Review Article - Interventional Cardiology (2011) Volume 3, Issue 5
Coronary stent thrombosis: incidence, predictors and triggering mechanisms
- Corresponding Author:
- Jochem van Werkum
St Antonius center for Platelet Function Research, St Antonius Hospital, Nieuwegein, The Netherlands
Tel: +31 306 099 111
Fax: +31 306 034 420
Abstract
Keywords
coronary stent thrombosis,high on-treatment platelet reactivity,risk factors,triggering mechanisms
The treatment of obstructive coronary artery disease has drastically revolutionized the field of clinical cardiology since the first coronary stent became available in the mid 1980s [1,2].The bare metal stent (BMS) was first introduced in 1985 to provide a mechanical scaffold for the artery wall to avoid elastic recoil of the vessel wall and to seal mechanical lacerations and (micro) dissection of the atherosclerotic plaque induced by ballon angioplasty [1,2].Further technological advances ultimately led to the development of drug-eluting stents (DES) [3-5].DES release antiproliferative drugs that reduce neointimal formation, thereby reducing in-stent restenosis rates to below 10%. As a result, the interventional community embraced the DES with a subsequent broadening of indications for interventional treatment of coronary artery disease (e.g., chronic total occlusions, multivessel disease, small vessel diameters, stent thrombosis (ST)-segment elevation myocardial infarction [STEMI]). At present, approximately 3 million percutaneous coronary interventions (PCI) are performed worldwide per year and as a result, coronary stent-related complications, even when occurring at a relatively low rate, have a major impact on total mortality and hospital stay. The most dreadful complication after coronary stenting is the abrupt thrombotic closure of the implanted stent: ST [6–16].
Coronary ST: definitions
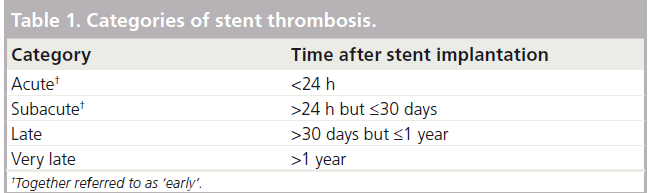
The true impact and incidence of ST has been unknown for at least three decades because its definition varied widely among randomized clinical trials and observational registries. Therefore, to allow consistency and a fair comparison across studies and registries, a new uniform definition of ST was proposed by the Academic Research Consortium (ARC) in 2007 [17].
In the ARC criteria, ST is classified according to 1) the elapsed time between index PCI and the ST and 2) the likelihood for its presence. Timing is categorized in four categories (Table 1). In parallel, the level of evidence is divided into three categories relating to varying degrees of certainty: possible, probable and definite ST.
Incidence of coronary ST
Despite being a relatively infrequent problem, ST has a major clinical impact because it results in life-endangering conditions such as myocardial infarction (in approximately 80%) and cardiac death (in up to 40% of cases) [10]. It is important to note that the risk of ST is directly related to the clinical presentation of the patient preceding stenting (the index PCI). For example, patients presenting with a STEMI have a fourfold increased risk for ST compared with patients undergoing coronary stent implantation for stable angina symptoms [6,18].
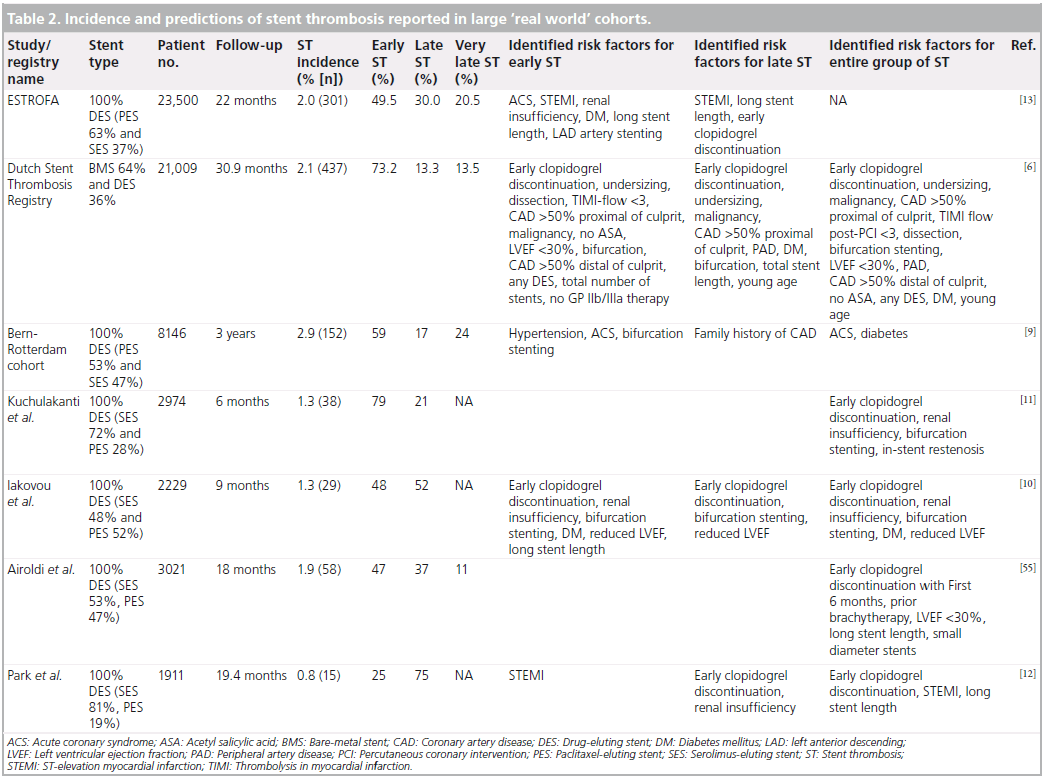
At present, it is fairly impossible to establish the true incidence of ST since an angiographic or pathologic confirmation is necessary to fulfil the ARC-criteria of definite ST. Nonetheless, large ‘real world’ and all-comer registries provide at least some insights into incidence rates of ST. In the large all-comer cohort of the Dutch Stent Thrombosis Registry (n = 21,009), the reported incidence of ST was 2.1% (number of cases: n = 437) after a median follow-up of 30.9 months [6,18,19]. Similarly, in the Spanish ESTROFA registry (n = 23,500), the reported incidence of ST was 2% (number of cases: n = 301) after a median follow-up of 22 months (Table 2) [13].
In the Bern-Rotterdam registry, definite ST occurred in 192 of 8146 patients (treated with either a sirolimus-eluting stent [n = 3823] or a paclitaxel-eluting stent [n = 4323]) with an incidence density of 1/100 patient-years and a cumulative incidence of 3.3% at 4 years [20].
Multiple studies and registries have shown that the incidence rates of early ST are quite similar between bare-metal stents and drug-eluting stents [21].
For late and very late ST, however, the incidence appears to be slightly higher for DES as compared with BMS. And more importantly, the phenomenon of late and very late ST may be less frequent in second-generation DES and tends to be more frequent with paclitaxel-eluting stents than with sirolimus-eluting stents [22–25].
The observational SCAAR registry reported the incidence of ST in patients treated with multiple types of stents. A total of 73,798 stents from 47,197 procedures and 42,150 patients were included [22]. During a follow-up period of 3 years, the risk of ST was lower in DES as compared with BMS with an adjusted risk ratio of 0.79; (99% CI: 0.63–0.99). However, after 6 months of follow-up, the risk for ST was higher in DES compared with BMS with an adjusted risk ratio of 2.02 (99% CI: 1.30–3.14). Likewise, the Western Denmark Heart Registry confirmed this SCAAR ‘real world’ observation that very late definite STs were significantly increased in DES patients, in particular with paclitaxel-eluting stents [23].
The reported incidence of late and very late ST in second-generation DES, such as zotarolimus- eluting (Endeavor®;) and everolimuseluting stents (Xience V®;) is very low, although ‘real world’ all-comer registries with a follow-up beyond a 2 year timeframe are limited [24–26].
Prognosis after a coronary ST
The majority of patients with ST present with a STEMI and mortality rates are high. De la Torre- Hernandez and colleagues followed 301 definite ST patients and at 1 year follow-up, mortality was 16%, and recurrent ST occurred in 4.6% of patients [13]. Several risk factors for mortality were identified in multivariate analysis, including older age, decreased left ventricular ejection fraction (<45%), thrombolysis in myocardial infarction flow grade 3 or less, and additional stent implantation at the time of emergent PCI for ST. In the Dutch Stent Thrombosis Registry, a consecutive cohort of 431 patients with definite ST were prospectively followed. After a median follow-up of 27.1 months, 12.3% patients had died and the cumulative incidence rate of definite recurrent ST was astonishingly high as 18.8% of the patients who had suffered a first ST also suffered a recurrent ST [19]. This high recurrence rate was also observed by Lemesle and coworkers who reported a recurrence rate of 36% in patients successfully treated for a first ST during a median follow-up of 40 months [27].
Predictors of coronary ST
Numerous predictors of ST, including clinical (e.g., diabetes, renal failure, young age, smoking, present malignancy and stenting for myocardial infarction, among many others), angiographic (e.g., small vessel diameter and multivessel disease) and procedural (e.g., bifurcation stenting, dissection) determinants have been identified (Table 2 & Box 1) [6–16]. And more recently, a heightened on-treatment platelet reactivity status as well as some genetic predisposing variants were added to this long list of risk factors [28– 33]. However, despite these known risk factors, a substantial group of patients develop ST in the absence or paucity of these risk factors. This implies that the pathophysiological mechanisms underlying ST are incompletely unraveled. The pathophysiology of ST is generally considered as a complex culmination of several distinct, but often overlapping, candidate pathological pathways, including alterations involved in inflammation, thrombosis, lipoprotein metabolism, vascular remodeling, endothelial function and oxidative stress. However, the body of evidence that these pathways are indeed involved is limited and mostly anecdotical. State-of-the-art genetic research has the potential to lead to a more fundamental understanding of underlying pathophysiological mechanisms that contribute to ST and will hopefully unravel some missing pathways in the near future.
Early versus late coronary ST
Although early and (very) late ST share several common risk factors, the impact of these factors vary substantially. In particular, the predominance of mechanical and anatomic factors underlying early ST has been commonly recognized for decades. Furthermore, a very important nonmechanical cause of ST occurring within the first 6 months after the index PCI is premature cessation of clopidogrel therapy, which is associated with hazard ratios up to 90 [6,10–13].
However, ST can also occur with appropriate use of dual antiplatelet therapy. A recent novel observation is the strong relationship between a high on-treatment platelet reactivity status and the occurrence of early ST [28–32].
Late ST might be less strongly related to mechanical factors, although acquired malapposition has been reported. Moreover, the influence of clopidogrel cessation on late and very late ST is less clear. The exact pathophysiologic mechanisms underlying late ST are not completely understood, but the following morphologic substrates have been identified and associated with late and very late ST [34–40]:
▪▪ Incomplete and delayed re-endothelialization with persistent fibrin deposition due to the cytotoxic or cytostatic drugs used in DES;
▪▪ Sirolimus- and paclitaxel-induced expression of endothelial tissue factor;
▪▪Delayed-hypersensitivity reactions to the durable polymer of DES;
▪▪Diffuse in-stent restenosis with thrombosis;
▪▪ Late malapposition due to a chronic inflammatory process and vascular remodeling;
▪▪ Stent fracture;
▪▪ In-stent neoatherosclerosis with plaque rupture.
Although these observations might be biased by the fact that they are derived from anecdotal case reports and autopsy studies, recent studies using intravascular imaging technologies (such as intravascular ultrasound and optical coherence tomography studies) and histopathologic findings from thrombi obtained with aspiration catheters point in the same direction [40].
Importance of adequate concomitant antithrombotic therapy
The occurrence of ST after DES implantation has focused attention on the adequacy of the current dual antiplatelet regimen of aspirin and clopidogrel. Multiple antithrombotic strategies have been evaluated for their ability to minimize the incidence ST without increasing the risk for bleeding complications. At present, current American College of Cardiology/ American Heart Association and European Society of Cardiology guidelines recommend the use of dual-antiplatelet therapy (aspirin and a thienopyridine) for all patients undergoing coronary stenting, although the optimal duration of this therapy, in particular after DES implantation, remains unclear. In view of this, a recently published analysis did not demonstrate any significant benefit associated with prolonged continuation of dual antiplatelet therapy as compared with the guideline-recommended clopidogrel duration of 12 months, although its sample size had limited power to detect a possible difference [41]. The cumulative incidence of definite ST at 24 months after DES implantation was similar between the two groups (0.4 vs 0.4%; p = 0.76). Ongoing trials such as the Double Randomization of a Monitoring Adjusted Antiplatelet Treatment Versus a Common Antiplatelet Treatment for DES Implantation, and Interruption Versus Continuation of Double Antiplatelet Therapy (ARCTIC, NCT00827411), the Dual Anti- Platelet Therapy Trial (DAPT, NCT00977938) and the Safety and Efficacy of Six Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE, NCT00661206), are adequately powered to assess the optimal duration of dual-antiplatelet therapy after DES implantation.
It is also important to note that novel P2Y12 inhibitors with a stronger and more predictable pharmacodynamic response (such as prasugrel and ticagrelor) have been shown to reduce the incidence of early ST drastically as compared with clopidogrel [42,43].
High on-treatment platelet reactivity
In recent years, it has become clear that the standard ‘one-size-fits-all’ dosing regimen of dual-antiplatelet therapy is not adequate in a substantial group of patients. Consistent findings across multiple investigations have shown heterogeneity in individual patient responses to antiplatelet therapy resulting in hyper-responsiveness at one end of the spectrum with a subsequent increase in bleeding complications, and hyporesponsiveness at the other end of the spectrum with a subsequent increase in atherothrombotic events [44,45]. As of yet, multiple observational studies using various techniques, different platelet agonists and definitions have demonstrated the predictive value of platelet function testing prior to coronary stenting to identify individuals who exhibit a ‘high on-treatment platelet reactivity state’, and these individuals are at increased risk for coronary ST.
The mechanisms leading to a heightened on-treatment platelet reactivity status are not fully understood and are likely multifactorial. Accelerated platelet turnover, unpredictable active metabolite generation, up- and downregulation of the platelet-receptor pathways, genetic polymorphisms, increased baseline platelet reactivity, poor compliance and drug– drug interactions, are among the multiple factors that have shown a significant effect [46]. A recent consensus document reports that several platelet function tests including the Multiplate®; system (Dynabyte), the VerifyNow®; system (Accumetrics), the flowcytometric vasodilatorstimulated- phosphoprotein assay (Biocytex, Marseille, France) and ‘classical’ light transmittance aggregometry, are capable of identifying patients at risk [45]. Of even more importance, the results of these tests have shown a clear relationship with the occurrence of atherothrombotic events, including ST.
Thus, high on-treatment platelet reactivity has emerged as an important risk factor for ST, in particular early ST. The possible role of high on-treatment platelet reactivity in the pathophysiology of late and verly late ST needs to be explored in sufficiently powered studies.
Triggering mechanisms of coronary ST
Despite the long list of ‘chronic’ risk factors for ST, the mechanisms underlying the actual moment of onset of ST are less well-established. An individual’s susceptibility to coronary ST is complex with many interrelations among endothelium, the naked stent struts, the blood (rheology, platelet reactivity, clotting factors and inflammatory cells), the myocardium and various endogenous triggering processes that can change from minute to minute. Unlike the ‘chronic’ risk factors of ST, acute risk factors – or triggering factors – act within a short time frame and are often a consequence of a disturbance in the balance of the autonomic nervous system (sympathic versus parasympathic). A sympathic trigger pattern (as a result of exertion or emotional stress) is characterized by increased blood and pulse pressures, a higher vascular tone (that can promote vasoconstrictive forces) and a relative prothrombotic state (increased platelet aggregability and decreased fibrinolysis) [47].
Recognition of the circadian variation in acute atherothrombotic events with a peak incidence between 6:00 am and noon has set the stage for the whole concept of triggering factors. The physiological changes early in the day produce a surge of typical sympathetic triggering patterns and cause a well-documented excess of approximately 30% of morning myocardial infarctions [48].
In the last two decades, several triggering factors have been identified in the setting of myocardial infarction, including heavy physical activity, emotional stress, eating, exposure to severe weather conditions, sexual intercourse, coffee and alcohol consumption and cocaine or marijuana use, and it is now accepted that triggering factors precede nearly half of myocardial infarction onsets [49,50].
Given the similarities in several pathophysiological pathways between ST and myocardial infarction, it could be expected that at least some of the reported triggering mechanisms preceding myocardial infarction might also account for coronary ST.
Thus far, three observational studies have examined whether the onset of ST varies in a circadian manner and all studies confirm the similar pattern as seen in acute myocardial infarction: coronary ST occurs more often in the early morning hours between 06:00 am and noon [51–53].
Only one observational study has identified the triggering role of vigorous physical exercise, emotional stress and infection preceding coronary ST [54]. In this substudy of the Dutch Stent Thrombosis Registry, all patients who suffered a ST were intensively interviewed using standardized questionnaires about the conditions and activities in the time frame preceding the ST. Patients were asked whether they had performed any physical activity in the 2 h preceding the ST or had suffered a life event (emotional stress) in the 2 weeks preceding the ST. The degree of physical activity intensity was quantified by the Compendium of Physical Activities, a coding scheme that classifies physical activity by rate of energy expenditure. To objectify the impact of emotional stress, the Social Readjustment Rating Scale by Holmes and Rahe was used. To search for the presence of an infection, all medical records were checked and laboratory charts were screened for parameters indicative for an infection, including positive cultures, antibiotics use, C-reactive protein, blood sedimentation rate, leukocyte count and leukocyte differentiation.
surprisingly high percentage (vigorous physical exercise 5%; emotional stress 11%; infection 10.5%) of interviewed patients reported a trigger. Moreover, analysis of the categories of ST revealed a higher prevalence of triggers with an increasing time interval between index PCI and ST. Interestingly, the prevalence of the studied triggering mechanisms was the highest (42%) in the group of patients presenting with a very late ST.
Conclusion
Coronary ST is a serious and potential lifethreatening complication after coronary stenting. Therefore, every possible effort should be directed to identify those patients at highest risk for ST. Risk factors associated with ST can be categorized as clinical-, angiographic- and procedural-related. Moreover, noncompliance and nonresponsiveness to the prescribed dual antiplatelet therapy are factors that also play a major role. Understanding of these important risk factors may aid in better prevention.
Future perspective
At present, approximately 3 million PCI procedures per year are performed worldwide and it is expected that this number will be doubled by the year 2020.
Given these huge numbers of PCI-procedures, any reduction in complication rates will have a tremendous clinical impact, in particular when such a complication results in substantial morbidity, mortality and prolonged hospital stay, as is the case for coronary ST.
In the last few years, improvements in interventional techniques and adjuvant-combined antithrombotic therapy have further reduced the incidence of ST, and given the wealth in the pipelines of both the pharmaceutical and device companies, it is hoped and expected that the ST rate will decline further. However, despite these remarkable steps of progress, specific issues require study in greater detail. First, the role of triggering mechanisms preceding ST are insufficiently studied and great efforts should be directed to this novel field of research, preferably in parallel with information on platelet inhibition status, coagulation status and other ‘chronic’ risk factors. Second, no matter what level of evidence is required, it will be necessary to develop simple clinical algorithms to aid physicians in their interpretation and use of platelet function testing in the cathlab. The issue of personalized antiplatelet therapy on the basis of platelet function testing is important and worthy of effort and further study, and the cardiology community eagerly awaits the results of ongoing clinical trials.
Financial & competing interests disclosure
Jochem van Werkum reports speakers fees from Accumetrics and Siemens, The Medicines Company; Jurriën ten Berg reports speakers fees from Sanofi-Aventis, Eli Lilly, BMS and MSD, Sanof i-Aventis, Schering-Plough and GlaxoSmithKline. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪▪ Coronary stent thrombosis (ST) is the most dreadful complication after coronary stenting impact because it results in life-endangering conditions such as myocardial infarction (in approximately 80%) and cardiac death (in up to 40% of cases).
▪▪ Incidence varies between 1 and 5%, dependent on clinical presentation of the patient preceding stenting (the index percutaneous coronary interventions).
▪▪ ST is classified according to the elapsed time between index percutaneous coronary interventions, the occurrence of ST (acute/subacute/late and very late) and the level of evidence for its presence (definite, probable and possible).
▪▪ Risk factors associated with ST can be categorized as clinical-, angiographic- and procedural-related.
▪▪ Noncompliance to antiplatelet drugs is the strongest predictor.
▪▪ A high on-treatment platelet reactivity status is also associated with ST.
▪▪ The onset of ST varies in a circadian manner with peak incidence between 06:00 am and noon.
▪▪ Triggering mechanisms such as vigorous exercise, emotional stress and infection are likely to play a superimposing role.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Palmaz JC, Sibbitt RR, Reuter SR et al. Expandable intraluminal graft: a preliminary study: work in progress. Radiology 156(1), 73–77 (1985).
- Sigwart U, Puel J, Mirkovitch V et al. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N. Engl. J. Med. 316(12), 701–706 (1987).
- Morice MC, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346(23), 1773–1780 (2002).
- Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315–1323 (2003).
- Stone GW, Ellis SG, Cox DA et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- van Werkum JW, Heestermans AA, Zomer AC et al. Predictors of coronary stent thrombosis:the Dutch stent thrombosis registry. J. Am. Coll. Cardiol. 53(16), 1399–1409 (2009).
- Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation 115(11), 1440–1455 (2007).
- Wenaweser P, Rey C, Eberli FR et al. Stent thrombosis following bare-metal stent implantation: success of emergency percutaneous coronary intervention and predictors of adverse outcome. Eur. Heart J. 26(12), 1180–1187 (2005).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562), 667–678 (2007).
- Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293(17), 2126–2130 (2005).
- Kuchulakanti PK, Chu WW, Torguson R et al. Correlates and long-term outcomes ofangiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation 113(8), 1108–1113 (2006).
- Park DW, Park SW, Park KH et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am. J. Cardiol. 98(3), 352–356 (2006).
- de la Torre-Hernandez JM, Alfonso F, Hernandez F et al. Drug-eluting stent thrombosis: results from the multicenter Spanish registry ESTROFA (Estudio ESpanol sobre TROmbosis de stents FArmacoactivos).J. Am. Coll. Cardiol. 51(10), 986–990(2008).
- Roy P, Torguson R, Okabe T et al. Angiographic and procedural correlates of stent thrombosis after intracoronary implantation of drug-eluting stents. J. Interv. Cardiol. 20(5), 307–313 (2007).
- Uren NG, Schwarzacher SP, Metz JA et al. Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur. Heart J. 23(2), 124–132 (2002).
- Cheneau E, Leborgne L, Mintz GS et al. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation 108(1), 43–47 (2003).
- Cutlip DE, Windecker S, Mehran R et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115(17), 2344–2351 (2007).
- Heestermans AC, van Werkum JW, Zwart B et al. Acute and subacute stent thrombosis afterprimary PCI for ST-segment elevation myocardial infarction: incidence, predictors and clinical outcome. J. Thromb. Haemost. 8(11), 2385–2393 (2010).
- van Werkum JW, Heestermans AA, de Korte FI et al. Long-term clinical outcome after a first angiographically confirmed coronary stent thrombosis: an analysis of 431 cases. Circulation 119(6), 828–834 (2009).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4‑year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 52(14), 1134–1140 (2008).
- Roukoz H, Bavry AA, Sarkees ML et al. Comprehensive meta-analysis on drug-eluting stents versus bare-metal stents during extended follow-up. Am. J. Med. 122(6), 581.e1–581.e10 (2009).
- Lagerqvist B, Carlsson J, Frobert O et al. For the Swedish coronary angiography and angioplasty registry study group. Stent thrombosis in Sweden. Circ. Cardiovasc. Interv.2(5), 401–408 (2009).
- Kaltolft A, Jensen LO, Maeng M et al. 2-year clinical outcomes after implantation of sirolimus-eluting, paclitaxel-eluting, and bare-metal coronary stents: results from the WDHR (western Denmark heart registry). J. Am. Coll. Cardiol. 53(8), 658–664 (2009).
- de la Torre Hernández JM, Alfonso F, Gimeno F et al. ESTROFA-2 study group. Thrombosis of second-generation drug-eluting stents in real practice results from the multicenter Spanish registry ESTROFA-2 (Estudio Español Sobre Trombosis de Stents Farmacoactivos de Segunda Generacion-2). JACC Cardiovasc. Interv. 3(9), 911–919 (2010).
- Kedhi E, Joesoef KS, McFadden E et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet 375(9710), 201–209 (2010).
- Kandzari DE, Leon MB, Popma JJ et al. For the ENDEAVOR III investigators. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J. Am. Coll. Cardiol. 48(12), 2440–2447 (2006).
- Lemesle G, Sudre A, Modine T et al. High incidence of recurrent in stent thrombosis after successful treatment of a first in stent thrombosis. Catheter Cardiovasc. Interv. 72(4), 470–478 (2008).
- Geisler T, Zurn C, Simonenko R et al. Early but not late stent thrombosis is influenced by residual platelet aggregation in patients undergoing coronary interventions. Eur. Heart J. 31(1), 59–66 (2010).
- Bouman HJ, van Werkum JW, Breet NJ et al. Platelet reactivity in coronary stent thrombosis. J. Thromb. Haemost. 9(5), 909–916 (2011).
- Gurbel PA, Bliden KP, Samara W et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST study. J. Am. Coll. Cardiol. 46(10), 1827–1832 (2005).
- Sibbing D, Braun S, Morath T et al. Platelet reactivity after clopidogrel treatment assessed with point of-care analysis and early drug-eluting stent thrombosis. J. Am. Coll. Cardiol. 53(10), 849–856 (2009).
- Breet NJ, van Werkum JW, Bouman HJ et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 303(8), 754–762 (2010).
- Harmsze AM, van Werkum JW, ten Berg JM et al. CYP2C19*2 and CYP2C9*3 alleles areassociated with stent thrombosis-a case-control study. Eur. Heart J. 31(24), 3046–3053 (2010).
- Finn AV, Nakazawa G, Joner M et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler. Thromb. Vasc. Biol.27(7), 1500–1510 (2007).
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193–202 (2006).
- Steffel J, Latini RA, Akhmedov A et al. Rapamycin, but not FK-506, increases endothelial tissue factor expression: implications for drug-eluting stent design. Circulation 112(13), 2002–2011 (2005).
- Stahli BE, Camici GG, Steffel J et al. Paclitaxel enhances thrombin-induced endothelial tissue factor expression via c-Jun terminal NH2 kinase activation. Circ. Res. 99(2), 149–155 (2006).
- Virmani R, Guagliumi G, Farb A et al. Localized hypersensitivity and late coronary thrombosis: should we be cautious? Circulation 109(6), 701–705 (2004).
- Nakazawa G, Finn AV, Vorpahl M et al. Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J. Am. Coll. Cardiol. 57(4), 390–398 (2011).
- Cook S, Ladich E, Nakazawa G et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 120(5), 391–399 (2009).
- Seung-Jung P, Duk-Woo P, Young-Hak K et al. Duration of dual antiplatelet therapyafter implantation of drug-eluting stents. N. Engl. J. Med. 362(15), 1374–1382(2010).
- Wiviott SD, Braunwald E, McCabe CH et al. For the TRITON-TIMI 38investigators. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 371(9621), 1353–1363 (2008).
- Cannon CP, Harrington RA, James S et al. For the PLATelet inhibition and patient outcomes investigators. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet 375(9711), 283–293 (2010).
- van Werkum JW, Heestermans AA, Deneer VH et al. Clopidogrel resistance: facts and fiction. Fut. Cardiol. 2(2), 215–228 (2006).
- Bonello L, Tantry US, Marcucci R et al. Working group on high on-treatment platelet reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 56(12), 919–933 (2010).
- Elsenberg EH, van Werkum JW, van de Wal RM et al. The influence of clinical characteristics, laboratory and inflammatory markers on ‘high on-treatment platelet reactivity’ as measured with different platelet function tests. Thromb. Haemost. 102(4), 719–727 (2009).
- Muller JE, Kaufmann PG, Luepker RV et al. For the mechanisms precipitating acute cardiac events participants. Mechanisms precipitating acute cardiac events participants. Review and recommendations of an NHLBI workshop. Circulation 96(9), 3233–3239 (1997).
- Cohen MC, Rohtla KM, Lavery CE et al. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am. J. Cardiol. 79(11), 1512–1516 (1997).
- Gabbay FH, Krantz DS, Kop WJ et al. Triggers of myocardial ischemia during daily life in patients with coronary artery disease: physical and mental activities, anger and smoking. J. Am. Coll. Cardiol. 27(3), 585–592 (1996).
- Culic V, Eterovic D, Miric D. Meta-analysis of possible external triggers of acute myocardial infarction. Int. J. Cardiol. 99(1), 1–8 (2005).
- Zwart B, van Werkum JW, Heestermans AC et al. Triggering mechanisms of stentthrombosis. Eurointervention 6(6), 722–728 (2011).
- Mahmoud KD, Lennon RJ, Ting HH et al. Circadian variation in coronary stent thrombosis. JACC Cardiovasc. Interv. 4(2), 183–190 (2011).
- Tamura A, Watanabe T, Nagase K et al. Circadian variation in symptomatic subacute stent thrombosis after bare metal coronary stent implantation. Am. J. Cardiol. 97(2), 195–197 (2006).
- Zwart B, Van Kerkvoorde TC, van Werkum JW et al. Vigorous exercise as a triggering mechanism for late stent thrombosis: a description of three cases. Platelet 21(1), 2–6 (2010).
- Airoldi F, Colombo A, Morici N et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 14 116(7), 745–754 (2007).