Editorial - Imaging in Medicine (2013) Volume 5, Issue 5
C-PIB and PET for the detection of cardiac amyloid
Gunnar Antoni and Per Westermark*
1Department of Medicinal Chemistry, Uppsala University, Uppsala, Sweden
2Department of Immunology, Genetics& Pathology, Uppsala University
- *Corresponding Author:
- Per Westermark
Department of Immunology
Genetics & Pathology, Uppsala University
Uppsala, Sweden
Tel: +46 18 4714819
Fax: +46 18 552739
Email: per.westermark@igp.uu.se
Abstract
Keywords
amyloid; amyloidosis; cardiac infiltration; heart; PET; PI; Pittsburg compound B n positron emission tomography
The systemic amyloidoses were earlier regarded as fairly intractable diseases and most therapies were directed to mitigate symptoms since no curative treatment was available. This situation made the development of diagnostic procedures of limited interest. During the last decades, the nature and pathogenesis of systemic amyloidoses have been elucidated with an increasing rapidity and novel therapies are being developed for many types. Therefore, in vivo assessment methods are becoming of greater importance. Particularly important are the possibilities to determine the amyloid load in whole organs or the whole body.
Systemic amyloidoses are a group of proteindeposition diseases in which one of 15 plasma proteins has adopted an abnormally high degree of b-structure, leading to a tendency to aggregate into long, stable fibrillar protein aggregates in which b-strands are oriented perpendicularly to the fibril axis [1,2]. Intermolecular association is very specific and in each amyloid type only one major fibril protein is usually present [3]. In addition, there are associated components that are more or less always present in all kinds of amyloid, irrespective of the type. The most important of these are serum amyloid P-component (SAP) and heparan sulfate proteoglycan [4,5]. The unique structure of the fibrils gives the amyloid certain specific properties, including the ability to bind dyes and other markers. Some dyes are used in the diagnostic work, particularly Congo red and Thioflavine T or S.
In systemic amyloidosis, the fibril protein precursor is expressed at one or a few sites, commonly in the bone marrow or liver, released to blood plasma and deposited extracellularly as fibrils in a diversity of organs, usually with the exception of the brain due to the blood–brain barrier. Deposits may grow to considerable size and lead to significantly enlarged organs. Symptoms depend on the affected organs and can vary enormously in some forms of systemic amyloidosis. In many forms, renal and cardiac involvement are most serious.
The most common systemic amyloidoses are those derived from immunoglobulin light chains (AL amyloidosis), serum amyloid A (AA amyloidosis) and transthyretin (ATTR amyloidosis: hereditary or wild-type TTR) [6]. In AL and ATTR amyloidosis, clinical heart involvement is common and a major cause of death. In 40% of AL amyloidosis patients, cardiomyopathy with right heart failure is present at diagnosis [7]. Heart involvement in ATTR amyloidosis depends on TTR mutation to a certain degree [8]; for some mutations, for example Val122Ile, which is prevalent in the African– American population, it is the predominant manifestation. In this and several other forms, including senile systemic amyloidosis, where wild-type TTR is the fibril protein, it is a major cause of death [9]. Senile systemic amyloidosis is probably the most prevalent form of systemic amyloidosis. Increasing awareness of this disease as well as a longer life expectancy in the developed parts of the world will put pressure on the development of imaging methods for cardiac amyloidosis.
Diagnosis of systemic amyloidosis is obtained by microscopical examination of a tissue biopsy, either from a symptom-giving organ or from a tissue known to be affected in most cases. Deposits of amyloid are recognized by affinity to certain dyes, particularly Congo red staining, which, when combined with polarization microscopy, offers a very sensitive and specific method to identify even small amyloid deposits. Furthermore, specific biochemical diagnosis, by which different disease forms are separated, can be obtained by using different methods, most commonly by the use of specific antibodies or proteomic techniques. However, these methods are not quantitative and usually rely on examination of small tissue pieces.
Although amyloidosis can be diagnosed with accuracy by histological and accessory methods, there are limited possibilities to determine the distribution of deposited material in the body.
Molecular imaging with SPECT and PET are the most advanced tools. SPECT is the standard at most large hospitals with a nuclear medicine department, whereas PET, to some extent, lags behind. In the future, PET will be available at the same level as SPECT and, during the last 15 years, PET has developed from being an expensive and exclusive research tool to a clinical routine ‘diagnostic imaging’ investigation. This progress also parallels the implementation of a personalized medicine concept in healthcare where imaging is a key method for patient stratification and differential diagnosis. The imaging potential of SPECT is, however, hampered by the available radionuclides with respect to which molecules and chemical structures can be labeled and thus produce a radiolabeled tracer targeting a specific protein or physiological process. PET on the other hand, using short-lived positron- emitting radionuclides, such as carbon-11, fluorine-18 and gallium-68, allows a very large variety of molecular structures to be labeled. Furthermore, PET, in contrast to SPECT, is a quantitative method exhibiting better spatial and temporal resolution, which, together with the resulting higher sensitivity, explains the increasing use of PET in clinical research as well as in a clinical setting.
“Although amyloidosis can be diagnosed with accuracy by histological and accessory methods, there are limited possibilities to determine the distribution of deposited material in the body.”
A few SPECT methods for the direct, noninvasive visualization of amyloid deposits in organs other than the brain have been presented. Hawkins and coworkers have developed the SAP scintigraphy method [10,11]. This method is based on the presence of the glycoprotein SAP in all kinds of human amyloid, irrespective of biochemical type or of organ involved. SAP, purified from healthy blood donors and labeled with iodine-131, is administered intravenously. Since SAP is bound to amyloid with high affinity, the distribution corresponds to amyloid infiltration. This method has been of great value and has been used to show the distribution and resolution of several biochemical types of amyloidosis, including those of AA, AL and ATTR nature. However, one problem with SAP scintigraphy has been the inability to visualize the heart involvement. Another limitation is that it is not quantitative, although it can show the distribution of amyloid.
“…a method to visualize cardiac deposits should have a resolution high enough to distinguish between affected and nonaffected areas.”
More recently, 99mTc-pyrophosphate scintigraphy, used for imaging of the skeletal system, has been found to visualize cardiac amyloid deposits [12–14]. This method seems to work better with ATTR amyloidosis compared with AL amyloidosis [14]. The reason why the compound binds to amyloid is uncertain, but may be due to the presence of small calcifications in the amyloid and inclusions of such material may vary between the disease type and may also depend on age of the deposited amyloid. 99mTcpyrophosphate scintigraphy is a good method to visualize TTR amyloid in cases with advanced disease [15], but it is more doubtful that small deposits can be shown. Another example is the polypeptide protease inhibitor aprotinin labelled with 99mTc [16]. Until recently, the monoclonal antibody, 124I-11-1F4, for AL amyloidosis was the only example of PET imaging of amyloid deposits in systemic amyloidosis [17]. However, of these examples, only 99mTc-aprotinin can be used for direct imaging of amyloid load in heart amyloidosis.
As mentioned above, the involvement of the heart is most commonly the factor determining survival in AL and ATTR amyloidosis. Proof of cardiac involvement is sometimes directly obtained by endomyocardial biopsy, but is more often dependent on indirect evidence. In addition, an endomyocardial biopsy can only visualize the involvement of a small part of the right ventricle. Combination diagnosis of amyloidosis, for example by a subcutaneous fat tissue biopsy, echocardiography and biochemical markers such as plasma proBNP, is used to monitor the degree of cardiac involvement, but there is lack of a direct, quantitative method to measure the amount and distribution of heart amyloid.
The distribution of amyloid within the heart is often highly variable even within the same biochemical type [18]. Therefore, a method to visualize cardiac deposits should have a resolution high enough to distinguish between affected and nonaffected areas.
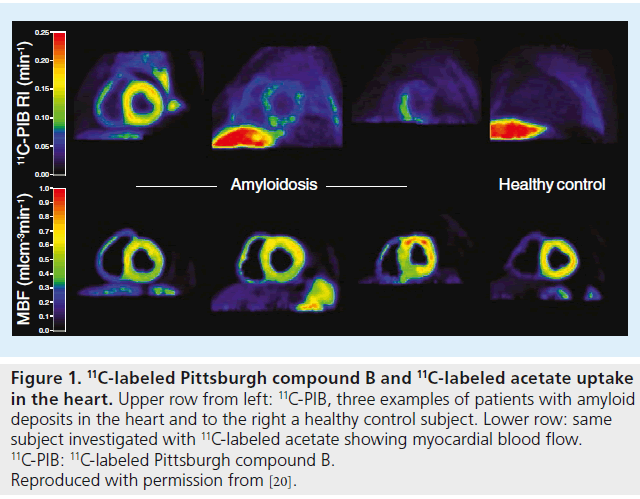
Amyloid imaging in the field of Alzheimer’s disease started with PET and 11C-labeled Pittsburgh compound B (11C-PIB) as presented in 2004, as the first noninvasive visualization of senile plaque consisting of aggregated amyloid-b deposits in the brain of a patient with Alzheimer’s disease [19]. PIB was, however, designed for imaging of the brain and, therefore, the pharmacokinetic properties were optimized for passage through the blood–brain barrier and not for imaging of other organs. It was, from our point of view, obvious that it would be of value to investigate whether the same tracer could also be used for imaging of amyloid deposits in other organs, especially in the heart. Preclinical ex vivo studies with PIB have convincingly shown that it would not be possible to image, for example, amyloid in the liver due to high nonspecific binding in this organ; however, the heart may be a viable option, as shown in in vitro binding studies with 11C-PIB and human heart tissue with amyloid of TTR type, which showed high specific binding [Hellström-Lindahl E et al., Unpublished Data].
It was possible to distinguish between the two groups by visual inspection of the summation images and quantification in terms of retention index gave statistically significant differences with no overlap. Myocardial blood flow was measured with 11C-acetate. It was found that amyloid patients had a significantly reduced myocardial blood flow, which verified that the higher 11C-PIB retention index was a measure of amyloid binding and not just reflecting tracer delivery. This study shows that it is possible to visualize and quantify 11C-PIB–amyloid binding in the heart noninvasively with PIB, which supports the future implementation of 11C-PIB and PET as a standard tool in the differential diagnosis of myocardial hypertrophy or an amyloid disease. However, before this can be realized, further validations are needed in larger populations to create an evidence-based method to be introduced in the healthcare system for diagnostic imaging and treatment monitoring of systemic amyloidosis affecting the heart. We can foresee developments in this direction and also improvements, such as even more specific PET tracers, potentially with selectivity for different types of amyloid proteins and with pharmacokinetic properties tailored to meet organ-specific requirements with special emphasis on imaging of the heart, pancreas, liver and kidney.
Figure 1.11C-labeled Pittsburgh compound B and 11C-labeled acetate uptake in the heart. Upper row from left: 11C-PIB, three examples of patients with amyloid deposits in the heart and to the right a healthy control subject. Lower row: same subject investigated with 11C-labeled acetate showing myocardial blood flow. 11C-PIB: 11C-labeled Pittsburgh compound B. Reproduced with permission from [20].
The authors conclude that PET imaging will probably be a useful method for the diagnosis and treatment monitoring of patients with systemic amyloidosis, potentially guiding the physician in the search for new treatment paradigms and improving the life span of the patients as a result of an early diagnosis before the aggregated proteins cause irreversible damage to the organ.
Financial & competing interest disclosure
This work was made possible via grants from the Swedish Research Council and from FAMY, FAMY Norrbotten and AMYL. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Merlini G, Westermark P. The systemic amyloidosis: clearer understanding of the molecular mechanisms offer hope for more effective therapies. J. Intern. Med. 255, 159–178 (2004).
- Sipe JD, Benson MD, Buxbaum JN et al. Amyloid fibril protein nomenclature: 2012 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid 19, 167–170 (2012).
- Eisenberg D, Jucker M. The amyloid state of protein in human diseases. Cell 148, 1188–1203 (2012).
- Snow AD, Willmer J, Kisilevsky R. A close ultrastructural relationship between sulfated proteoglycans and AA amyloid fibrils. Lab. Invest. 57, 687–698 (1987).
- Pepys MB, Herbert J, Hutchinson WL et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature 417, 254–259 (2002).
- Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 349, 583–596 (2003).
- Nuvolone M, Palladini G, Merlini G. Amyloid diseases at the molecular level: general overview and focus on AL amyloidosis. In: Amyloid and Related Disorders. MM Picken, A Dogan, GA Herrera (Eds). Springer, NY, USA, 9–29 (2012).
- Zeldenrust SR. Genotype–phenotype correlation in FAP. Amyloid 19(Suppl. 1), S22–S24 (2012).
- Dungu JN, Anderson LJ, Whelan CJ et al. Cardiac transthyretin amyloidosis. Heart 98, 1546–1554 (2012).
- Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N. Engl. J. Med. 323, 508–513 (1990).
- Hawkins PN, Richardson S, MacSweeney JE et al. Scintigraphic quantification and serial monitoring of human visceral amyloid deposits provide evidence for turnover and regression. Quart. J. Med. 86, 365–374 (1993).
- Rapezzi C, Guidalotti P, Salvi F et al. Usefulness of 99mTc-DPD scintigraphy in cardiac amyloidosis. J. Am. Coll. Cardiol. 51, 1509–1510 (2008).
- Castaño A, Bokhari S, Brannagan TH 3rd, Wynn J, Maurer MS. Technetium pyrophosphate myocardial uptake and peripheral neuropathy in a rare variant of familial transthyretin (TTR) amyloidosis (Ser23Asn): a case report and literature review. Amyloid 19, 41–46 (2012).
- Bokhari S, Castaño A, Pozniakoff T et al. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ. Cardiovasc. Imaging 6, 195–201 (2013).
- Quarta CC, Guidalotti PL, Longhi S et al. Defining the diagnosis in echocardiographically suspected senile systemic amyloidosis. JACC Cardiovasc. Imaging 5, 755–758 (2012).
- Glaudemans AW, Slart RH, Zeebregts CJ et al. Nuclear imaging in cardiac amyloidosis. Eur. J. Nucl. Med. Mol. Imaging 36, 702–714 (2009).
- Wall JS, Kennel SJ, Paulus M et al. Radioimaging of light chain amyloid with a fibril-reactive monoclonal antibody. J. Nucl. Med. 47, 2016–2024 (2006).
- Leone O, Longhi S, Quarta CC et al. New pathological insights into cardiac amyloidosis: implications for non-invasive diagnosis. Amyloid 19, 99–105 (2012).
- Klunk WE, Engler H, Nordberg A et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 55, 306–319 (2004).
- Antoni G, Lubberink M, Estrada S et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J. Nucl. Med. 54, 213–220 (2013).