Review Article - Interventional Cardiology (2011) Volume 3, Issue 1
Covered-stent implantation in coarctation of the aorta: indications, materials, techniques and outcomes
- Corresponding Author:
- Carlos AC Pedra
Catheterization Laboratory for Congenital Heart Disease
Instituto Dante Pazzanese de Cardiologia
Av. Dr. Dante Pazzanese, 500. 04012-180 São Paulo, SP Brazil
Tel: +55 115 085 6114
Fax: +55 115 085 6196
E-mail: cacpedra@uol.com.br
Abstract
Keywords
coarctation of the aorta, congenital heart disease, covered-stent implantation, interventional cardiology
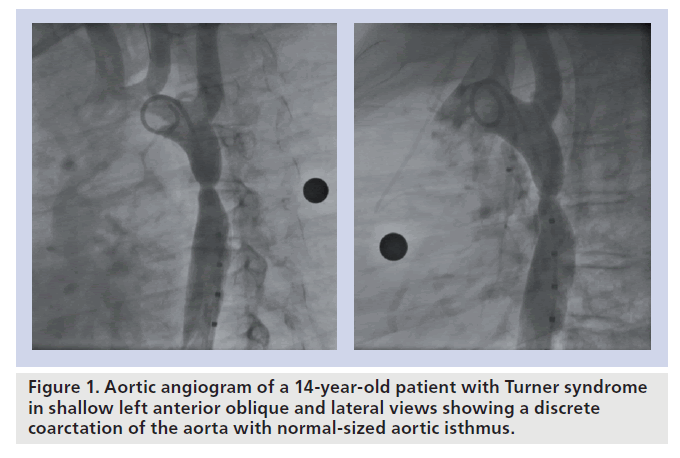
Coarctation of the aorta (CoA) accounts for 6–8% of live births with congenital heart disease [1]. In those patients presenting after infancy, the lesion is typically a discrete and eccentric narrowing of the proximal descending aorta located near the aortic end of the arterial duct or ligament (Figure 1). Aortic tortuosity and variable degrees of transverse aortic arch (TAA) or isthmic hypoplasia can occasionally be present. The obstruction may also persist or recur following successful surgical repair or balloon dilation (ReCoA). If left untreated, CoA has a poor prognosis, with most patients suffering from significant morbidity or even dying before the age of 40 years owing to heart failure, endocarditis, cerebral vascular accidents or premature coronary artery disease [2].
Surgical repair of CoA, first performed by Crafoord and Nylin in 1944 [3], was once the initial treatment option for these patients. Currently, it is still indicated as the initial intervention during the newborn period and infancy. The various repair techniques include excision of the obstruction and end-to-end re-anastomosis, patch repair or insertion of a tube bypass graft when the anatomy is more complex. However, in older patients, surgery can be challenging and complications include spinal cord damage, excessive bleeding, paradoxical hypertension, pleural effusion and infection [4].
First employed by Singer et al. in 1982 [5], balloon dilation was the earliest form of nonsurgical intervention for CoA. Despite the time period that has passed, this technique is still considered controversial for native CoA owing to histological data showing that successful relief of stenosis is accompanied by uncontrolled damage to the aortic intima and media, leading to a relatively high incidence of aneurysm formation of 2–20% [6,7]. In adult patients, the aorta in close proximity to the coarctation may be markedly tortuous or thinned and cystic medial necrosis [8] and calcification may be present. Accordingly, these factors predispose adult patients to dissection, aneurysm formation or even rupture.
Bare-metal stent implantation in CoA has been increasingly used to treat patients who would previously have been treated with surgery or balloon angioplasty. This treatment modality was first used in 1991 [9] and has become the procedure of choice in many institutions. However, after initial encouraging results, complications have been encountered such as aneurysm formation, stent malposition, stent fracture and vascular complications [10]. The rationale for the use of stents is based on the fact that overdilation, dissection and elastic recoil of the aorta are avoided using this strategy [11]. Furthermore, tacking of the intimal flaps to the aortic wall after tearing of the intima and media allows healing to occur. Moreover, the stent may reinforce weakened areas within the aortic wall and provide a framework for neointima formation over the tear, offsetting a predisposition to aneurysm formation. In conclusion, stenting may result in less aortic vascular injury than balloon angioplasty alone [12].
A covered stent was first utilized in 1999 to treat a young man presenting with coexistent CoA and a localized aneurysm [13]. Since then, the use of covered stents for the treatment of CoA has steadily grown, but the literature is often limited to small series and case reports [13–19]. Since balloon dilation and bare-metal stent implantation for CoA carry the risk of aneurysm formation or rupture, covered-stent implantation may be the preferred strategy in specific situations to reduce the incidence of aortic wall injury. Indications for the use of covered stents to treat CoA are discussed later.
Indications for CoA stenting
For patients with either native or recurrent CoA, it is generally accepted that relief of the obstruction should be undertaken in the clinical setting of an arm–leg gradient greater than 20 mmHg [10,11,20,21]. However, some have suggested that patients with milder obstruction and borderline systolic gradients may benefit from stent implantation, decreasing high left ventricular filling pressures and preserving systolic and diastolic left ventricular function in the long term [22,23]. It is also accepted that a mild obstruction should be relieved when associated with hypertension at rest, abnormal blood pressure response during exercise, progressive left ventricular hypertrophy or unequivocal systolic or diastolic dysfunction [20,22,23].
Stenting is indicated in cases of long tubular narrowings or associated hypoplasia of the aortic isthmus in which balloon angioplasty alone has yielded sub-optimal outcomes [10,11,21–27]. It has been demonstrated that patients with complex obstructions within the aortic arch may also benefit from stenting [28,29]. These are difficult patients to manage because the surgical repair involves an extended arch anastomosis, which frequently requires cardiopulmonary bypass and circulatory arrest with their attendant risks.
In cases of more straightforward anatomy, stenting reduces the gradient at the coarctation site more effectively than balloon dilation alone [24]. Therefore, in the adult patient, stenting is gradually being considered as the treatment of choice in any variant of CoA. In small children, aortic stenting is limited by the size of the current delivery systems and the need for redilation to match somatic growth. However, recently, with the appearance of new technologies including lower-profile balloons and stents, there has been a trend to also treat the younger patient with CoA by primary stenting [18]. It has been our policy to offer stenting to patients over a weight of 20 kg. In addition, our current policy for implanting a covered stent in patients with CoA includes:
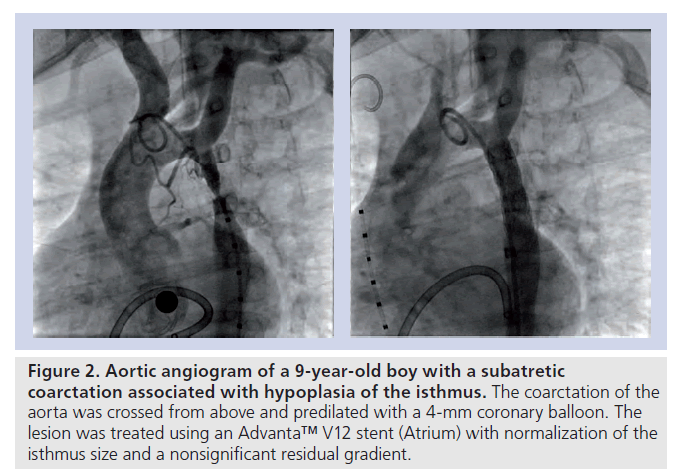
▪ Critical or subatretic obstructions, defined by a minimum diameter at the CoA site of 2–3 mm or less on angiography; CoA associated with atresia of the aortic lumen (‘blind’ or atretic CoA) (Figure 2);
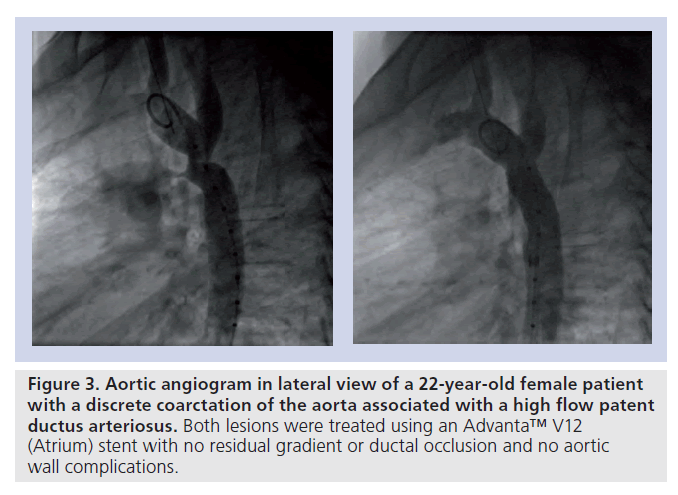
▪ CoA associated with a patent ductus arteriosus (Figure 3);
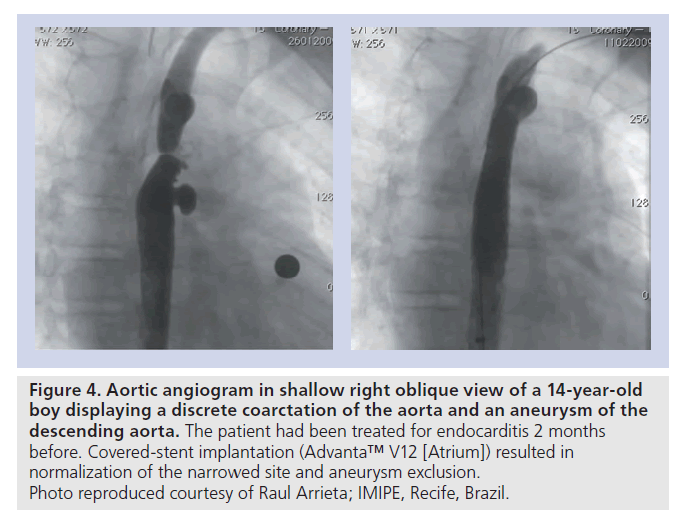
▪ CoA associated with adjacent aneurysms or degenerative changes of the aortic wall, suggested by the presence of an aneurysmal ascending aorta or TAA and/or significant aortic tortuosity (Figure 4);
▪ CoA in patients over 30 years of age (Figures 5 & 6);
▪ CoA in Turner’s patients (Figure 1) [30];
▪ Aneurysm formation, dissection or rupture of the aorta following standard balloon dilation or stent implantation, detected either acutely (as a ‘bail-out’ procedure) or at follow-up (Figure 7);
▪ Presence of circumferential fractures within a previously implanted stent in the aorta with malalignment between the proximal and distal ends and/or protrusion of the stent struts into the aortic wall detected at a repeat angiography or MRI/multislice spiral computed tomography (CT) during follow-up.
Figure 2. Aortic angiogram of a 9-year-old boy with a subatretic coarctation associated with hypoplasia of the isthmus. The coarctation of the aorta was crossed from above and predilated with a 4‑mm coronary balloon. The lesion was treated using an Advanta™ V12 stent (Atrium) with normalization of the isthmus size and a nonsignificant residual gradient.
Figure 3. Aortic angiogram in lateral view of a 22-year-old female patient with a discrete coarctation of the aorta associated with a high flow patent ductus arteriosus. Both lesions were treated using an Advanta™ V12 (Atrium) stent with no residual gradient or ductal occlusion and no aortic wall complications.
Figure 4. Aortic angiogram in shallow right oblique view of a 14-year-old boy displaying a discrete coarctation of the aorta and an aneurysm of the descending aorta. The patient had been treated for endocarditis 2 months before. Covered-stent implantation (Advanta™ V12 [Atrium]) resulted in normalization of the narrowed site and aneurysm exclusion. Photo reproduced courtesy of Raul Arrieta; IMIPE, Recife, Brazil.
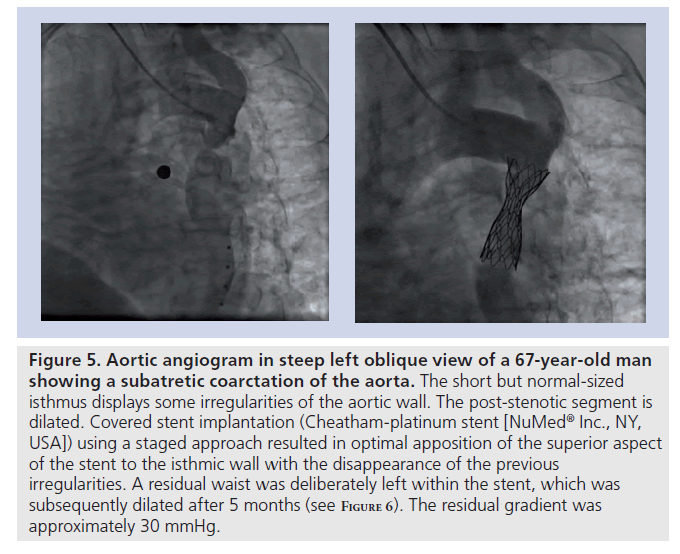
Figure 5. Aortic angiogram in steep left oblique view of a 67-year-old man showing a subatretic coarctation of the aorta. The short but normal-sized isthmus displays some irregularities of the aortic wall. The post-stenotic segment is dilated. Covered stent implantation (Cheatham-platinum stent [NuMedR Inc., NY, USA]) using a staged approach resulted in optimal apposition of the superior aspect of the stent to the isthmic wall with the disappearance of the previous irregularities. A residual waist was deliberately left within the stent, which was subsequently dilated after 5 months (see F igure 6). The residual gradient was approximately 30 mmHg.
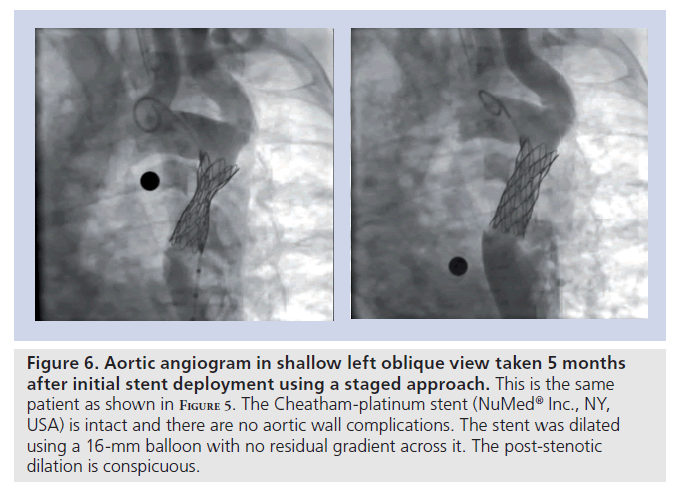
Figure 6. Aortic angiogram in shallow left oblique view taken 5 months after initial stent deployment using a staged approach. This is the same patient as shown in F igure 5. The Cheatham-platinum stent (NuMedR Inc., NY, USA) is intact and there are no aortic wall complications. The stent was dilated using a 16‑mm balloon with no residual gradient across it. The post-stenotic dilation is conspicuous.
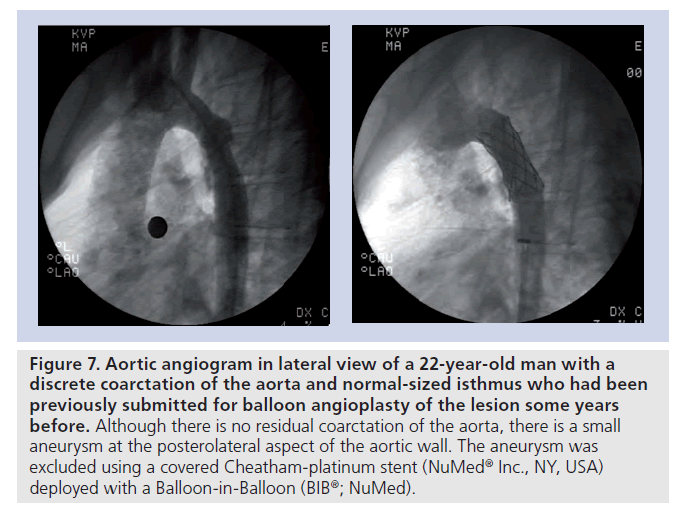
Figure 7. Aortic angiogram in lateral view of a 22-year-old man with a discrete coarctation of the aorta and normal-sized isthmus who had been previously submitted for balloon angioplasty of the lesion some years before. Although there is no residual coarctation of the aorta, there is a small aneurysm at the posterolateral aspect of the aortic wall. The aneurysm was excluded using a covered Cheatham-platinum stent (NuMedR Inc., NY, USA) deployed with a Balloon-in-Balloon (BIBR; NuMed).
It is worth mentioning that all patients with CoA should undergo an integrated imaging assessment of the aortic arch and the ascending and descending aorta before and after coarctation stenting. The use of CT or MRI along with angiography plays a key role in assessing this aortic arch anomaly. It is mandatory to obtain a detailed description of the lesion in order to plan an intervention using a stent, as well as to evaluate the arch and the implanted stent for position, integrity and aortic wall complications during follow-up [31–34].
Balloons & covered stents
Standard single-balloon catheters or the ‘Balloon-in-Balloon’ (BIB®; NuMed® Inc., NY, USA) may be used to deploy the stent according to operator preference and patient characteristics [11]. Single large-diameter balloons tend to first expand at their ends, which may predispose to balloon rupture or cause injury to the adjacent vessel wall resulting in the development of aneurysm or aortic dissection [11,16]. The development of the BIB catheter has much improved the delivery of large-diameter stents in the aorta, especially in the transverse arch (Figure 8) [29]. This catheter has an inner balloon and a 1-cm longer outer balloon that is twice the diameter of the inner balloon and available in outer-balloon sizes of 8–24 mm. It offers the important advantage of opening the stent more uniformly along its length, resulting in more control over its precise placement and preventing stent flaring and migration [11]. However, lower-profile single-balloon catheters can be preferable in smaller patients to reduce risk of damage to the femoral artery at the access site [16]. On occasion, higher-pressure balloons such as Mullins (NuMed), Atlas® (Bard, NJ, USA) or Conquest™ (Bard) may be needed to open up a residual waist, especially for postsurgical CoA [20]. Generally, a balloon that is slightly longer (0.5–1.0 cm) than the stent is used for implantation.
Currently, the most used covered stents are:
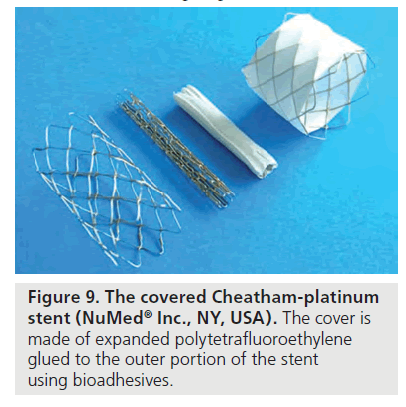
▪ The Cheatham-platinum balloon expandable stent (CP stent) [11], covered with an ultrathin stretchable expanded polytetrafluoroethylene (ePTFE) membrane applied to the stent using biodegradable adhesive (NuMed) (Figure 9). This stent is composed of heat-tempered 90% platinum and 10% iridium metal alloy, with the metal wire arranged in a ‘zig’ pattern [10,11,16]. Stents with eight ‘zigs’ enable expansion up to 25 mm and those 34–45 mm in length usually provide appropriate covering for the CoA lesion. They are MRI compatible and highly radiopaque on fluoroscopy. The CP stents display less foreshortening than the bare-metal Palmaz (Johnson & Johnson Interventional Systems, NJ, USA) after expansion, are slightly more flexible and have rounded edges. They have excellent radial strength and optimal resistance to fatigue, although the occurrence of localized or circumferential fractures has been described in their first version. Refinements in the welding process using gold have been employed by the manufacturer to solve this problem [17]. A premounted version on a BIB has recently been introduced (Figure 10).
Figure 10. The premounted Cheathamplatinum stent (NuMed ® Inc., NY, USA). At the bottom of the picture, a bare Cheathamplatinum stent premounted over a Balloon-in- Balloon (BIBR; NuMedR Inc., NY, USA) is displayed. The gold weldings on the stent are easily seen. At the top of the picture, an opened covered Cheatham-platinum stent (NuMed) is seen over the BIB balloon.
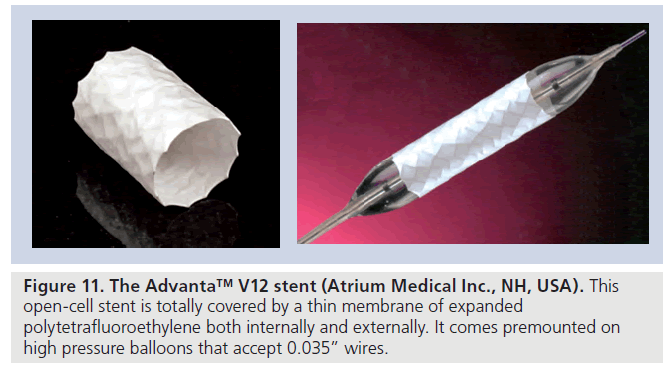
▪ The Advanta™ V12 LD stent (Atrium Medical Inc., NH, USA) [18] is a balloon-expandable laser-cut 316L stainless steel open-cell stent with a covering made of expanded PTFE on the interior and exterior aspects (Figure 11). It is available in three lengths (29, 41 and 61 mm) and premounted on balloons of 12-, 14- and 16‑mm diameters. The stent can be dilated up to a maximal diameter of 22 mm. To avoid tears and/or ruptures of the ePTFE membranes, subsequent dilation is not recommended after initial deployment of more than 4 mm at the same setting.
A variety of self-expanding stents have also been employed in the interventional management of CoA [13–15,19], mainly to treat aortic wall complications, such as dissections and aneurysm formation, either acutely or during follow-up. The primary use of such implants to dilate a native or a recurrent lesion is limited owing to its lack of radial strength. Therefore, their characteristics will not be reviewed herein.
Finally, in some countries such as the USA where the availability of covered stents is limited, reports on self-fabricated covered stents utilized in high-risk patients or acute aortic wall complications have been presented [35].
Technique
The technique used to implant bare and covered stents varies among different institutions and with different materials [9–30,35–44]. The most important steps are described below. The procedure is generally performed under general anesthesia since it is painful, and because patient movement at the time of stent deployment is hazardous [36]. After obtaining femoral arterial access, a second vascular access may occasionally be required for serial angiograms in order to assist proper positioning of the stent during deployment. This may be achieved transseptally through a femoral vein or using a radial or brachial artery. A second access is indicated for more complex cases such as TAA stenting, in subatretic CoA and in aneurysmal aortas [10]. Venous access may also be required for the use of a temporary pacemaker during stent implantation. This is of importance when stenting the aortic arch or when significant aortic regurgitation is present [20]. Rapid right ventricular pacing reduces the pulse pressure in a controlled manner during stent deployment, facilitating precise positioning. After vascular access is obtained, heparin sulfate (100–150 IU/kg; max: 10,000 IU in total) is administered intravenously and the activated clotting time is usually maintained for more than 200 s.
The lesion is crossed from the descending aorta with the aid of an end-hole catheter and a hydrophilic guide wire, and after routine left heart catheterization is carried out, aortic angiograms are obtained in anteroposterior and lateral views or using shallow left anterior oblique, left lateral and shallow right anterior oblique projections with caudal angulation. Measurements of the diameters of the transverse arch, aortic isthmus, post-stenotic dilation and descending aorta at the level of the diaphragm are obtained with correction for magnification using marker catheters, grids or spheres. Occasionally, a subatretic lesion can only be crossed from above (Figure 2), requiring snaring maneuvers for catheter progression coming from below. Likewise, in patients with atretic CoA, the very discrete atretic segment may be perforated from above using a stiff or the stiff end of a coronary wire or a radiofrequency catheter [15].
Prior to intervention, a long, stiff wire with a soft tip, such as a 260-cm 0.035” Rosen wire or Amplatz Super-Stiff wire (AGA Medical Corp., MN, USA) is parked in the ascending aorta or right or left subclavian artery depending on the straightest wire course and angulation of the lesion. The length of the selected stent should cover the entire lesion, which can extend from the head vessels (origin of the left subclavian artery or the left common carotid artery if the status is postsubclavian flap repair) to approximately 15 mm beyond the site of the CoA [10,20] into the area of the post-stenotic dilation. Segmental lesions may need longer or overlapping stents (Figure 4). The diameter of the balloon is chosen to equal that of the proximal isthmus, usually at the level of the take-off of the left subclavian artery or of the distal transverse arch [10,20]. Occasionally, the balloon is a little larger than these measurements depending on the adjacent anatomy.
If the selected covered stent is not premounted (Figures 9 & 10), it is manually crimped onto the catheter balloon. A piece of umbilical tape looped around the stent and pulled at both ends to help tighten the stent onto the balloon may be used, especially when crimping the higher-profile covered CP stent. Some operators slightly inflate the balloon, so that it grips the stent a bit more tightly and some coat the balloon with a little contrast medium, to help the stent stick to the balloon (‘Mullins glue’) [16]. During the crimping maneuver, the guide wire should be left in the catheter-balloon shaft in order to straighten the system, avoid damage to the balloon and crushing of the shaft. Manual crimping of the covered CP stent onto the catheter balloon and loading it into the long sheath requires extra care [10]. Wetting the ePTFE layer should be avoided at all times to ensure it keeps its original shape around the stent, preventing unfolding and damage. A cut-off short sheath (with the same profile as the long sheath) is used to protect the covered CP stent while advancing it through the hemostatic valve and to prevent it billowing out.
Although the use of the BIB has facilitated precise stent deployment, some maneuvers have been employed to reduce cardiac output for precise stent positioning. In the past, adenosine or b-blockers were used to produce transient asystole during balloon inflation, but more recently, rapid right ventricular pacing has been used with good effect [20]. The rate of pacing varies between 180 and 220 beats per minute. The exact rate can be determined from the outset by monitoring the pressure in the ascending aorta while pacing the heart at different rates. The rate for pacing, which reduces the pulse pressure in the aorta, is used. Rapid pacing is strongly recommended for TAA stenting [20,29].
Predilation of the lesion is controversial and was associated with a higher incidence of aortic wall abnormalities at follow-up in a recent multicenter trial [34]. However, in the presence of a critical lesion, the CoA site may be predilated using small- to moderate-diameter balloons (6–8 mm), just to make enough room for the passage of the long sheath [10]. To theoretically reduce the risk of catastrophic vascular complications, the prospective US protocol for CoA stenting (COAST) included a test to assess the compliance of the coarctation lesion, as described by some groups [23]. A predilation is performed using a compliant balloon dilated up to 80% of the final diameter of the stent (or 2 mm smaller) at low pressures (<4 atm). This maneuver is intended as a diagnostic measure, not as an angioplasty prior to stent placement. The disappearance of the waist on the balloon diagnoses a compliant lesion, which is in turn amenable to stenting. If there is a significant waist remaining on the balloon, a slightly smaller balloon is chosen and complete expansion of the stent is postponed until a second catheterization approximately 6 months later. In our viewpoint, although postsurgical lesions can be noncompliant, almost all native CoA (except those that are part of the middle aortic syndrome) are very compliant and do not need to be routinely pre-dilated.
The most popular sheath for stent delivery is the straight 75-cm long Cook RB-Mullins design sheath (Cook Cardiology, IN, USA), which has a radiopaque tip and a sidearm fitting for contrast injections and pressure measurements. The sheath should be left in the artery for as little time as possible. The ultimate profile of the long sheath required for implantation depends on the profile of the catheter balloon, generally being 1–2 Fr larger than it. When using a covered CP stent, the sheath should be 1–2 Fr larger than that needed for implantation of a bare stent [20].
The balloon-stent assembly is back-loaded into the sheath and advanced to its tip. The sheath is slowly retracted, exposing the stent in the vascular lumen. Repeat angiograms via the side arm of the long sheath during retraction or via the second angiographic catheter positioned in the arch are performed to ensure proper stent positioning prior to balloon inflation. When using the BIB, the inner balloon is inflated first and the stent is repositioned if needed before inflation of the outer balloon [11]. Most patients require only one or two dilations for stent deployment. When using a BIB, it is recommended that a second inflation of the outer balloon is performed while keeping the inner balloon deflated. In this way, the vector forces are better distributed outwards to the aortic wall. Although some have advocated flaring at the ends of the stent to optimize endothelialization [25], it is unlikely that complete stent apposition in the poststenotic region can be achieved and it may be detrimental (Figure 6). If the main goal of stenting in CoA is gradient relief, this is attained regardless of complete apposition of the stent in the poststenotic area, and since the stent is covered, the direction of flow is directed towards the lumen and not the aortic wall, protecting the wall, allowing the dilation to possibly regress [40–43]. The formation of clots in this area has not been reported or found in our experience. A staged approach with serial stent dilations to fully achieve the desired diameter may be advisable in higher-risk patients (Figure 5 & 6) [15,23,40].
We (Elchanan Bruckheimer) have described methods for covered CP stent implantation using the lowest-profile system possible by anchoring the stent in the coarctation with a high-pressure balloon (8–10 mmHg) and then carefully removing this balloon and further dilating the stent through the same sheath with a MaxiLD balloon (Cordis, NJ, USA). We have managed to implant and dilate stents up to 16 mm on a regular basis using a 9‑Fr Avanti 55‑cm sheath (Cordis) [44]. The Advanta V12 LD stent can be implanted on a 12‑mm balloon through an 8‑Fr sheath (manufacturer’s recommendation is 9 Fr) and the 14‑ and 16‑mm delivery systems need an 11‑Fr sheath [18]. The methods for implantation are similar to those described previously.
After stent deployment, care should be taken so as not to dislodge the stent. Once the balloon is deflated, the sheath is gently advanced over it followed by balloon withdrawal. Excessive catheter and wire manipulation over the recently stented area should be avoided. Repeat pressure measurements and aortic angiograms are performed immediately after stent implantation. Cephazolin (20 mg/kg; maximum: 2 g) is administered during the procedure and at 8-h intervals (total: three doses). Heparin sulfate is partially neutralized using protamine if the activated coagulation time is greater than 200 s. A Perclose (Abbott Vascular®, IL, USA) suture has been commonly employed and inserted in the femoral artery prior to the introduction of the large sheath. This helps to achieve fast hemostasis at the end of the procedure and prevent hemorrhagic complications at the puncture site [20]. Occasionally, two sutures are used to repair the artery, especially when a large (14 Fr) sheath is to be used. However, this may be too costly in some countries and reliable hemostasis may well be achieved solely by judicious manual compression [10,15,24]. Patients are awakened in the catheterization laboratory and transferred to the recovery room for routine clinical observation. They are usually discharged home the following day on aspirin (2–5 mg/kg/day; maximum: 300 mg) and instructed to avoid contact sports for 6 months. A chest radiograph, a 12-lead electrocardiogram and a transthoracic echocardiogram are obtained before discharge and scheduled after 1–3 months, 6 months, 12 months and yearly thereafter in conjunction with clinical visits.
Owing to the possibility of late aneurysm formation after CoA stenting, close follow-up using imaging techniques is mandatory in all patients [31–34]. Therefore, a repeat catheterization, MRI or CT should be scheduled 6–12 months after the procedure and probably at fixed periods during the patient’s lifetime especially before and after pregnancy. Elective recatheterization is performed after 6–12 months in order to carry out stent redilation when there is residual stenosis as a result of the stent having been intentionally underinflated initially (Figures 5 & 6).
Outcomes
The initial use of covered stents was limited to treat complications encountered after balloon angioplasty due to aortic wall injury such us aneurysm formation and wall dissections [13,14,37–39]. Subsequently, Ewert et al. from Berlin, Germany, reported their successful experience in treating long, extreme subatretic coarctations in four adult patients by the implantation of covered stents 39–50 mm long [40]. During follow-up, the stents were redilated and one stent fractured requiring the implantation of a second stent. In addition, in 2004 Qureshi et al. from the UK, reported the implantation of covered CP stents in patients with native CoA or ReCoA ranging from 12 to 19 years [17]. The gradients were significantly reduced, the diameters increased and no complications were encountered.
A larger experience was published by Ewert et al. in 2005 with the use of the CP stent in a broad spectrum of heart malformations [41]. Among 60 stents implanted, 37 were indicated for CoA and ReCoA and 11 of these patients successfully received 12 covered stents. The indications included subatretic or severe lesions, aneurysm formation and previously implanted stent fracture. All stents were placed in the target lesion without complications. During follow-up, one covered stent fractured at 6 months, requiring the implantation of an additional covered stent.
In 2005, our group (Carlos Pedra) in Sao Paulo, Brazil [15], reported nine patients who received ten covered stents (ten CP stents and two self-expandable Braile stent grafts). Indications included severe or atretic CoA, aneurysm formation, coexistent patent arterial duct and circumferential fracture of a previously implanted stent. Median age and weight were 31 years and 65 kg, respectively. The procedure was successful in all subjects. However, we found the development of aneurysms in two patients despite the use of covered stents. One patient had a small aneurysm requiring conservative management and the other was successfully treated using a second covered custom-made self-expandable Braile stent. During follow-up, all patients had either reduced the dose or suspended the use of antihypertensive drugs.
Tzifa et al. published their experience with the implantation of 33 covered CP stents in 30 patients as a rescue for CoA aneurysms and previous stent-related complications, and in patients at risk of developing complications because of complex CoA anatomy or advanced age [42]. Mean age and weight were 28 years (range: 8–65 years) and 62 kg (range: 28–86 kg), respectively. They obtained both a significant reduction in systolic gradients across the CoA and an improvement in the diameter of the CoA site. During long-term follow-up, all stents were visualized either with CT or MRI and showed adequate patency and correct position. In 43% of the patients, antihypertensive medication was either decreased or stopped.
A multicenter experience from Italy was published by Butera et al. which included 33 patients with a median age of 13 years (range: 6–66 years) and complex aortic coarctations who received covered CP stents [43]. The gradient across the stenosis decreased significantly and the vessel diameter increased accordingly. All stents were correctly positioned and there were no complications. On follow-up angiography, if performed, the aneurysms were completely excluded. During follow-up, one patient developed intrastent restenosis due to a significant endothelial proliferation that was successfully redilated using a high-pressure balloon.
Kenny et al. reported in 2008 their experience implanting 38 covered stents of which three were self-expanding stents, in 37 patients [19]. Median age and weight were 29.6 years (range: 9–65 years) and 71.5 kg (range: 35–95 kg), respectively. Indications included native coarctation, recurrent coarctation following surgical treatment, aneurysm formation, aorto-bronchial fistula, previous stent fracture and CoA associated with an arterial duct. Gradients across the stenosis and diameters improved significantly after the interventions. There were no deaths and one patient developed aortic rupture requiring emergency surgery. In conclusion, the authors stated that self-expanding stent grafts may be preferable to balloon expandable stents when there is aneurysm formation in the setting of aortopathy.
We (Elchanan Bruckheimer) reported the experience at the Schneider Children’s Medical Center in Petach Tikva, Israel, 2 years ago [44], using a covered CP stent in native CoA and subsequent serial redilations. Initially, we implanted covered stents on balloons of a diameter sufficient to anchor the stent in the coarctation site, using the smallest delivery system possible. Dilation with larger diameter balloons was performed until the pressure gradient was less than 20 mmHg and the stent was opposed to the aortic wall. A total of 22 patients with native coarctation were included. The CoA diameter increased and the pressure gradient decreased significantly. Nine patients underwent further dilation at an average of 5 months later. Complications included a small tear at further dilation, which was treated with a second stent and a femoral pseudoaneurysm. At short-term follow-up, all patients were alive and well with no evidence of recoarctation or aneurysm.
Recently, we (Elchanan Bruckheimer and Carlos Pedra) have reported the acute results treating 25 patients with CoA using the large-diameter covered Advanta V12 premounted stent [18]. The stents were placed through 8–11 Fr delivery systems and the technique included an initial inflation of balloons to a diameter sufficient to anchor the stent in the coarctation site using the smallest available delivery system, followed by a secondary dilation using larger diameter balloons until the pressure gradient was less than 20 mmHg and the stent was opposed to the aortic wall. The CoA diameter increased and the peak pressure gradient between the ascending and descending aorta decreased, both reaching statistical significance. In all cases, the stent reached the initial planned diameter and there were no complications. Median follow-up was 4.9 months and the patients had neither symptoms nor evidence of ReCoA.
It has been documented that the risk of aortic rupture after implanting covered stents is lower compared with balloon angioplasty or a baremetal stent alone, but this risk is not completely eliminated [33]. Aortic rupture after covered-stent implantation was reported some years ago [45].
▪ Redilation
Covered stents can be re-expanded in a similar ways as the redilation of bare-metal stents [46]. Ewert and colleagues reported covered-stent redilation 6 months after initial under-dilation and residual gradients were abolished [40]. In one of the four patients involved, a stent fractured and the implantation of an additional covered stent was required. Furthermore, Butera et al. also demonstrated the feasibility of re-dilating covered stents in seven children at a mean of 20 months after initial implantation [47]. In this series, no complications were encountered. Finally, we (Elchanan Bruckheimer) have also reported the feasibility and safety of covered stent redilation in nine children with an average of 5 months post initial implantation [44].
▪ Crossing head & neck vessels
It is obviously important to avoid covering the origins of the carotid arteries with a covered stent. Covering the origin of a subclavian artery may be of concern although sometimes it cannot be avoided, especially if there is an adjacent aneurysm. In these cases, an intact vertebral-basilar circulation should be documented [11,48]. Old surgical data from patients in whom the subclavian artery was used as a flap, and thus sacrificed, and recent experience with stent graft implantation for distal aortic disease, which invariably involves covering the origin of the left subclavian artery, suggest that these complications are rare [20]. However, if it is felt that restoration of flow to an excluded left subclavian artery is mandatory, it is possible to create a pathway through the stent struts of an implanted covered stent. Tsai et al. have reported an elegant technique to puncture the ePTFE membrane of a NuMed stent that was used to treat an aortic aneurysm. After the membrane was perforated, the stent cell was dilated to re-establish flow to the excluded left subclavian artery [49].
▪ Spinal cord injury
The use of covered stents may carry the risk of spinal cord injury owing to the possibility of occlusion of spinal arteries or the artery of Adamkiewicz. Generally, these arise well below the level of coarctation, usually at the level of T9–12 vertebrae. If the coarctation is located in the thoracic aorta at a lower level than usual, it is recommended to portray beforehand the origin and the distribution of these arteries, in order to determine the lowest thoracic level at which it is safe to use a covered stent.
Recent data from patients suffering from spinal cord injury after thoracic endovascular aneurysm repair, suggest that covering the origin of the spinal arteries is crucial in the development of paraplegia [50].
Conclusion
Covered stent implantation for native or recurrent CoA is accepted as an attractive alternative to surgical therapy, balloon angioplasty and bare-metal stent insertion in older children, adolescents and adults. Indications are mainly related to preventing aortic wall complications and the use of these stents is increasing. Indeed, in adults, covered stents may be considered the treatment of choice for native CoA. Although the implantation technique can be challenging and demanding, its use is relatively safe and extremely effective as a treatment strategy. During follow-up, reinterventions including redilations and additional stent placement may be required.
Future perspective
Current data, although limited by nonrandomized study design, small sample size and short-term follow-up, have shown the procedural and mid-term safety and effectiveness of covered- stent implantation for CoA. Future directions in stent development should include equipment refinement and stent design to decrease the profile of the devices preventing injuries at the vascular access site, expandable membranes for adequate redilation over time, the development of recapturable and repositionable devices as well as the capability of the stent to adapt to somatic growth (‘growth stent’).
Financial & competing interests disclosure
Carlos Pedra and Elchanan Bruckeimer receive a clinical research grant from Atrium. Elchanan Bruckeimer is the Primary Investigator in an international multicenter study to assess the feasibility, safety and efficacy of the Advanta V12 stent in a larger cohort of patients and Carlos Pedra is Co-Investigator.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Coarctation of the aorta & surgery
▪ Limited to neonatal period and infancy.
▪ Recurrent coarctation of the aorta (CoA) and aneurysm formation may be encountered during follow-up.
▪ Complications include spinal cord damage, paradoxical hypertension, pleural effusion and infection.
CoA & balloon dilation
▪ Balloon dilation is the earliest form of nonsurgical intervention.
▪ It is a controversial technique owing to relatively high incidence of recurrent CoA and aneurysm formation (2–20%).
CoA & bare-metal stent implantation
▪ Bare-metal stent implantation lowers the risk of aneurysm formation, dissection and elastic recoil.
▪ It may result in less vascular injury than balloon angioplasty alone.
▪ It has a low complication rate, including aneurysm formation, stent malposition, stent fracture and vascular complications.
CoA & covered-stent implantation
▪ Covered-stent implantation has a decreased incidence of aortic wall injuries (dissection, aneurysm and aortic rupture). Its use is relatively safe and extremely effective.
▪ This is the treatment of choice in patients with subatretic and atretic CoA, with an adjacent aneurysm and with coexistence of a patent ductus arteriosus or a circumferential fracture of a previously implanted stent.
▪ This is the primary indication in patients older than 30 years of age.
Technical considerations
▪ The implantation technique is challenging and demanding.
▪ Redilation over time is feasible.
References
- Izukawa T, Mulholland HC, Rowe RD: Structural heart disease in the newborn. changing profile: comparison of 1975 with 1965. Arch. Dis. Child. 54, 281–285 (1979).
- Benson L, Mc Laughlin PR: Coarctation of the aorta. In: The Natural and Modified History of Congenital Heart Disease. Freedom R, Yoo SJ, Mikailian H, Williams WG (Eds). Blackwell, NY, USA 251–275 (2004).
- Crafoord C, Nylin G: Congenital coarctation of the aorta and its surgical treatment. J. Thorac. Surg. 14, 347–361 (1945).
- Bromberg B, Beekman R, Rocchini A et al.: Aortic aneurysm after patch aortoplasty repair of coarctation: a prospective analysis of prevalence, screening tests and risks. J. Am. Coll. Cardiol. 14, 734–741 (1989).
- Singer MI, Rowen M, Dorsey JT: Transluminal aortic balloon angioplasty for coarctation of the aorta in the newborn. Am. Heart J. 103, 131–132 (1982).
- Tynan M, Finley JP, Fontes V, Hess J, Kan J: Balloon angioplasty for the treatment of native coarctation: results of Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am. J. Cardiol. 65, 790–792 (1990).
- Fontes V, Esteves C, Braga S et al.: It is valid to dilate native aortic coarctation with a balloon catheter. Int. J. Cardiol. 27, 311–316 (1990).
- Isner JM, Donaldson RF, Fulton D, Bhan I, Payne DD, Cleveland RJ: Cystic medial necrosis in coarctation of the aorta: a potential factor contributing to adverse consequences observed after percutaneous balloon angioplasty of coarctation sites. Circulation 75, 689–695 (1987).
- O’Laughlin MP, Perry SB, Lock JE, Mullins CE: Use of endovascular stents in congenital heart disease. Circulation 83, 1923–1939 (1991).
- Pilla CB, Fontes VF, Pedra CA: Stenting for aortic coarctation. Expert Rev. Cardiovasc. Ther. 3, 879–890 (2005).
- Cheatham JP: Stenting of coarctation of the aorta. Catheter Cardiovasc. Interv. 54, 112–125 (2001).
- Ohkubo M, Takahashi K, Kishiro M, Akimoto K, Yamashiro Y: Histological findings after angioplasty using conventional balloon, radiofrequency thermal balloon, and stent for experimental aortic coarctation. Pediatr. Int. 46, 39–47 (2004).
- Gunn J, Cleveland T, Gaines P: Covered stent to treat coexistent coarctation and aneurysm of the aorta in a young man. Heart 82, 351 (1999).
- De Giovanni JV: Covered stents in the treatment of aortic coarctation. J. Interv. Cardiol. 14, 187–190 (2001).
- Pedra C, Fontes V, Esteves C et al.: Use of covered stents in the management of coarctation of aorta. Pediatr. Cardiol. 26, 431–439 (2005).
- Golden A, Hellenbrand W: Coarctation of the aorta: stenting in children and adults. Catheter Cardiovasc. Interv. 69, 289–299 (2007).
- Qureshi SA, Zubrzycka M, Brzezinska-Rajszys G, Kosciesza A, Ksiazyk J: Use of covered Cheatham-platinum stents in aortic coarctation and recoarctation. Cardiol. Young 14, 50–54 (2004).
- Bruckheimer E, Birk E, Santiago R, Dagan T, Esteves C, Pedra CA: Coarctation of the aorta treated with the Advanta V12 large diameter stent: acute results. Catheter Cardiovasc. Interv. 75, 402–406 (2010).
- Kenny D, Margey R, Turner MS, Tometzki AJ, Walsh KP, Martin RP: Self-expanding and balloon expandable covered stents in the treatment of aortic coarctation with or without aneurysm formation. Catheter Cardiovasc. Interv. 72, 65–71 (2008).
- Qureshi SA: Stenting in aortic coarctation and transverse arch/isthmus hypoplasia. In: Percutaneous Interventions for Congenital Heart Disease. Sievert H, Qureshi SA, Wilson N, Hijazi ZM (Eds). Informa Healthcare, London, UK 475–486 (2007).
- Zabal C, Attie F, Rosas M, Buendia-Hernandez A, Garcia-Montes J: The adult patient with native coarctation of the aorta: balloon angioplasty or primary stenting? Heart 89, 77–83 (2003).
- Marshall AC, Perry SB, Keane JF, Lock JE: Early results and medium-term follow-up of stent implantation for mild residual or recurrent aortic coarctation. Am. Heart J. 139, 1054–1060 (2000).
- Qureshi AM, McElhinney DB, Lock JE, Landzberg MJ, Lang P, Marshall AC: Acute and intermediate outcomes, and evaluation of injury to the aortic wall, as based on 15 year experience of implanting stents to treat aortic coarctation. Cardiol. Young 17, 307–318 (2007).
- Pedra CA, Fontes VF, Esteves CA et al.: Stenting vs. balloon angioplasty for discrete unoperated coarctation of the aorta in adolescents and adults. Catheter Cardiovasc. Interv. 64, 495–506 (2005).
- Hamdan M, Maheshwari S, Fahey J, Hellenbrand W: Endovascular stents for coarctation of the aorta: initial results and intermediate-term follow-up. J. Am. Coll. Cardiol. 38, 1518–1523 (2001).
- Harrison DA, McLaughlin PR, Lazzam C, Connelly M, Benson LN: Endovascular stents in the management of coarctation of the aorta in the adolescent and adult: one year follow up. Heart 85, 561–566 (2001).
- Lezo S, Pan M, Romero M et al.: Immediate and follow-up findings after stent treatment for severe coarctation of aorta. Am. J. Cardiol. 83, 400–406 (1999).
- Pihkala J, Pedra CA, Nykanen D, Benson LN: Implantation of endovascular stents for hypoplasia of the transverse aortic arch. Cardiol. Young 10, 3–7 (2000).
- Holzer RJ, Chisolm JL, Hill SL, Cheatham JP: Stenting complex aortic arch obstructions. Catheter Cardiovasc. Interv. 71, 375–382 (2008).
- Fejzic Z, van Oort A: Fatal dissection of the descending aorta after implantation of a stent in a 19-year-old female with Turner’s syndrome. Cardiol. Young 15, 529–531 (2005).
- Therrien J, Thorne SA, Wright A, Kilner PJ, Somerville J: Repaired coarctation: a ‘cost-effective’ approach to identify complications in adults. J. Am. Coll. Cardiol. 35, 997–1002 (2000).
- Chakrabarti S, Kenny D, Morgan G et al.: Balloon expandable stent implantation for native and recurrent coarctation of the aorta: prospective computed tomography assessment of stent integrity, aneurysm formation and stenosis relief. Heart 96, 1212–1216 (2010).
- Tanous D, Collins N, Dehghani P, Benson LN, Horlick EM: Covered stents in the management of coarctation of the aorta in the adult: initial results and 1‑year angiographic and hemodynamic follow-up. Int. J. Cardiol. 140, 287–295 (2010).
- Forbes TJ, Moore P, Pedra CA et al.: Intermediate follow-up following intravascular stenting for treatment of coarctation of the aorta. Catheter Cardiovasc. Interv. 70, 569–577 (2007).
- Holzer R, Concilio K, Hijazi Z: Selffabricated covered stent to exclude an aortic aneurysm after balloon angioplasty for post-surgical recoarctation. J. Invasive Cardiol. 17, 177–179 (2005).
- Forbes TJ, Garekar S, Amin Z et al.: Procedural results and acute complications in stenting native and recurrent coarctation of the aorta in patients over 4 years of age: a multi-institutional study. Catheter Cardiovasc. Interv. 70, 276–285 (2007).
- Ovaert C, Benson L, Nykanen D, Freedom R: Transcatheter treatment of coarctation of the aorta: a review. Pediatr. Cardiol. 19, 27–44 (1998).
- Forbes T, Matisoff D, Dysart J, Aggarwal S: Treatment of coexistent coarctation and aneurysm of the aorta with covered stent in a pediatric patient. Pediatr. Cardiol. 24, 289–291 (2003).
- Qureshi SA: Use of covered stents to treat coarctation of the aorta. Korean Circ. J. 39, 261–263 (2009).
- Ewert P, Abdul-Khaliq H, Peters B, Nagdyman N, Schubert S, Lange PE: Transcatheter therapy of long extreme subatretic aortic coarctation with covered stents. Catheter Cardiovasc. Interv. 63, 236–239 (2004).
- Ewert P, Schubert S, Peters B, Abdul-Khaliq H, Nagdyman N, Lange P: The CP stent – short, long, covered – for the treatment of aortic coarctation, stenosis of pulmonary arteries and caval veins, and Fontan anastomosis in children and adults: an evaluation of 60 stents in 53 patients. Heart 81, 948–953 (2005).
- Tzifa A, Ewert P, Brzezinska-Rajszys G et al.: Covered Cheatham-platinum stents for aortic coarctation. Early and intermediate results. J. Am. Coll. Cardiol. 47, 1457–1463 (2006).
- Butera G, Piazza L, Chessa M et al.: Covered stents in patients with complex aortic coarctations. Am. Heart J. 154, 795–800 (2007).
- Bruckheimer E, Dagan T, Amir G, Birk E: Covered Cheatham-platinum stents for serial dilation of severe native aortic coarctation. Catheter Cardiovasc. Interv. 74, 117–123 (2009).
- Collins N, Mahadevan V, Horlick E: Aortic rupture following a covered stent for coarctation: delayed recognition. Catheter Cardiovasc. Interv. 68, 653–655 (2006).
- Duke C, Rosenthal E, Qureshi S: The efficacy and safety of stent redilatation in congenital heart disease. Heart 89, 905–912 (2003).
- Butera G, Gaio G, Carminati M: Redilation of e-PTFE covered CP stents. Catheter Cardiovasc. Interv. 72, 273–277 (2008).
- Magee A, Brzezinska-Rajszys G, Qureshi S, Rosenthal E, Zubrzycka M, Ksiazyk J: Stent implantation for aortic coarctation and recoarctation. Heart 82, 600–606 (1999).
- Tsai SF, Hill SL, Cheatham JP: Treatment of aortic arch aneurysm with a NuMED-covered stent and restoration of flow to excluded left subclavian artery: perforation and dilation of e-PTFE can be done! Catheter Cardiovasc. Interv. 72, 385–389 (2009).
- Matsuda H, Fukuda T, Iritani O et al.: Spinal cord injury is not negligible after TEVAR for lower descending aorta. Eur. J. Vasc. Endovasc. Surg. 39, 179–186 (2010).