Review Article - Interventional Cardiology (2012) Volume 4, Issue 3
Coronary slow flow phenomenon: more than just an angiographic curiosity
- Corresponding Author:
- Michael P Savage
Jefferson Heart Institute 925 Chestnut St Philadelphia, PA 19107, USA
Tel: +1 215 955 6478
E-mail: michael.savage@jefferson.edu
Abstract
Keywords
angina pectoris, coronary angiography, coronary flow reserve, coronary slow flow phenomenon, microvasculature, Syndrome Y, vasodilator
Chest pain presentations including acute coronary syndrome and noncardiac chest pain comprise an inordinate amount of emergency room admissions and overall healthcare costs. Coronary angiography, seen as the gold standard for the diagnosis of coronary artery disease, can often be the ultimate test in the chest pain diagnostic algorithm. Even at the end of this diagnostic road, a diagnosis of ‘normal coronary arteries’ based on the absence of epicardial coronary stenosis may not convey the entire story in terms of the patient’s condition or prognosis.
Coronary slow flow phenomenon (CSFP) describes a slow progression of contrast medium in filling the coronary arteries during coronary angiography. CSFP occurs in the absence of epicardial coronary stenoses or other ‘secondary’ conditions that can impair coronary flow velocity. ‘Primary’ or ‘idiopathic’ CSFP has been described in up to as many as 7% of patients undergoing diagnostic coronary angiography [1]. This clinical entity, which can present identically to most acute coronary syndromes, may account for up to 4% of unstable angina admissions [2,3]. The clinical course can be quite challenging to physicians and debilitating to patients, with recurrence of chest pain occurring in up to 80% and hospital readmission in almost 20% of cases in less than a 2-year follow-up period [2].
The purpose of this paper is to raise awareness and understanding of this clinical condition, CSFP, through review of the qualitative and quantitative observations, the proposed physiologic mechanisms and the limited studies on medical treatment.
Historical background
Tambe et al. first described this slow coronary blood flow using angiography in 1972, which gave origin to its clinical interest that has accelerated over the past decade [4]. From the initial description, small vessel dysfunction appeared to be implicated. Subsequent myocardial biopsy pathology analysis in the 1980s and 1990s further suggested this mechanism. Over the course of the past decade, both observational and control studies by Beltrame et al. have helped delineate this disorder from Syndrome X or other chest pain presentations in the absence of significant epicardial coronary artery disease [2]. Following this observation, more has been reported on the topic, including analysis of the coronary artery microvascular resistance, reports of the not-so benign clinical impact and finally novel therapeutic approaches beyond traditional antianginal regimens, which often do not improve the symptomatology.
Definition
Assigning a definitive diagnosis for clinical use and also clinical trial design is challenging in this patient population, especially when considering the wide range of acute coronary syndrome presentations and the various forms of coronary microvascular disorders. CSFP is defined angiographically by delayed distal opacification in the absence of coronary artery disease [5]. Slow coronary flow can be quite subjective to make matters even more difficult. For the purposes of many of the clinical trials and the papers investigating potential mechanisms, a more specific, standardized definition was developed. CSFP was defined by more than one expert angiographer as the presence of: angiographically normal or near normal coronary arteries (i.e., <40% stenosis in any of the epicardial coronary arteries); and Thrombolysis In Myocardial Infarction (TIMI)-2 f low (i.e., requiring ≥3 beats to opacify prespecified branch points in the distal vasculature of at least one of the three major epicardial coronary vessels) [6,7]. Patients are excluded from this CSFP diagnosis if they have other conditions that would confound impaired coronary flow such as distal embolism/slow flow following coronary reperfusion intervention (no reflow), coronary artery ectasia, coronary arterial spasm, left ventricular myocardial dysfunction (ejection fraction <50% [with or without prior myocardial infarction (MI)]), sudden increases in intracavitary pressure, valvular heart disease, air embolism or connective tissue disorders [7,8]. These exclusion criteria separated out the ‘secondary’ coronary slow flow patients from the true ‘primary’ and ‘idiopathic’ CSFP. In the trials, corrected TIMI frame counts based on previously established criteria and definitions by Gibson et al. and Beltrame et al. were used [7,9]. These observational studies and trials also corrected for the length and diameter of coronary vessels. They also accounted for reduced epicardial coronary perfusion pressures by assessing important hemodynamic data such as heart rate, mean arterial pressure and rate–pressure product [2,7].
Interestingly the left anterior descending artery, even when corrected for length, was the artery that was most often involved (50–90% of the time) followed by the right coronary artery (28–45%) and the left circumflex (~20%) [2,7].
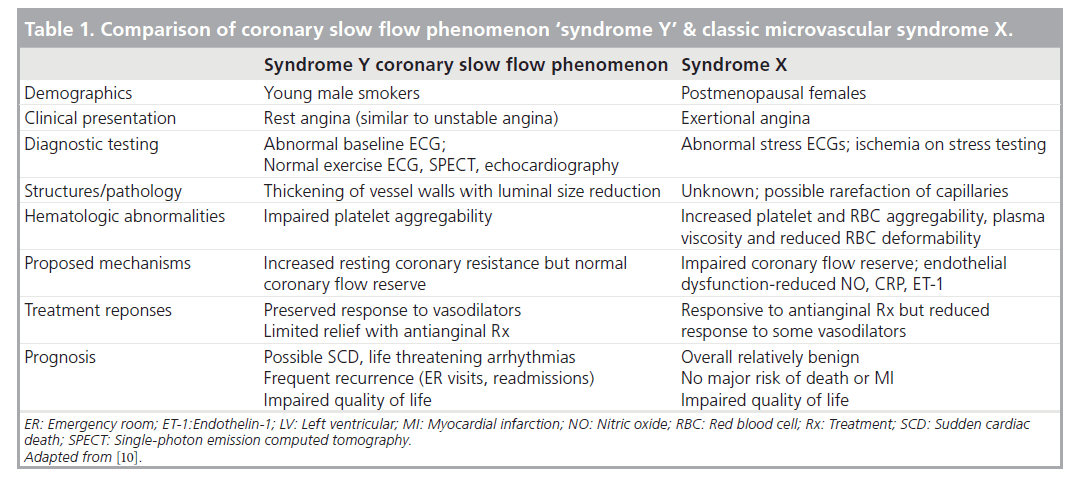
The literature presents a confusing landscape with regards to CSFP and microvascular flow abnormalities. Not all microvascular flow abnormalities are created equal. While CSFP as a description might be observed along with microvascular dysfunction in the classic coronary syndrome X, some authors view CSFP, alternatively labeled as ‘syndrome Y’, as a distinct entity separate from syndrome X [10,11]. As Table 1 shows, there are proposed inherent differences mechanistically, clinically and prognostically. These differences will also be reviewed in further detail in the subsequent sections.
Table 1. Comparison of coronary slow flow phenomenon ‘syndrome Y’ & classic microvascular syndrome X.
Another important distinction to be made is the difference between CSFP, which is a primary and spontaneous flow phenomenon, versus the no or slow reflow phenomenon, which occurs following urgent coronary revascularization in acute coronary syndrome. This separate entity is believed to result from a secondary cause following downstream distal embolization into the microvascular bed, but not spontaneously from the intrinsic condition of the microvasculature.
Clinical presentation
The most common demographic described as having CSFP is in young male smokers. These patients present with rest angina rather than with exertional symptoms, often bringing them to the emergency department. They often have resting ECG abnormalities (in up to a third of patients) yet normal exercise ECGs, normal myocardial stress ECGs and imaging, as well as normal resting ECGs [12]. It is not until proceeding to cardiac catheterizations, sometimes more than once, that the observation and diagnosis is made, which is often a source of frustration for patients and clinicians alike. Their typical initial clinical diagnosis is of unstable angina (Braunwald Class IIIB); a minority of patients may have ECG and cardiac biomarker evidence of MI (5–10%), which is clearly different than in patients with the classic and relatively benign syndrome X [2,13,14].
Previous authors have suggested the possibility that CSFP might not actually be only associated with, but a possible cause for MI in patients with otherwise normal coronary angiography [15]. Although ischemia is not usually documented by stress testing, the presence of demonstrable ischemia appears to identify patients with CSFP at increased risk of adverse outcomes [16–19]. At least one patient was reported to have sudden cardiac death, presumed to be related to persistent ischemia, while another case report made an association between CSFP and a subsequent acute MI [2,20]. Ventricular arrhythmias were also reported on initial presentations with sustained and nonsustained ventricular tachycardia, both of which are documented in these CSFP patients irrespective of prior MI and/or a structurally abnormal heart [2]. Other reports support these causal associations of increased corrected QT dispersions and ventricular arrhythmias leading to sudden cardiac death as a result of CSFP [21]. One illustrative case report implicated CSFP in the pathogenesis of ventricular fibrillation in a 20-year-old with aborted sudden cardiac death [22].
Proposed mechanisms
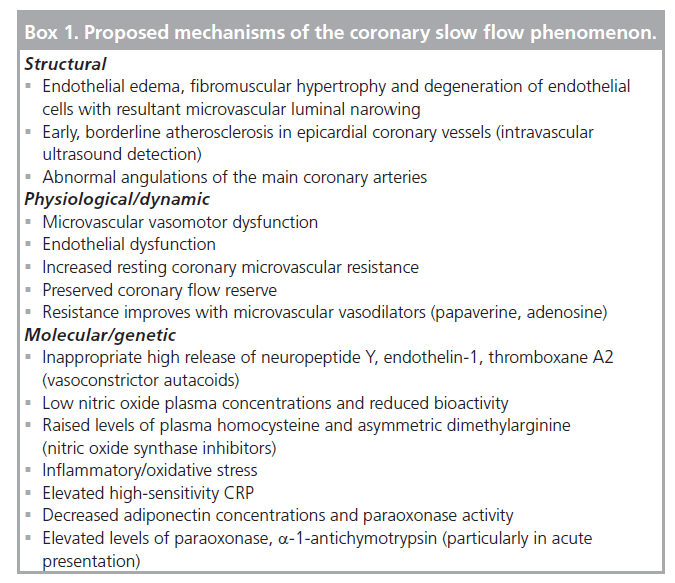
The initial descriptions implicating the distal coronary microvasculature were supported by early pathologic findings. Left and right ventricular myocardial biopsy specimens from patients with CSFP have demonstrated the presence of coronary microvascular disease [1,23]. Pathology showed myofiber hypertrophy, hyperplastic fibromuscular thickening of small arteries, swelling and degeneration of endothelial cell with luminal narrowing [23]. Consistent with these findings, another group revealed thickening of the vessel walls with reduced luminal size but also showed at the cellular level mitochondrial abnormalities and glycogen content reduction in the left ventricular biopsy specimens [1].
CSFP has been associated with hemorrheological abnormalities [24,25]. Celik et al. demonstrated evidence for increased platelet activation in patients with CSFP. Compared with normal controls, patients with slow coronary flow had larger mean platelet volume and higher levels of plasma P-selectin [24]. Other authors have noted additional rheological factors associated with CSFP that may contribute to decreased blood flow velocity including increased blood viscosity and increased erythrocyte aggregation [25].
The mechanism of CSFP though goes beyond structural and hematological abnormalities and has now moved to a paramount dynamic physiologic component (Box 1). Early observations noted that CSFP episodes could be improved or reversed by intravenous small vessel vasodilators, such as dipyridamole, while large vessel vasodilators did not have much of an impact [1]. Further dynamic implication of the small vessel dysfunction is suggested by CSFP being associated with ST elevation during coronary angiography in the notable absence of large vessel coronary spasm [26,27].
In these early studies, CSFP could not be accounted for by other possible hemodynamic explanations in reduced epicardial perfusion pressure and/or larger caliber coronary epicardial vessels requiring longer contrast filling time. It is important to recognize that uniformally in these investigations, systemic hemodynamics (based on heart rates and mean arterial pressures at the time of angiography) and vessel caliber were not significantly different between CSFP patients and control patients with normal flow.
Biochemically, elevations in serum uric acid level, 2 h postprandial blood glucose and blood platelet counts are found in patients with CSFP [28]. In the same recent clinical analysis, high-sensitivity CRP (hs-CRP), a marker of systemic inflammation, was the only significant independent predictor and the most likely to be implicated in the causal pathway of CSFP [24]. Results from another recent study also demonstrated a significant elevation of hs-CRP in CSFP patients. The same study also performed plasma proteomic profiling by western blot analysis and found that a-1-antitrypsin, a-1-antichymotrypsin, paraoxonase (an antioxidant), corticosteroid binding globulin and leucine-rich a-2-glycoprotein were all significantly elevated in the acute presentations of CSFP. The finding of and the association of these proteins should further implicate the inf lammatory/oxidative process in the pathogenesis of CSFP particularly in the acute coronary syndrome-like presentation [29].
On the molecular level, endothelin-1 and neuropeptide Y (another reason for the label ‘syndrome Y’) have been implicated as possible mediators of the microvascular constriction response. Dog and human model experiments have reproduced CSFP when infusing both of these proteins via the intracoronary route [30,31]. High release of these autocrine vasoconstrictors may underlie the mechanism for CSFP. In addition, nitric oxide, an endothelial-independent vasodilator, plasma concentrations were lower with reduced bioactivity in patients with CSFP [32]. Increased homocysteine and increased asymmetric dimethylarginine, a nitric oxide synthase inhibitor, along with decreased adiponectin and paraoxonase activity are all found to have detrimental reflections on endothelial function and may contribute to the development of CSFP [33,34]. These findings support the role of endothelial dysfunction along with increased resting coronary resistance. Beltrame et al. showed decreased resting oxygen saturation in CSFP patients as a surrogate for increased resting coronary resistance along with blunted responses to endothelial stimuli – cold pressor or acetylcholine testing [35]. However, despite abnormalities of baseline coronary flow as a likely result of endothelial dysfunction, coronary flow reserve was preserved and not impaired, as demonstrated by fast (up to 150 bpm) atrial pacing, which showed normal microvascular responsiveness [35].
Conf licting data and viewpoints were expressed by Erdogan et al. who reported impaired coronary flow reserve (CFR) in CSFP patients by dipyridamole stress and transthoracic Doppler echocardiography [36]. The authors speculated that CFR assessment and impairment in the epicardial and microvasculature may reflect a precursor to clinical epicardial coronary artery disease [36]. There is also conflicting data and opinions when it comes to the ‘normalization’ of the coronary flow in relation to the timing of clinical episodes. Beltrame et al. demonstrated that even inbetween acute presentations coronary microvascular tone is chronically elevated in CSFP patients [35]. Years later, Sharman et al. explored the same question but interestingly found no difference in local myocardial blood flow, myocardial blood flow reserve and ascending aortic blood pressure by stress contrast echocardiography and radial tonometry between rest- and stress-induced episodes [37]. Likewise they also found no difference in systemic blood f low by arterial wave ref lections and aortic stiffness by aortic and brachial pulse wave velocities [37]. These findings were interpreted to indicate that arterial tone and blood flow are normal in CSFP patients between their acute exacerbations. Given the complexity of the disorder and the many mechanisms in play, it is unclear whether these surrogate systemic measures reflect vascular dynamics at the level of the coronary microcirculation.
Fineschi et al. furthered our understanding of CSFP from these conflicting data with the first human in vivo study [8]. By using an invasive thermodilution method (CFR thermo, Radi Medical Systems, MA, USA) they were able to assess resting and hyperemic coronary artery resistances and CFR in patients with CSFP [38–40]. Fractional flow reserve was also measured in all cases to exclude the presence of epicardial coronary stenoses [41]. Under baseline resting conditions, indexes of resting microvascular resistances were signif icantly elevated in patients with CSFP when compared with normal controls. This difference was reversible following administration of intracoronary papaverine. While hyperemic mean transit times and indexes of resting microvascular resistance were still in the higher range of normal, they were not significantly different when compared with normal controls following response to vasodilator intervention. The key take home message was that CFR was preserved in CSFP despite a significantly elevated and abnormal resting microvascular resistance [8].
Functionally, the main difference between the classic cardiac syndrome X and CSFP is the preserved CFR. It is the dynamic vasodilator (dipyridamole, adenosine, papaverine and exercise) response that improves the inherent high vascular resistance in CSFP but not in cardiac syndrome X [10].
Another interesting perspective under consideration is whether or not the slow flow phenomenon is exclusive to the coronaries or pervades throughout the circulatory system. Wang et al. summarized the current context of f low mediated dilatation of the brachial artery and carotid intima-media thickness abnormalities in patients with CSFP linked to endothelial dysfunction and coronary intimamedia thickening, respectively [42]. They hypothesized that CSFP is part of a larger systemic vascular disturbance and not isolated to the coronaries. Elevated hs-CRP in these patients combined with these observations gives stronger credence to inflammation as a prime cause of CSFP, not only at the coronary but at the broader systemic level [28].
Clinical implications
Prior to more recent investigations and explanations of the root causes, CSFP had been associated with adverse clinical conditions such as increased atherosclerotic burden [43], impaired diastolic function [44], endothelial dysfunction [45] and left bundle branch block [46]. How clinical cardiologists deal with this condition and its associations hinges very much on the recognition and understanding of the entity of CSFP.
With recognition on initial angiography, invasive and general cardiologists can have an important impact making a correct diagnosis. CSFP is a diagnosis that has now been better characterized with a certain degree of clinical distinction and prognosis. By establishing a correct diagnosis, cardiologists can avoid the need for additional noninvasive and repeat invasive testing. The impact can be influential to patients with reassurance and avoidance of emergency room visits, which can have a profound impact on patients psychologically and on our healthcare system economically with cost savings [12].
Understanding this disorder is also critical from a prognostic standpoint. Previously the reassurance given to patients with ‘normal coronary arteries’ may have been reasonably appropriate in those patients with classic syndrome X; to give that same information to a patient with true CSFP may not be appropriate. Given the reported examples of significant ventricular arrhythmias and even sudden cardiac death being linked to this condition, physicians should not trivialize the importance of these findings. By contrast, long-term follow-up (mean of 7 years) of patients with syndrome X confirmed no deaths, MIs or signif icant worsening of left ventricular function [47]. Clearly there is potential morbidity and even mortality connected with CSFP. Whether or not pharmacologic interventions can improve these outcomes still remains an open question.
Proposed treatment strategies
In the acute setting in the cardiac catheterization lab, certain vasodilators that have effects on the microvasculature (<200 μm in diameter), which include dipyridamole, adenosine and papaverine, can all yield significant improvements in coronary flow. Mangieri et al. observed that in this CSFP patient population flow could return to normal limits with infusion of dipyridamole, which was attributed to the microvascular dilation effect [1]. Vasodilators, which dilate epicardial coronary arteries >200 μm in diameter, such as nitroglycerin do not successfully normalize the TIMI frame count (i.e., coronary flow) in the acute or chronic situations.
Reported clinical experience has shown that basic antianginal medical therapy has been limited in effectiveness in chronic treatment of the CSFP condition. Calcium channel blockers have shown some promise in treating microvascular dysfunction (although those more appropriately labeled as syndrome X) broadly, not those specifically with CSFP [48]. One major exception to these generalizations has been a unique calcium T-channel blocker in addition to calcium L-channel blockade, with mibefradil. Rat studies by Gustafsson et al. demonstrated the predominance of T-channels in the microvasculature [49]. This abundance of T- versus L-channels in the microperiphery of the coronary beds may account for the lack of response to classic antianginal therapies, specif ically the calcium channel blocker verapamil [7]. Beltrame et al. sought to take advantage of these properties and use such an agent, mibefradil, to block these particular calcium channels [7]. The designs and results of this trial in the CSFP population will be discussed in further detail below.
An important nonpharmacologic intervention that should not be overlooked in treating CSFP patients is smoking cessation. Smoking has clearly been associated with CSFP. This association is consistent with the observed impaired postischemic skin blood flow motion (depicted by laser Doppler flowmetry of the skin microcirculation) present in chronic healthy smokers, which suggests an early sign of endothelial and smooth muscle microvascular dysfunction [50,51]. Patients with chronic obstructive pulmonary disease, a systemic inflammatory disorder, were associated with a higher TIMI frame count (slow flow) [52]. This link could reflect microvascular impairment throughout vascular beds in chronic smokers. In addition, several authors have demonstrated that chronic smoking can result in increased plasma viscosity and fibrinogen levels and thus further increase microvascular resistance contributing to increased slow coronary flow [53,54]. Although clinical studies exploring the impact of smoking cessation on CSFP are lacking, from a theoretical standpoint it would be appropriate to promote smoking cessation in addition to general coronary artery disease prevention measures.
Clinical trials
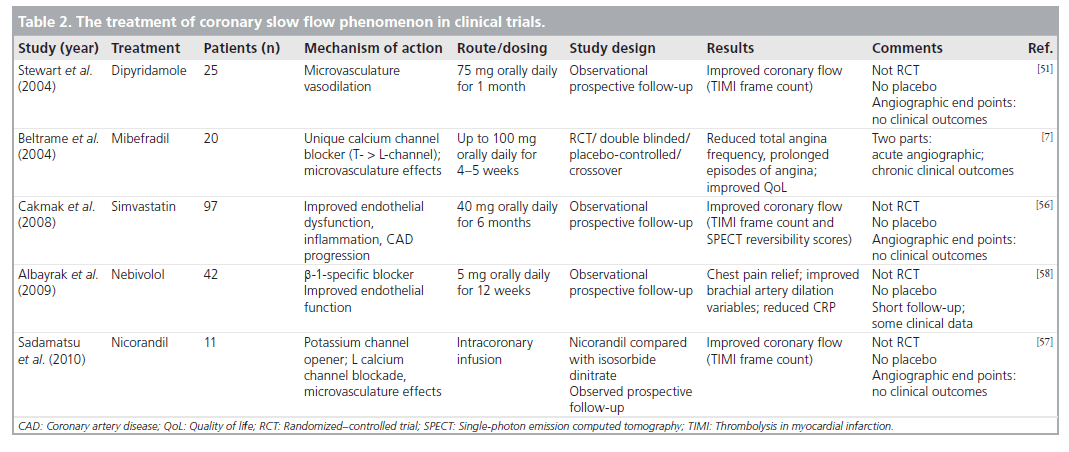
Dipyridamole, which mainly acts by blocking the uptake of adenosine in erythrocytes, platelets and endothelium resulting in antiplatelet and vasodilator effects, is primarily used chronically for secondary stroke prevention in conjunction with aspirin. Could or would chronic oral therapy with such microvascularspecific vasodilators such as dipyridamole, lead to continual improved flow and improved clinical outcomes ? That question was evaluated in a small trial by Kurtoglu et al. of 25 CSFP patients. Treatment with 75 mg of dipyridamole three-times daily for up to 1 month [55], demonstrated clear improvement of TIMI frame counts (representing coronary blood flow) from the beginning of therapy to the completion of therapy. As with Mangieri’s sentinel intracoronary studies, coronary flow normalized in the majority of vessels [1]. This observational only, not randomized or controlled, trial limited itself to angiographic and not clinical outcomes. While theoretically appealing, further controlled trials are necessary to determine the clinical utility of dipyridamole in the management of CSFP.
One of the first randomized, double-blinded, placebo-controlled trials was performed by Beltrame et al. in a total of 20 patients with CSFP; half were assigned to receive mibefradil 100 mg daily. This trial demonstrated acutely improved coronary blood flow as demonstrated by angiography [7]. In the chronic setting, mibefradil demonstrated efficacy by reducing spontaneous angina episodes by 56%, by reducing prolonged (>20 min) angina episodes by 74% and finally by improving quality of life (assessed by the Health Outcome Study Short Form-36) [7]. There was no significant increase in the side effects or adverse events reported. Beltrame’s study was really one of the few trials that examined clinical end points rather than surrogate imaging or angiographic data, as with many of the other trials (Table 2). Unfortunately mibefradil was voluntarily withdrawn from general therapeutic availability by the manufacturing company, primarily because of its inhibition of cytochrome P450 3A4, resulting in potential accumulation of coadministered drugs, such as simvastatin.
One of the few other trials that examined improving CSFP (with regards to myocardial perfusion abnormality and coronary flow) was with simvastatin. Cakmak et al. theorized that statins could potentially improve myocardial perfusion via their counteractions on endothelial dysfunction, mild diffuse atherosclerosis and inflammation, which have been implicated in CSFP [56]. A total of 97 patients with CSFP (as determined by TIMI frame count methods previously described) were given 40 mg of simvastatin once daily for a period of 6 months with single-photon emission computed tomography imaging and lipid measurements performed at the beginning and end of the trial. They reported a significant positive correlation between mean TIMI frame count and basal reversibility score (r = 0.84, p = 0.0001). Analysis of the reversibility scores demonstrated an improvement in myocardial perfusion after 6 months in patients with CSFP [56]. In the absence of a control group, it remains unclear to what degree the simvastatin therapy contributed to the improvement in myocardial perfusion; other investigators have observed an improvement in TIMI flow over time in patients with CSFP who were not treated with statin therapy [35].
Pharmacologic interventions can also be performed in the cardiac catheterization laboratory during acute presentations of CSFP. A small study (11 patients) from Japan showed the microvascular vasodilator, nicorandil, which works on potassium channel permeability as well as L-calcium channel closure, had greater improvement in coronary flow (as assessed by TIMI frame counts angiographically) than isosorbide dinitrate, a commonly used antianginal medication, when administered via the intracoronary route [57]. Unfortunately for practitioners in the USA, this drug is also not available for clinical use.
Most recently Albayrak et al. explored the efficacy of nebivolol, a relatively new b-1 selective blocker, in patients with CSFP [58]. In this study, 42 CSFP patients received 5 mg of nebivolol orally daily for a 12-week period. Clinical improvements were observed with chest pain relief in 38 out of the 42 (90%) patients and improved systolic and diastolic blood pressures. From the perspective of a potential mechanistic explanation, hs-CRP was decreased, while brachial artery dilation variables (basal resistive index, postf low mediated dilation resistive index and postnitrate mediated dilation) significantly decreased following treatment [58]. These improvements with treatment could reflect improvements in both vascular inflammation and endothelial function, which as described above are possible key components in the pathophysiology of CSFP. Traditional antianginal therapy, including b-blockers, have reportedly not had great clinical success in this patient population [58]. Whether or not nebivolol, as a newer b-blocker, has unique beneficial properties or whether other b-blockers have just not been adequately studied in this setting is unknown.
Novel or even previously known therapeutics developed for other reasons (i.e., angina, coronary artery disease secondary prevention) have not evolved for acute and chronic treatments of CSFP. No breakthroughs have emerged over the last several years and nothing appears to be forthcoming. A proposed trial in Israel, exploring the role of dipyridamole for chronic treatment of CSFP, never materialized for unknown reasons [101]. Trials and development in this area appear to be at a standstill for now.
Conclusion
Recognition and definition of CSFP continues to evolve. CSFP denotes delayed contrast filling of the epicardial arteries, which is believed to be a result of increased resting baseline microvascular resistance. It is the reversal of this resistance with a preserved response to adenosine-dependent vasodilators and a preserved normal CFR that distinguishes CSFP from other microvascular disorders such as syndrome X. There is an increasing appreciation that CSFP is a relevant angiographic finding with a dynamic physiologic substrate. There is also accumulating evidence that CSFP can be responsible for serious clinical outcomes: recurrent emergency room visits for angina; hospitalizations; repeat diagnostic testing; ventricular arrhythmias; and even sudden cardiac death.
Future perspective
Although encouraging based on the information just reviewed, the overall survey of the literature on CSFP is somewhat discouraging, with respect to the paucity of novel publications, especially in regards to therapeutic advances. Yet, there is a growing understanding of CSFP as a unique microvascular, and more importantly a clinical, disorder. Much of the explanations for the mechanisms and pathogenesis are not entirely resolved (Box 1). Our understanding of the disorder, although improved when compared with a few decades ago, is still in its infancy. The lack of a unifying paradigm, as well as the poor recognition of the disorder have resulted in the paucity of clinical trials and proven interventions. Some of the most studied and potentially effective therapies, mibefradil and nicorandil, are either no longer on the market or are unavailable for use in the USA. At the time of the submission of this review, there are currently no ongoing trials examining treatments for this condition [101]. Given the lack of proven efficacy with clinically available drugs, appropriate therapy for this condition remains unsettled. Based on the scant data with mibefradil, some authors such as Lanza and Crea have suggested that calcium channel antagonists be used as first-line therapy with the caveat that this recommendation requires conf irmation by appropriate prospective studies [14]. With wider recognition of this condition and its clinical significance, it is hoped that future trials of drugs with effects on microvasculature function will be forthcoming.
Financial & competing interests disclosure
The authors have no relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership, or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
▪ Slow coronary blood flow was first described in 1972 and was initially thought of as just an angiographic observation.
▪ Not as uncommon as originally thought (up to 7% of diagnostic angiography).
Definition
▪ Delayed contrast progression through coronaries during angiography in the absence of secondary conditions that can lead to impaired coronary flow.
Clinical presentation
▪ Similar to unstable angina, usually with normal stress testing.
Proposed mechanisms
▪ Microvasculature dysfunction with structural and dynamic pathophysiology abnormalities.
▪ Endothelial abnormalities – inflammatory, early atherosclerosis.
▪ Abnormal coronary artery vascular resistance but with preserved coronary flow reserve (with reversibility with microvascular dilators).
Clinical impact/practice
▪ Mimics acute coronary syndrome resulting in extensive diagnostic testing, emergency room visits, admissions and healthcare costs.
▪ Impacts patients’ quality of life significantly with recurrent, significant chest pain.
▪ Linked to more serious outcomes, such as myocardial infarctions, recurrent ischemia, ventricular arrhythmias and rare cases of sudden cardiac death.
Clinical trials
▪ Few randomized, controlled trials; most studies assessed only angiographic end points.
▪ Angiographic flow improvement with dipyridamole (adenosine-dependent vasodilator) simvastatin.
▪ Symptom relief and possible improved endothelial function with nebivolol.
▪ Symptom and clinical benefits plus angiographic flow improvement with calcium channel blockers, specifically mibefradil (a calcium T-channel blocker, although no longer clinically commercially available).
Conclusion
▪ Coronary slow flow phenomenon is clinically relevant angiographic finding, for which the pace of discovery has accelerated in the last several decades.
▪ Recognition of coronary slow flow phenomenon has potential for great clinical benefit.
▪ Understanding and unifying the mechanisms of coronary slow flow phenomenon are still a work in progress.
▪ Proven and available treatments for this condition are undefined.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Mangieri E, Macchiarelli G, Ciavolella M et al. Slow coronary flow: clinical and histopathological features in patients with otherwise normal epicardial coronary arteries. Cathet. Cardiovasc. Diagn. 37, 375–381 (1996).
- Beltrame JF, Limaye SB, Horowitz JD. The coronary slow flow phenomenon – a new coronary microvascular disorder. Cardiology 97, 197–202 (2002).
- Diver DJ, Bier JD, Ferreira PE et al. Clinical and arteriographic characterization of patient with unstable-angina without coronary arterial narrowing (from TIMI-IIIA trial). Am. J. Cardiol. 74, 531–537 (1994).
- Tambe AA, Demany MA, Zimmerman HA et al. Angina pectoris and slow flow velocity of dye in coronary arteries. A new angiographic finding. Am. Heart J. 84, 66–71 (1972).
- Beltrame JF. Chest pain and normal angiography. In: Braunwald Heart Disease (e-Edition). Braunwald E (Ed.), Elsevier, PA, USA, 1329 (2006).
- Chesebro JF, Knatterud GL, Roberts R et al. Thrombolysis in Myocardial Infarction (TIMI) trial, Phase 1: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 76, 142–154 (1987).
- Beltrame JF, Turner SP, Leslie SL et al. The angiographic and clinical benefits of mibefradil in the coronary slow flow phenomenon. J. Am. Coll. Cardiol. 44, 57–62 (2004).
- Fineschi M, Bravi A, Gori T. The “slow coronary flow” phenomenon: evidence of preserved coronary flow reserve despite increased resting microvascular resistances. Int. J. Cardiol. 127, 358–361 (2008).
- Gibson CM, Cannon CP, Daley WL et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation 93, 879–888 (1996).
- Leone MC, Gori T, Fineschi M. The coronary slow flow phenomenon: a new cardiac “Y” syndrome? Clin. Hemorheol. Microcirc. 39(1–4), 185–190 (2008).
- Gori T, Fineschi M. Two coronary “orphan” diseases in search of clinical consideration: coronary syndromes X and Y. Cardiovasc. Ther. 30, e58–e65 (2012).
- Beltrame JF, Ganz P. Evaluating patients with persistent chest pain and no obstructive coronary artery disease. JAMA 302 (6), 622–624 (2009).
- Nava Lopez G, Monteverde C, Jauregui R et al. X syndrome. Angiographic findings. Arch. Inst. Cardiol. Mex. 59(3), 257–265 (1989).
- Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation 121, 2317–2325 (2010).
- Yetkin E, Turhan H, Erbay AR et al. Increased thrombolysis in myocardial infarction frame count in patients with myocardial infarction frame count in patients with myocardial infarction and normal coronary arteriogram: a possible link between slow coronary flow and myocardial infarction. Atherosclerosis 81, 193–199 (2005).
- Kapoor A, Goel PK, Gupta S. Slow coronary flow – a cause for angina with ST segment elevation and normal coronary arteries. A case report. Int. J. Cardiol. 67, 257–261 (1998).
- Celik T, Ivyisoy A, Kursaklioglu H et al. ST elevation during treadmill exercise test in a young patient with slow coronary flow: a compared with report and review of literature. Int. J. Cardiol. 112, e1–e4 (2006).
- Goel PK, Gupta SK, Agarwal A et al. Slow coronary flow: a distinct angiographic subgroup in syndrome X. Angiology 52, 507–514 (2001).
- Tatli E, Yildirim T, Aktoz M. Does coronary slow flow phenomenon lead to myocardial ischemia? Int. J. Cardiol. 131, e101–e102 (2009).
- Przybojewski J, Becker P. Angina pectoris and acute myocardial infarction due to ‘slow-flow phenomenon’ in non-atherosclerotic coronary arteries: a case report. Angiology 37, 751–761 (1986).
- Saya S, Hennebry TA, Lozano P et al. Coronary slow flow phenomenon and risk for sudden cardiac death due to ventricular arrhythmias: a compared with report and review of the literature. Clin. Cardiol. 31, 352–355 (2008).
- Amasyali B, Turhan H, Kose S et al. Aborted sudden cardiac death in a 20-year-old man with slow coronary flow. Int. J. Cardiol. 109, 427–429 (2006).
- Mosseri M, Yarom R, Gotsman MS et al. Histologic evidence for small-vessel coronary artery disease in patients with angina and patent large coronary arteries. Circulation 74, 964–972 (1986).
- Celik T, Yuksel UC, Bugan B et al. Increased platelet activation in patients with slow coronary flow. J. Thromb. Thrombolysis 29, 310–315 (2010).
- Demaske A, Muxel S, Fasola F et al. Peripheral hemorheological and vascular correlates of coronary blood flow. Clin. Hemorheol. Microcirc. 49(1–4), 261–269 (2011).
- Murakami H, Urabe K, Nishimura M. Inappropriate microvasculature constriction produced transient ST-segment elevation in patients with syndrome X. Circulation 32, 1287–1294 (1998).
- Beltrame JF, Horowitz JD. ST elevation secondary to microvascular dysfunction. J. Am. Coll. Cardiol. 34, 312–313 (1999).
- Xia S, Deng SB, Wang Y et al. Clinical analysis of the risk factors of slow coronary flow. Heart Vessels 26, 480–486 (2011).
- Kopetz VA, Penno MAS, Hoffman P et al. Potential mechanisms of at the acute coronary syndrome presentation in patients with the coronary slow flow phenomenon – insight from a plasma proteomic approach. Int. J. Cardiol 156(1), 84-91 (2011).
- Larkin SW, Clarke JG, Keogh BE et al. Intracoronary endothelin induces myocardial ischemia by small vessel constriction in the dog. Am. J. Cardiol. 64, 956–958 (1989).
- Clarke J, Davies G, Kerwin R et al. Coronary artery infusion of neuropeptide Y in patients with angina pectoris. Lancet I, 1057–1059 (1987).
- Camsarl A, Pekdemir H, Cicek D et al. Endothelin-1 and nitric oxide concentrations and their response to exercise in patients with slow coronary flow. Circ. J. 67, 1022–1028 (2003).
- Riza Erbay A, Turhan H, Yasar AS et al. Elevated level of plasma homocysteine in patient with slow coronary flow. Int. J. Cardiol. 102, 418–423 (2005).
- Selcuk MT, Selcuk H, Temizhan A et al. Asymmetric dimethylarginine plasma concentrations and l-arginine/asymmetric dimethylarginine ratio in patients with slow coronary flow. Coron. Artery Dis. 18, 545–551 (2007).
- Beltrame JF, Limaye SB, Wuttke RD et al. Coronary hemodynamic and metabolic studies of the coronary slow flow phenomenon. Am. Heart J. 146, 84–90 (2003).
- Erdogan D, Caliskan M, Gullu H et al. Coronary flow reserve is impaired in patients with slow coronary flow. Atherosclerosis 191(1), 168–174 (2007).
- Sharman JE, Moir S, Kostner KM et al. Patients with coronary slow flow phenomenon demonstrate normal myocardial blood flow and arterial reflection between acute episodes. Int. J. Cardiol. 131, 321–325 (2009).
- Barbato E, Aarnoudse W, Aengevaeren WR et al. Validation of coronary flow reserve measurements by thermodilution in clinical practice. Eur. Heart J. 25, 219–223 (2004).
- Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation 113, 2054–2061 (2006).
- Fearon WF, Balsam LB, Farouque HM et al. Novel index for invasively assessing the coronary microcirculation. Circulation 107, 3129–3132 (2003).
- Pijls NH, VanGelder B, Van der Voort P et al. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis of myocardial blood flow. Circulation 92, 3183–3193 (1995).
- Wang X, Geng LL, Shao-Ping N. Coronary slow flow phenomenon: a local or systemic disease? Med. Hypotheses 75, 334–337 (2010).
- Pekdemir H, Cin VG, Cicek D et al. Slow coronary flow may be a sign of diffuse atherosclerosis. Contribution of FFR and IVUS. Acta Cardiol. 59, 127–133 (2004).
- Sezgin AT, Topal E, Barutcu I et al. Impaired left ventricle filling in slow coronary flow phenomenon: an echo-Doppler study. Angiology 56, 397–401 (2005).
- Sezgin, Barutcu I, Sezgin AT et al. Plasma nitric oxide level and its role in slow coronary flow phenomenon. Int. Heart J. 46, 373–382 (2005).
- Acikel S, Bozkaya OA, Akdemir R. The relationship between intermittent left bundle branch block and spontaneous coronary flow in a patient presenting with acute coronary syndrome. Blood Coagul. Fibrinolysis 21(6), 595–597 (2010).
- Kaski JC, Rosano GM, Collins P et al. Cardiac syndrome X: clinical characteristics and left ventricular function. Long term follow-up study. J. Am. Coll. Cardiol. 25, 807–814 (1995).
- Shaw LJ, Merz CN, Pepine CJ et al.; Women’s Ischemia Syndrome Evaluation (WISE) Investigators. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Circulation 114(9), 894–904 (2006).
- Gustafsson F, Andreasen D, Salomonsson M et al. Conducted vasoconstriction in rat mesenteric arterioles: role for dihydropyridine-insensitive Ca(2+) channels. Am. J. Physiol. Heart Circ. Physiol. 280, H582–H590 (2001).
- Rossi M, Carpi A, Di Maria C et al. Absent post-ischemic increase of blood flow motion in the cutaneous microcirculation of healthy chronic cigarette smokers. Clin. Hemorheol. Microcirc. 36, 163–171 (2007).
- Stewart S, Kohen A, Brouder D et al. Noninvasive interrogation of microvascular for signs of endothelial dysfunction in patient with chronic renal failure. Am. J. Heart Circ. Physiol. 1287, H2687–H2696 (2004).
- Selcuk H, Maden O, Selcuk MT et al. Documentation of impaired coronary blood flow in chronic obstructive pulmonary disease patients. Circ. J. 74, 346–352 (2010).
- Haustein KO, Krause J, Hausten H et al. Effects of cigarette smoking or nicotine replacement on cardiovascular risk factors and parameters of haemorheology. J. Int. Med. 252, 130–139 (2002).
- Meade TW, Imeson J, Stirling Y. Effects of changes in smoking and other characteristics on clotting factors and the risk of ischaemic heart disease. Lancet 2, 986–989 (1987).
- Kurtoglu N, Akcay A, Dindar I. Usefulness of oral dipyridamole therapy for angiographic slow coronary flow. Am. J. Cardiol. 87(6), 777–779 (2001).
- Cakmak M, Tanriverdi H, Cakmak N et al. Simvastatin may improve myocardial perfusion abnormality in slow coronary flow. Cardiology 110(1), 39–44 (2008).
- Sadamatsu K, Tashiro H, Yoshida K et al. Acute effects of isosorbidedinitrate and nicorandil on the coronary slow flow phenomenon. Am. J. Cardiovasc. Drugs 10(3), 203–208 (2010).
- Albayrak S, Ordu S, Yuksel H et al. Efficacy of nebivolol on flow-mediated dilation in patients with slow coronary flow. Int. Heart J. 50(5), 545–553 (2009).
▪ In this series, coronary slow flow phenomenon (CSFP) was observed in up to 7% of patients with chest pain and normal epicardial coronary arteries; improved flow was seen after dipyridamole but not after intracoronary nitroglycerin. Endomyocardial biopsy frequently demonstrated wall thickening of microvessels with luminal narrowing.
▪ The study of 47 patients demonstrated that CSFP most often presents as an acute coronary syndrome. During a median followup of 21 months, 84% of patients had recurrent chest pain.
▪ This seminal article of CSFP described six patients with angina pectoris who had ‘strikingly’ slow coronary flow in the absence of epicardial disease. The author speculated that microvascular disease was the likely underlying etiology.
▪▪ This is one of the few placebo-controlled prospective trials in patients with CSFP. The effects of mibefradil, a unique calcium T-channel blocker, was evaluated in 20 patients with CSFP in a randomized cross-over design. Angina frequency and sublingual nitrate consumption were reduced by more than 50% with mibefradil.
▪▪ This human in vivo study utilized intracoronary thermodilution techniques to assess coronary flow reserve and coronary microvascular resistance in eight patients with CSFP. Although resting microvascular resistance was significantly increased with CSFP, the microvasculature had retained capacity to vasodilate: following papaverineinduced hyperemia, microvascular resistance normalized and coronary flow reserve was not impaired.
▪ An excellent review that contrasts the clinical and pathophysiologic difference between syndrome X and syndrome Y (coronary slow flow).
▪ Website
101. Normal coronary artery with slow flow improved by adenosine injection, dipyridamole treatment and clinical follow-up. www.clinicaltrials.gov/ct2/show/NCT00960817?term=coronary+slow+flow+phenomenon(Accessed 4 April 2012)