Special Report - Interventional Cardiology (2015) Volume 7, Issue 3
Coronary intramural hematomas: a focused review
- Corresponding Author:
- Michael Shenoda
Sansum Clinic
317 W. Pueblo St. Santa Barbara
CA 93105, USA
Tel: +1 805 898 3138
Fax: +1 805 898 3416
E-mail: mshenoda@gmail.com
Abstract
Intramural hematomas are defined as an accumulation of blood in the media with entry and exit points that may or may not be identifiable. Intramural hematomas can occur during percutaneous coronary interventions and have been reported to occur spontaneously. While a majority of intramural hematomas are identifiable on coronary angiography, intravascular ultrasound should be considered the gold standard for diagnosis given its superior imaging capabilities. Outcomes are variable and there are no guidelines for the treatment of intramural hematomas. Therefore, treatment of intramural hematomas should be based on patient characteristics and clinical scenario.
Intramural hematomas are defined as an accumulation of blood in the media with entry and exit points that may or may not be identifiable. Intramural hematomas can occur during percutaneous coronary interventions and have been reported to occur spontaneously. While a majority of intramural hematomas are identifiable on coronary angiography, intravascular ultrasound should be considered the gold standard for diagnosis given its superior imaging capabilities. Outcomes are variable and there are no guidelines for the treatment of intramural hematomas. Therefore, treatment of intramural hematomas should be based on patient characteristics and clinical scenario.
Keywords
acute coronary syndrome, coronary angiography, coronary dissection, intramural hematoma, intravascular ultrasound, percutaneous coronary intervention
An intramural hematoma is defined as blood accumulation in the media which displaces the internal elastic membrane inward and the external elastic membrane outwards [1]. Entry and exit points may or may not be identifiable. Previous studies have suggested an occurrence rate of 6.7% of percutaneous coronary interventions (PCIs) [2] as demonstrated by intravascular ultrasound (IVUS). Additionally, there have also been reports of spontaneous intramural hematomas presenting as acute coronary syndrome [3,4]. Intramural hematomas are classified as dissections [1] and more commonly occur in de novo lesions, in diabetics and near transitions of arterial walls [2]. This paper will present two cases of intramural hematomas and discuss the proposed pathophysiology, diagnosis, natural history and contemporary treatment of intramural hematomas.
Case 1
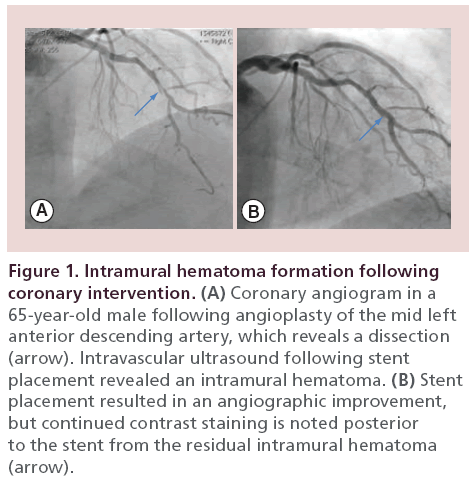
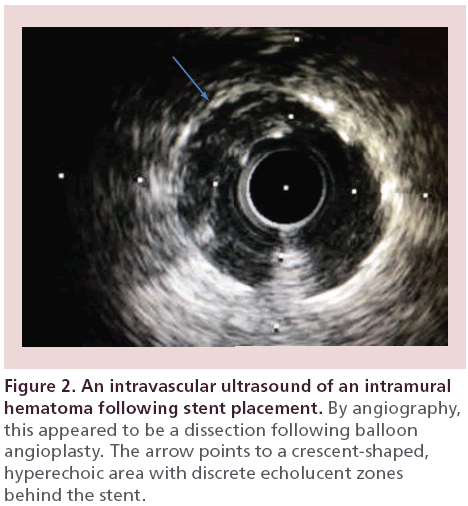
A 65‐year-old male with hypertension presented with progressive angina symptoms in the outpatient setting. A myocardial perfusion imaging study revealed ischemia in the anterior and lateral walls. A coronary angiogram revealed a high-grade stenosis of the mid left anterior descending artery (LAD), just before a diagonal artery bifurcation and a second high-grade stenosis of the left circumflex artery (LCx). Following balloon angioplasty of the LAD stenosis, there appeared to be a resultant dissection by angiography (Figure 1A), which was successfully covered by stent placement (Figure 1B). An IVUS revealed an intramural hematoma posterior to the stent placement (Figure 2). Following postdilatation with a noncompliant balloon, there was residual contrast staining on angiography in the area that was treated with stent placement (Figure 1B, arrow). However, a repeat IVUS revealed that the stent was well opposed and the intramural hematoma was covered with no evidence of progression. Given the contrast load, we elected to perform a staged intervention of the LCx several weeks later. Repeated angiography at the time of the LCx PCI revealed that the contrast straining posterior to the mid LAD stent had resolved, signifying resolution of the previously seen intramural hematoma.
Figure 1: Intramural hematoma formation following coronary intervention. (A) Coronary angiogram in a 65‐year-old male following angioplasty of the mid left anterior descending artery, which reveals a dissection (arrow). Intravascular ultrasound following stent placement revealed an intramural hematoma. (B) Stent placement resulted in an angiographic improvement, but continued contrast staining is noted posterior to the stent from the residual intramural hematoma (arrow).
Case 2
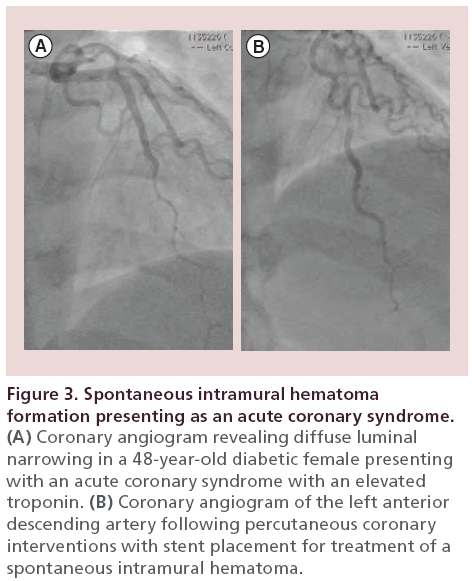
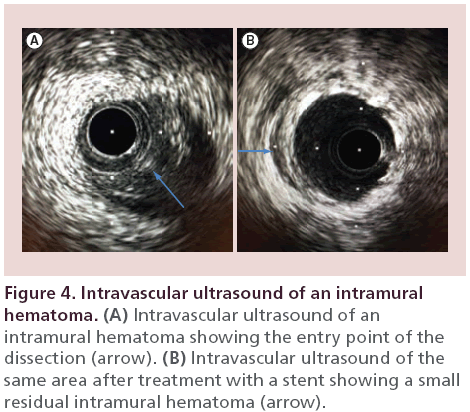
A 48‐year-old diabetic female presented to the emergency room with chest pain radiating to the neck and jaw. Her electrocardiogram revealed anterior T-wave inversions. Her initial troponin was mildly elevated at 0.33 ng/ml which later rose to 18 ng/ml. Coronary angiography revealed diffuse and smooth luminal narrowing of the mid LAD (Figure 3A). Intracoronary nitroglycerin was given to treat possible coronary vasospasm, which did not improve the mid LAD narrowing. An IVUS revealed a spontaneous dissection with a defined entry point, resultant intramural hematoma and no identifiable exit point (Figure 4). Given her ongoing symptoms and elevated troponin, we proceeded with stent placement, which was successful at treating the spontaneous intramural hematoma (Figure 3B). A repeat IVUS following stent placement revealed a well-opposed stent with a small residual intramural hematoma which was covered by the stent. The patient had complete resolution of her symptoms following the PCI and continued to do well at her outpatient follow-up appointment.
Figure 3: Spontaneous intramural hematoma formation presenting as an acute coronary syndrome. (A) Coronary angiogram revealing diffuse luminal narrowing in a 48-year-old diabetic female presenting with an acute coronary syndrome with an elevated troponin. (B) Coronary angiogram of the left anterior descending artery following percutaneous coronary interventions with stent placement for treatment of a spontaneous intramural hematoma.
Pathophysiology
Balloon angioplasty leads to plaque compression, plaque rupture, medial stretching and intimal splitting [5]. Intimal splitting occurs at the thinnest and weakest portion of the plaque [5]. Once the intima splits and separates from the media, the media and adventitia can be stretched by an inflated balloon, creating a space for blood pooling. Eccentric lesions, which compromise approximately 70% of coronary lesions [6] are more likely to develop intramural hematomas. In eccentric lesions, the media is thicker in the diseasefree portion of the artery and thinner/atrophied in the diseased area of the artery [7]. Therefore, these lesions are more likely to have medial disruption at the transition of the diseased and nondiseased sections following balloon angioplasty, leading to a hematoma formation within the normal segment of the coronary artery. Similarly, stent placement across these transition points in the artery may leads to edge dissections and intramural hematoma formation. Media scarring in the diseased section of the coronary artery may resist propagation of medial dissection and hematoma formation [2]. This may explain why intramural hematomas are more commonly seen in de novo lesions and in patients with less severe coronary artery disease [2].
Spontaneous coronary intramural hematomas/dissections are well-documented phenomena, which can lead to acute coronary syndrome. The underlying mechanisms are not well defined. Proposed mechanisms include intimal tears from weakening of the coronary artery wall and vasa vasorum bleeding [8]. Associated risk factors include pre- and postpartum periods, trauma, hormonal use, vasculitis, fibromuscular dysplasia and illicit drug use [3]. Recently, a retrospective study has suggested that coronary tortuosity may also be another mechanistic factor in spontaneous coronary artery dissections [9].
Diagnosis
Coronary angiography can detect a majority of intramural hematomas. However, their angiographic characteristics can vary widely, and may include dissection (a majority of the presentations) (Figure 1), diffuse luminal narrowing (Figure 3), a new lesion following stent deployment, coronary spasm and haziness in the vessel. Additionally, approximately 30% of intramural hematomas are undetected by coronary angiography [2] and thus IVUS should be considered the gold standard for the diagnosis of intramural hematomas.
IVUS can reveal the entry point of the dissection (Figure 4), propagation direction, underlying arterial plaque, severity of the intramural hematoma and luminal compromise. Characteristics of an intramural hematoma on IVUS imaging includes a homogeneous, crescent-shaped, hyperechoic area (Figure 2) [2]. A dramatic increase in backscatter intensity has been reported to occur with stagnant blood flow, presumably from red blood cell aggregation, leading to increased echogenicity [10]. Intramural hematomas can also contain echolucent areas (Figure 2), which presumably represents accumulation of saline or radiographic contrast [2].
Optical Coherence Tomography has also been reported as an important imaging tool in the diagnosis of intramural hematomas [11]. Given its superior resolution, it may provide more insight into the mechanisms of intramural hematomas.
Natural history of intramural hematomas
Literature case reports suggest that the natural history of intramural hematomas is quite variable. Several studies have suggested gradual absorption and resolution without percutaneous intervention [3,11]. However, the propensity for progression of intramural hematomas has also been documented. These include both shortterm complications, such as myocardial infarction and repeat revascularization and long-term complications, such as death [2]. Stenting intramural hematomas have been reportedly associated with late stent malapposition following the hematoma absorption [12]. Given these unpredictable outcomes and lack of randomized trials, treatment should be considered on a case-by-case basis.
Treatment
Treatment options for intramural hematomas include conservative medical therapy, close angiographic followup and percutaneous coronary intervention. The later usually entails stent placement [13], although other techniques have also been reported. A scoring balloon angioplasty was performed after an intramural hematoma did not resolve with stent placement [14]. The scoring balloon was used to create a fenestration between the true and false lumens [14]. Similarly, a cutting balloon was also used to create a fenestration to treat an intramural hematoma [14]. A chronic total occlusion-dedicated guidewire was also used to successfully fenestrate an intramural hematoma [16]. There are currently no guidelines on the treatment of intramural hematomas, and therefore appropriate clinical judgment is imperative as these patients are generally at high risk for adverse outcomes.
Conclusion
Intramural hematomas can occur during PCI and may present as an acute coronary syndrome in spontaneous cases. While a majority of intramural hematomas are identifiable on coronary angiogram, up to 30% are undetectable by angiography only. IVUS should be considered the gold standard in the diagnosis of intramural hematomas and may facilitate management strategies. There is no clear consensus on the treatment of intramural hematomas, therefore, treatment should be individualized based on patient characteristics and the clinical scenario.
Future perspective
In the current era of second generation drug-eluting stents, a randomized clinical trial of stenting versus conservative therapy for intramural hematomas would be difficult to conduct, require a large number of patients and prolonged follow-up to be clinically relevant. However, continued evaluation and research, through case reports and reviews, is imperative for a better understanding of treatment outcomes.
The initial bioabsorbable stent data have shown promising outcomes with regards to vessel healing and recovery of endothelial function. Therefore, the use of bioabsorbable stents for the treatment of coronary intramural hematomas, especially those that occur in the setting of spontaneous dissections, would make clinical sense as the burden of vessel atherosclerosis is minimal in certain cases. Clinical trials evaluating the role of bioabsorbable stents in the setting of coronary intramural hematomas should be considered.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
• An intramural hematoma is defined as blood accumulation in the media which displaces the internal elastic membrane inward and the external elastic membrane outwards.
Diagnosis
• Intravascular ultrasound should be considered the gold standard for diagnosing intramural hematomas as up to 30% are undetectable by angiography.
Treatment
• There is no clear consensus on the treatment of intramural hematomas, therefore, treatment should be individualized based on patient characteristics and the clinical scenario.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- Mintz GS, Nissen SE, Anderson WD et al. ACC Clinical Expert Consensus Document on standards for the acquisition, measurement and reporting of intravascular ultrasound studies: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents (Committee to develop a Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies [IVUS]). J. Am. Coll. Cardiol. 37, 1478–1492 (2001).
- Maehara A, Mintz GS, Bui AB et al. Incidence, morphology, angiographic findings, and outcomes of intramural hematomas after percutaneous coronary interventions: an intravascular ultrasound study. Circulation 105, 2037–2042 (2002).
- Fujikura H, Hata Y, Morino Y et al. Acute coronary syndrome due to intramural hematoma. Circulation 114, e644–e645 (2006).
- Flaherty MP, Dawn B, Solankhi N. Iatrogenic submedial coronary artery intramural hematoma presenting subacutely. J. Invasive Cardiol. 21, e128–e131 (2009).
- Farb A, Virmani R, Atkinson JB et al. Plaque morphology and pathologic changes in arteries from patients dying after coronary balloon angioplasty. J. Am. Coll. Cardiol. 16, 1421–1429 (1990).
- Vlodaver Z, Edwards JE. Pathology of coronary atherosclerosis. Prog. Cardiovasc. Dis. 14 256–274 (1971).
- Waller BF. The eccentric coronary atherosclerotic plaque: morphologic observations and clinical relevance. Clin. Cardiol. 12, 14–20 (1989).
- Alfonso F. Spontaneous coronary artery dissection: new insights from the tip of the iceberg? Circulation 126, 667–670 (2012).
- Eleid MF, Guddeti RR, Tweet MS et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ. Cardiovasc. Interv. 7, 656–662 (2014).
- Yamada EG, Fitzgerald PJ, Sudhir K et al. Intravascular ultrasound imaging of blood: the effect of hematocrit and flow on backscatter. J. Am. Soc. Echocardiogr. 5, 385–392 (1992).
- Johnson TW, Smith D, Strange JW et al. Spontaneous multivessel coronary intramural hematoma. An insight with OCT. JACC Cardiovasc. Imaging 5, 1070–1074 (2012).
- Sanchez-Recalde A, Moreno R, Jimenez-Valero S. Stenting of spontaneous intramural coronary haematoma: long-term consequences. Eur. Heart J. 29, 1593 (2008).
- Werner GS, Figulla HR, Grosse W et al. Extensive intramural hematoma as the cause of failed coronary angioplasty: diagnosis by intravascular ultrasound and treatment by stent implantation. Cathet. Cardiovasc. Diagn. 36, 173–178. (1995).
- Okuya Y, Fukushima K, Hori Y et al. Fenestration using a scoring balloon Scoreflex® as troubleshooting for acute vessel closure due to intramural hematoma complication in percutaneous coronary intervention. Cardiovasc. Interv. Ther. (2014) (Epub ahead of print).
- Ito S, Ojio S, Suzuki T. A novel use of cutting balloon in treating coronary artery dissection that developed during PCI. J. Invasive Cardiol. 15, 216–220 (2003).
- Taniguchi N, Takahashi A, Sakamoto S. Successful fenestration using a chronic total occlusion-dedicated guidewire in a patient with catheter-induced dissection of the right coronary artery. J. Invasive Cardiol. 23, 84–86 (2011).
• Defines what an intramural hematoma is by Intravascular Ultrasound Studies imaging.
•• This is the largest reported case series of intramural hematomas following percutaneous coronary intervention.