conference 2014 [8].
Primary end point
The primary end point is 9-month target lesion failure (TLF) rate, defined as any ischemia-driven revascularization of the TLR, myocardial infarction (MI; Q-wave and non-Q-wave) related to the target vessel or cardiac death related to the target vessel.
Additional end points
Clinical end points measured in hospital and at 30 days, 9 months and 12 months:
• TLR rate
–– TLF rate (primary end point at 9 months);
–– Target vessel revascularization (TVR) rate;
–– Target vessel failure (TVF) rate;
–– MI (Q-wave and non-Q-wave) rate;
–– Cardiac death rate;
–– Noncardiac death rate;
–– All death rate;
–– Cardiac death or MI rate;
–– All death or MI rate;
–– All death/MI/target vessel revascularization rate;
–– Stent thrombosis rate (definite or probable by Academic Research Consortium definitions).
All subjects enrolled in the trial (including those who do not have a study stent implanted) will undergo clinical follow-up at 30 days, 9 months and 12 months postprocedure.
During the index procedure, subjects may have a maximum of two lesions treated.
• Subjects may have one target lesion, or one target lesion and one nontarget lesion, treated. If a nontarget lesion is treated, it must be treated prior to the target lesion and must be a clinical angiographic success.
At the time of the index procedure, there must be no revascularizations planned for the target or nontarget vessels after the index procedure.
Sample size parameters
Due to historical BMS 9-month TLF rate, the expected OMEGA (test) rate is 12.2%. With a delta of 6.0%, a test significance level of ≤0.05 (one-sided), a power of 90% and an expected rate of attrition of 5%, the number of patients to be included was 328 subjects.
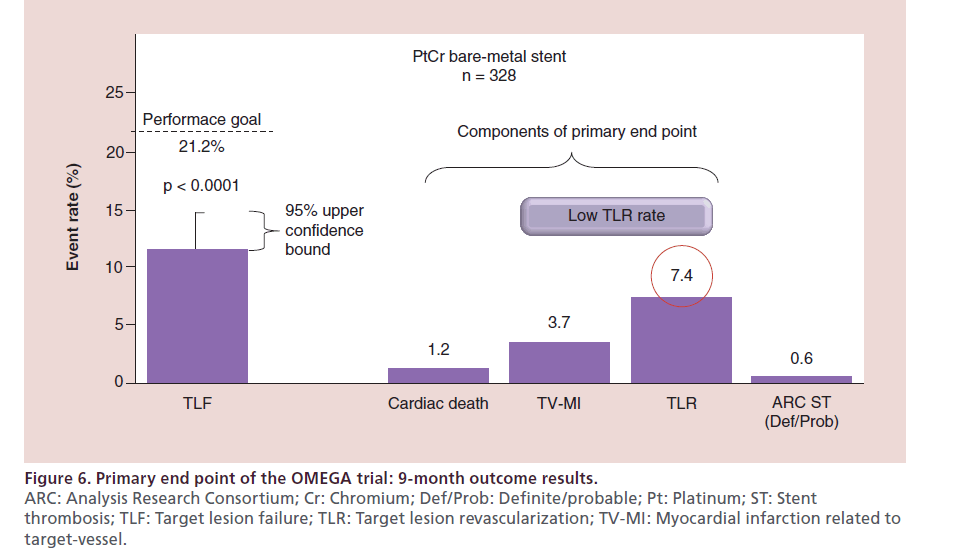
Nine-month clinical results
At 9 months 99% of the patients were available for follow-up. The primary end point, freedom from TLF at 9 months was 88.5% in total study population (Figure 6). The additional end points, such as stent thrombosis and clinically driven rates of TLR at 9 months were 0.6 and 7.4%, respectively.
Figure 6: Primary end point of the OMEGA trial: 9-month outcome results.
ARC: Analysis Research Consortium; Cr: Chromium; Def/Prob: Definite/probable; Pt: Platinum; ST: Stent
thrombosis; TLF: Target lesion failure; TLR: Target lesion revascularization; TV-MI: Myocardial infarction related to
target-vessel.
Discussion
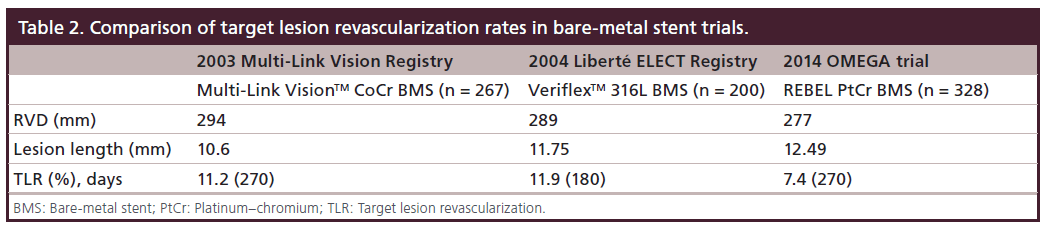
BMS play an important role to treat single lesion less than 15 mm and/or greater than 2.5 mm of diameter. Kirtane et al. in a large meta-analysis on 34 observational studies of BMS and DES reported a significant reduction in mortality (hazard ratio: 0.78; 95% CI: 0.71–0.86) and MI (hazard ratio: 0.87; 95% CI: 0.78–0.97) with DES [9]. Moreover, it has been demonstrated that vessel diameter influences restenosis rates, with an increased risk of restenosis related to smaller vessel diameters after BMS implantation [10]. However Steinberg et al. supported the hypothesis that low risk populations do not receive benefit from DES. With regard to BMS, even a late loss of 1 mm in a greater than 3.5 mm vessel would render a 2.5 mm diameter vessel at long-term follow up. This translates into binary restenosis of less than 50% and no compromission of hemodynamics [11]. With the new REBEL system, the surface of the stent that is exposed to the vessel is a passive corrosion resistant oxide surface comparable to that of stainless steel stents which undergo the same passive process [12]. The primary objective of OMEGA study was to evaluate safety and efficacy of the REBEL Stent and to compare it with other contemporary BMS such as Vision, Driver, Liberte and Tsunami. Availability of published data and absence of any relevant differences in coronary risk factors and cardiac history between the OMEGA study and previous trials with those stents allow us to compare patient and lesion characteristics and outcome data [13–18]. Despite the fact that definitions of TVF or its individual components was not fully unified across the trials, our comparison showed similar TVF rate observed in OMEGA study to the TVF rates observed in other published studies which were between 6.0 and 16.0%.
Moreover, the PtCr BMS in the OMEGA trial had only 7.4% TLR. Previous BMS data on older platforms showed higher TLR rates (Table 2).
Conclusion
Our results indicate comparable safety and effectiveness of the REBEL stent to other contemporary BMS. We can conclude that the REBEL PtCr BMS system provides a useful treatment option for patients with ischemic heart disease caused by lesions in native coronary arteries.
Third-generation BMS have emerged, with advanced design characteristics leading to improved performance compared with prior generations. Furthermore, most of the new BMS are considered as platforms for nextgeneration DES and therefore during their development several important mechanical characteristics to enhance procedures are taken into consideration.
Future perspective
Over the last 30 years PCI has undergone progressive and very remarkable improvements in acute and long-term outcomes and safety. Newer generation compared to early generation DES represent a step forward in the treatment of coronary artery due to the structure amelioration, such as reduced thickness of the struts, higher effectiveness of stent architecture and enhanced delivery systems. The fourth revolution and new challenge in the stent era is now represented by the bioresorbable vascular scaffolds system. Its structure completely biodegradable over time leaving no foreign material on vessel wall, as well as temporary coverage of side branches and no permanent fracture, the ability of the vessel wall to expand and to regain physiologic vasomotion, the possibility of performing further percutaneous or surgical treatment of the same site with a potential improvement in long-term outcome are some of the characteristics, which make the development of this technology attractive and, in the future, may turn bioresorbable vascular scaffolds into the workhorse device for coronary intervention.
Executive summary
• Coronary artery disease affects over 5,000,000 people worldwide. Despite technology evolution and extension of indications of drug-eluting and bioresorbable stents, bare-metal stents (BMS) play an important role to treat single lesions less 15 mm of length and/or greater than 2.5 mm of diameter.
• Although clinical trials have demonstrated that drug-eluting stents have pronounced ability to reduce restenosis and target lesion revascularization, the selection of an appropriate stent for each individual patient is still an unresolved question.
• Third-generation BMS have emerged, with advanced design characteristics leading to improved performance compared with prior generations.
• The newly developed coronary BMS, REBEL™ stent system, (Boston Scientific, USA), is a balloon expandable platinum–chromium (PtCr) stent presenting three key components (PtCr alloy, customized stent architecture and an enhanced delivery system), which offers advantages over earlier generations of BMS.
• The current clinical Omega study was specifically designed to evaluate the safety and effectiveness of the newly developed REBEL stent in percutaneous coronary interventions.
• The target vessel failure rate observed in OMEGA study at 9-month clinical outcome is similar to the target vessel failure rates observed in literature.
• The results of OMEGA study indicate comparable safety and effectiveness of the REBEL stent to other contemporary BMS.
Financial & competing interest disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Morice M-C, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346, 1773–1780 (2002).
- Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349, 1315–1323 (2003).
- Gualano SK, Gurm HS, Share D et al. Temporal trends in the use of drug-eluting stents for approved and off-label indications: a longitudinal analysis of a large multicenter percutaneous coronary intervention registry. Clin. Cardiol. 33(2), 111–116 (2010).
- Krone RJ, Rao SV, Dai D et al. ACC/NCDR Investigators. Acceptance, panic, and partial recovery the pattern of usage of drug-eluting stents after introduction in the US (a report from the American College of Cardiology/National Cardiovascular Data Registry). JACC Cardiovasc. Interv. 3(9), 902–910 (2010).
- Lopez JJ, Keyes MJ, Nathan S et al. Rapid adoption of drugeluting stents: clinical practices and outcomes from the early drug-eluting stent era. Am. Heart J. 160(4), 767–774 (2010).
- Venkitachalam L, Lei Y, Stolker JM et al. EVENT Registry Investigators. Clinical and economic outcomes of liberal versus selective drug-eluting stent use: insights from temporal analysis of the multicenter Evaluation of Drug Eluting Stents and Ischemic Events (EVENT) registry. Circulation 124(9), 1028–1037 (2011).
- Kereiakes DJ, Popma JJ, Cannon LA et al. Longitudinal stent deformation: quantitative coronary angiographic analysis from the PERSEUS and PLATINUM randomised controlled clinical trials. EuroIntervention 8(2), 187–195 (2012).
- John CW, Didier C, Monica M, et al. CRT-152 ninemonth primary endpoint results of the Omega study: clinical outcomes after implantation of a modern platinum chromium bare metal stent Am. Coll. Cardiol. Intv. 7(Suppl. 2), S1 (2014).
- Kirtane AJ et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation 119, 3198–3206 (2009).
- Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349, 1315–1323 (2003).
- Steinberg DH et al. Comparison of effectiveness of bare metal stents versus drug-eluting stents in large (>3.5 mm) coronary arteries. Am. J. Cardiol. 99, 599–602 (2007).
- Haidopoulos M. Development of an optimized electrochemical process for subsequent coating for 316 stainless steel for stent applications. J. Mater. Sci. Mater. Med. 17, 647–657 (2006).
- Legrand V, Kelbaek H, Hauptmann KE et al. Clinical and angiographic analysis with a cobalt alloy coronary stent (driver) in stable and unstable angina pectoris. Am. J. Cardiol. 97, 349–352 (2006).
- Sketch MH Jr, Ball M, Rutherford B et al. Evaluation of the Medtronic (Driver) cobalt-chromium alloy coronary stent system. Am. J. Cardiol. 95, 8–12 (2005).
- ELECT study. www.fda.gov
- Bonnier HJ, van den Heuvel P, Legrand V et al. Clinical and angiographic outcomes after Tsunami coronary stent placement. J. Invasive Cardiol. 16, 252–256 (2004).
- Kereiakes DJ, Cox DA, Hermiller JB et al. Usefulness of cobalt chromium coronary stent alloy. Am. J. Cardiol. 92, 463–466 (2003).
- Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 92, 2333–2342 (1995).