Editorial - Imaging in Medicine (2010) Volume 2, Issue 3
Cranial development in the first trimester: the use of 3D in the study of complex structures 1
Lucia Rosignoli, Gabriele Tonni* and Giovanni Centini
1Prenatal Diagnostic Unit, Policlinic Hospital “Le Scotte”, University of Siena, Italy
2Prenatal Diagnostic Service, Guastalla Civil Hospital, AUSL Reggio Emilia, Reggio Emilia, Italy
3Prenatal Diagnostic Unit, Policlinic Hospital “Le Scotte”, University of Siena, Italy
- *Corresponding Author:
- Gabriele Tonni
Prenatal Diagnostic Service
Guastalla Civil Hospital, AUSL Reggio Emilia
Reggio Emilia, Italy
Tel: +39 052 283 7232
Fax: +39 052 283 7412
E-mail: tonni.gabriele@ausl.re.it
Abstract
Embryology
The differentiation of facial tissues occurs early in the embryonic period, between week 5 and 7, and begins to be characterized by the first two branchial arches, in respect to the tissues underlying the forebrain that begin to develop during the second month.
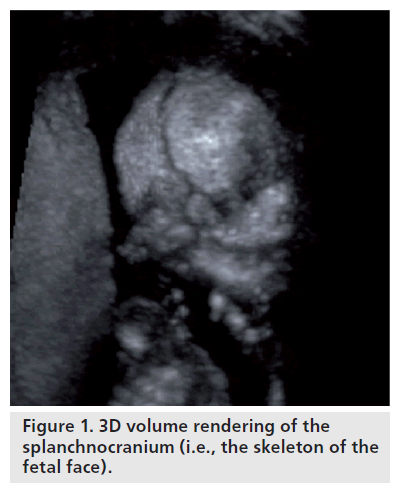
The concept of growth is described as the manifestation of quantitative and structural changes that occur in regular succession, as changing parts of the body and organs begin to function, developing new features while improving the activities of the existing bones [1,2]. There are two distinguishing parts in the cranium: the neurocranium or cavity of the brain, which is subdivided into the area at base of the cranium and cranial vault; and the splanchnocranium, the skeleton of the face (Figure 1). Both areas are initially formed by mesenchymal thickening, which then hardens into bone by either endochondral or membrane ossification. The bones at the base of the cranium develop through chondrocyte ossification, whereas the bones of the cranial vault are derived by membrane ossification. Cranial bone growth is virtually identical to bone growth in other regions of the body, that being the ossification of cartilage during bone replacement. There are two fundamental mechanisms: apposition and reabsorption.
This can be better described using the theory proposed by Moss [3–7], “the origin, growth and maintenance of all tissues and skeletal formations are a secondary phenomena and offset of other previous events which verify the nonskeletal tissue, organs or functional areas specifically related to them”. The cranial cavity represents a classic example of a matrix function, meaning that the cranium grows under the pressure of the brain, which during the prenatal period, develops faster than any other part of the fetus. The splanchnocranium, however, remains considerably smaller since it is not used by the fetus before birth.
From 2D to 3D/4D
The 2D ultrasound technique
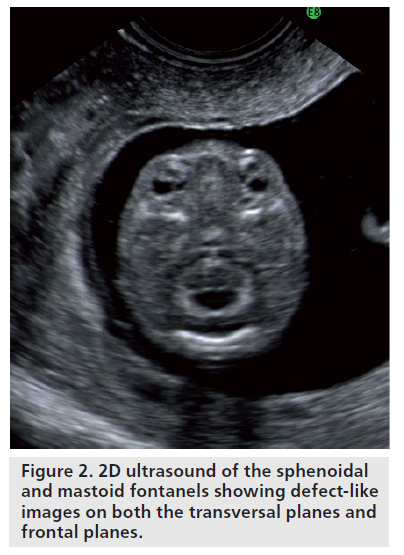
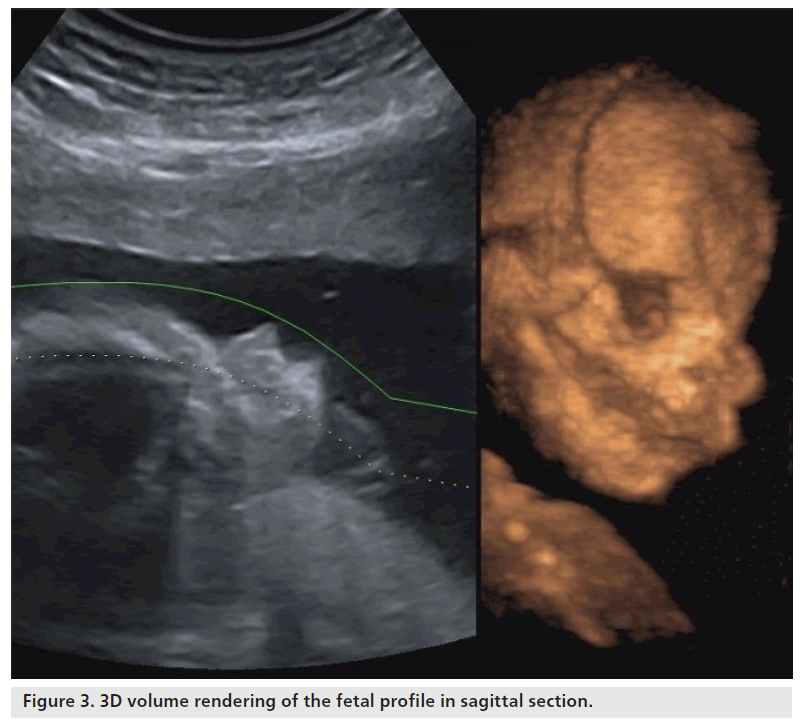
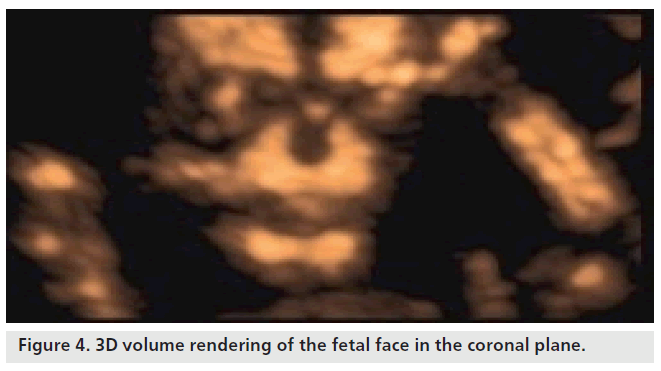
The cranium, which is already visible from week 7, takes on a recognizable profile at week 10, considering progressive ossification allows easy identification of cranial bones. When using 2D ultrasound scans, the cranial curvature generally hinders the assessment of the majority of the sutures and only occasionally may the fontanels be seen before week 20. Regarding the transverse scans, the coronal and lambdoid sutures are interpreted as a defected profile image, while the sphenoidal and mastoid fontanels give the impression that there is a defect on both the transversal and frontal planes (Figure 2). The identification of facial features of the fetus has been consequential owing to the acquisition of 3D ultrasound, which allows volume reconstruction on the three orthogonal planes: the sagittal scans, which provide information relative to the profile (Figure 3), forehead and chin; the axial planes, which display the orbital cavity; and finally, the coronal plane for the face (Figure 4). Many of the facial bones, or parts of them, have a V‑shape since apposition occurs on the inside and reabsorption occurs externally [8]. A more precise analysis of the anomalies of the face requires a step-by-step scan on three planes, which is only possible with a 3D ultrasound. This method allows three orthogonal planes to be seen simultaneously, in great detail and in a multiplanar form, therefore allowing for a more complete and accurate assessment.
Structural growth of the cranium
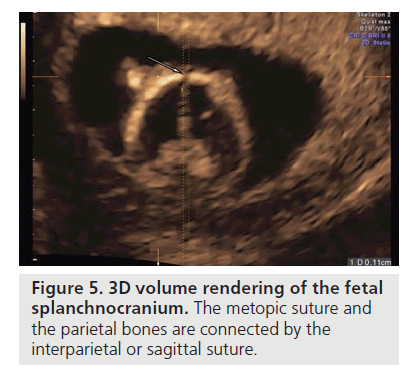
The teca cranium is formed bilaterally by the frontal and parietal bones, and the temporal and optical folds of the cranium. The process of ossification begins around week 10, starting at the frontal and parietal zones. The frontal bones on the symmetry plane are united and divided by a metopic suture, the parietal bones are connected by a interparietal or sagittal suture (Figure 5).
Figure 5.3D volume rendering of the fetal splanchnocranium. The metopic suture and the parietal bones are connected by the interparietal or sagittal suture.
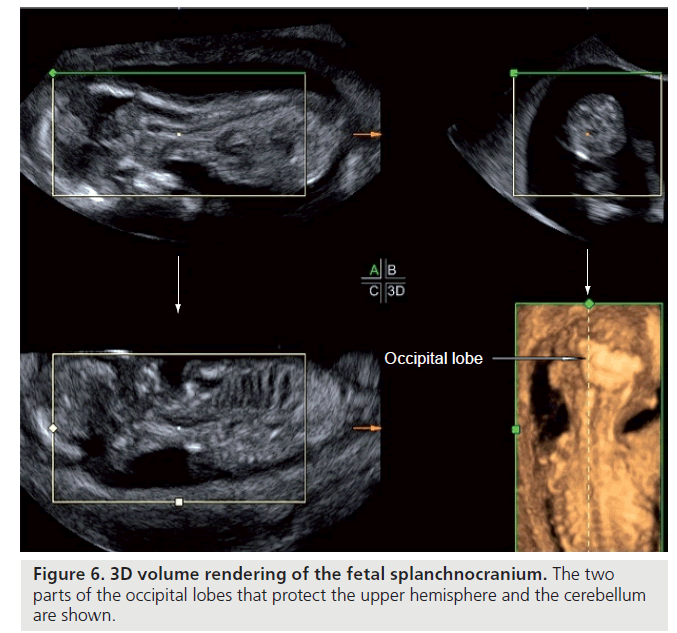
The coronal suture is formed between the frontal and parietal bone on both sides. The temporal bone grows in tandem with the cranial base, while the parietal follows the growth pattern of the teca cranium. The union between a temporal fold and parietal bone is called a fold suture, a suture with rounded edges, where the overlap, placed at the level of the temporal suture, causes the growth at the base of the cranium to the teca and vice versa. The folds of the occipital lobe follow two types of ossification: the upper part forms by chondral ossification and the lower part by intramembranous ossification. The joint of the upper and lower parts is considered an anchor point for the tentorium of the cerebellum, the two parts of the occipital lobe protect the upper hemispheres and the cerebellum (Figure 6).
Altered formations of the optical folds are seen in Meckel–Gruber syndrome [9]. This is due to the missing closure, or suture, in the regions where, in normal circumstances, the parts of bone are fused together by chondral ossification [10].
Sutures
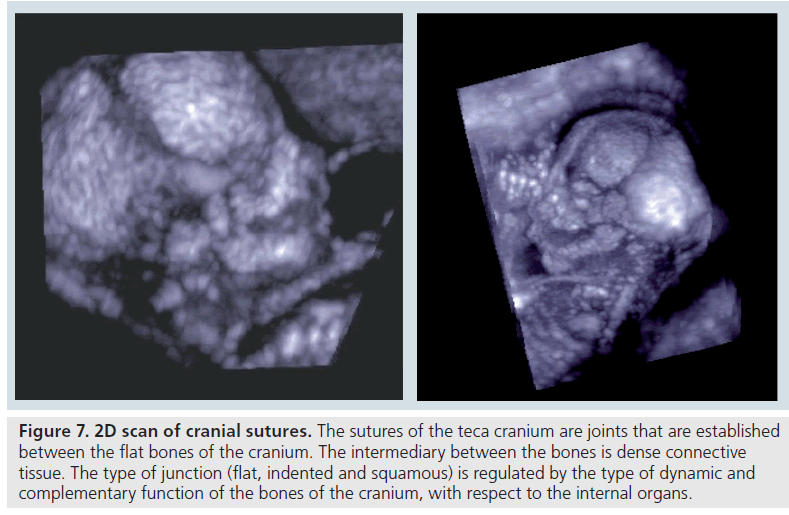
The sutures of the teca cranium are joints that are established between the flat bones of the cranium, the intermediary between the bones is dense connective tissue. The type of junction, flat, indented and squamous, is regulated by the type of dynamic and complementary function of the bones of the cranium, with respect to the internal organs (Figure 7).
Figure 7.2D scan of cranial sutures. The sutures of the teca cranium are joints that are established between the flat bones of the cranium. The intermediary between the bones is dense connective tissue. The type of junction (flat, indented and squamous) is regulated by the type of dynamic and complementary function of the bones of the cranium, with respect to the internal organs.
Visualization and detection of the sutures is important mainly for the recognition of craniosynostosis, which occurs in premature ossification of sutures that are present in the most complex types of syndromes, such as Apert, Crouzon and Pfeiffer syndrome [11–14].
Deformity of the cranium may also affect other bones of the maxillary face, creating truly defined dysmorphisms.
Fontanels
The fontanels along the sutures are the connecting lines of the bones in the cranium that retain their flexibility, both at the time of childbirth and during the first year of life, in order to adapt and form along with the growth of the brain. They are fibrous areas resulting from connections of the periosteum with the intracranial and dura madre. There are six fontanels: one anterior, one posterior and four lateral – two anterior and two posterior. The anterior fontanel is the most visible owing to its size and distinctive rhomboid shape. The posterior fontanel, on the other hand, is the most difficult to detect as it is the smallest.
The fontanels have differential ossifications: the posterior closes within the second month of life, whereas the anterior tends to reduce after approximately the sixth month, completing the process at 1 year of age. However, in some cases, this could also depend on the size at birth. In any case, they continue to maintain a certain flexibility in order to adapt to the development of the intracranial structures [9].
Maxillofacial district
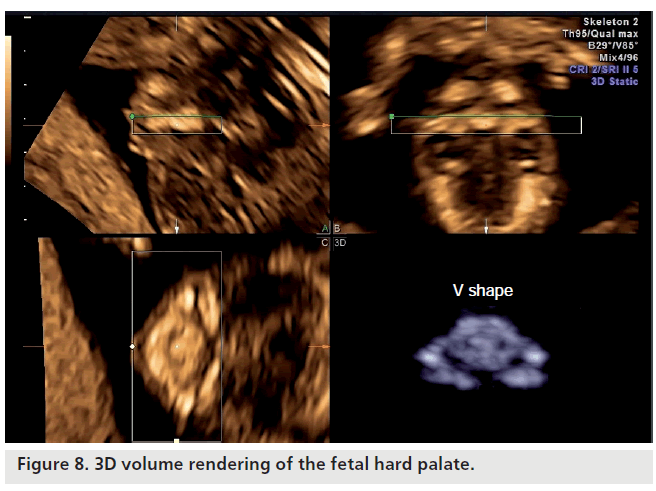
The mandible is the first bone of the skull to grow. The development starts from Meckel’s cartilage (bilaterally) and fuses at the center of the symphysis on the median sagittal plane. After the formation of bone has reached the lingual surface of Meckel’s cartilage, the jaw will have the ability to move, while the mandibular condyle is formed independently of the cartilage. The bone tissue of the mandible begins to form around weeks 7–8 and is located before the cartilage, precisely at the point where there is the bud of the permanent canine tooth. It is here that the bone proceeds in forward and backward directions, taking on an ‘S’ shape. The body of the mandible is divided into anterior and posterior parts (Figure 8).
The mandibular condyle has a conical shape in the first trimester. From week 15 the cartilaginous tissue covering the condyle has a mushroom shape. The shape of the jaw is characterized in an early stage of development with the absence of a mandibular branch and the condyle. The modifying condyle contributes to the formation of the mandibular branch and, together with these changes, there is a reabsorbtion of tissue in the anterior aspect with concurrent apposition in the posterior aspect, until the full formation of the mandible at the time of birth.
Jaw bone
Following the mandible, there is the initial appearance of the jawbone at weeks 9–10 in the area below the infraorbital nerves. If we look head-on at the disposition of the bones, it is evident that the ossification of the mandible and maxilla grow simultaneous and symmetrically in height with respect to the canine teeth, being synchronous with the functions and development of the peripheral nervous system. In its primitive stage, the jawbone has a horseshoelike shape. The lifting of the first palatine processes, which start laterally and bilaterally from the maxillary processes adjacent to the tongue, give shape to the palate. Immediately after the conjunction of the midline palatine processes, the oral cavity is established with the subdivision of the nasal and oral cavity. However, at this stage, ossification of the cranium base has not started. There are three recognized areas of growth for the maxillofacial district: transversal, sagittal and coronal [9]. The transversal growth happens late in the first trimester and is subject to progressive separation of the alveolar process of the molars, with respect to the canines, which in the first period run parallel to the median palatine suture. This process will change the morphology of the palate, which will then show greater posterior amplitude than anterior.
Nasal cavity
The nasal cavities are divided on the sagittal plane of the nasal septum, laterally by the hollows and anteriorly from the cartilage of the outer nose and nasal bone.
Vomer
The bilateral bone forms the support of the nasal septum and the morphology changes gradually between the first and second trimesters. Around week 12, the vomer is established by two bony plates that become fused around week 16–17 and this forms the support of the median palatal suture.
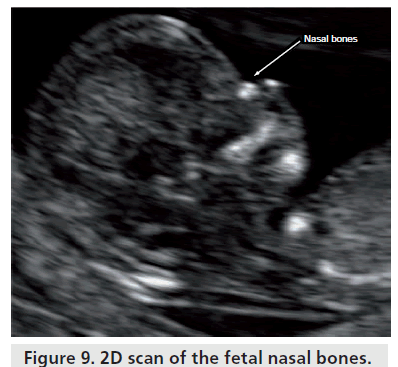
Nasal bone
The nasal bone is a bilateral bone that originally appears as a small fragment above the septal cartilage and the process of ossification happens later than the maxilla and vomer (Figure 9). The development of these small bones is very fast and they are configured in the first quarter as thin and elongated structures [15].
Division between the cavity & cranium base
The division between the cranium cavity and the cranium base, known as the neurocranium, is marked by a line that coincides with the craniofacial line. From a lateral view, this line, starting from the zygomatic frontal articulation, follows the upper temporal line and continues along the upper nuchal line until the outer prominence.
Cranium base
In early development, the cranium base is first derived from endochondral ossification and later, as cartilage tissue. The cranium base is designed to support the brain and divides the neurocranium from the skeleton of the face, more specifically, the ossification is subdivided in the median sagittal plane and in its bilateral parts.
On the median plane, the observed process of ossification starts from the optical forum to the frontal bone, lasting the whole course of pregnancy. The sequence of ossification is as follows: first, the optical folds and the great wing of the sphenoid develop at week 11, with the occipital condyle and the tympanic rings developing subsequently.
From the beginning of ossification, the bones grow together at various synchondrosis, which are formed between the components of bone. The synchondrosis at the base of the cranium, with respect to the sutures, are centers of autonomous growth; meaning that they are not subject to change by environmental factors. The developmental process of the median sagittal segment of the cranium base is divided into seven stages. Initially there is the appearance of the frontal bone, then the base of the occipital bone, the base of the sphenoid and the elements of the ethmoid.
Basic anthropometry
Different populations have varying characteristics with regards to the cranium. To simplify these differences, craniometric measurements were created for the postnatal period, cranial indices or centesimal relationships between the values of some measurements. Through cephalometric measurements, distance can be measured between fixed anatomical points, and from these points, cephalometric reference planes can be constructed, which are essential for orthodontic evaluations. The cephalometric layout allows for the diagnosis and guidance for therapy [16,17].
Prenatal measurements by 3D/4D ultrasound
Craniofacial features of Down’s syndrome (also present in other syndromes)
It has been demonstrated that Down’s syndrome is related to premature phenotypic expression in weeks 6–12 of the intrauterine life.
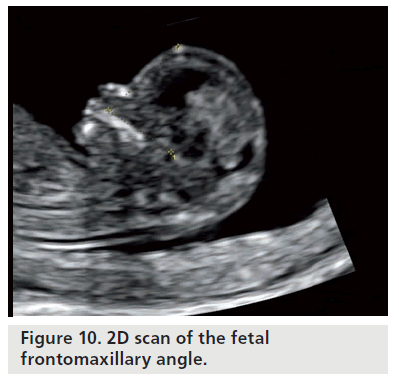
The most general features of deficit skeletal development in Down’s syndrome are remarkably effected, including the facial maxillofacial district [18–20]. The skeletal changes predominantly involve the middle-third of the face, which is shown as a reduction in the size of the upper jaw and cranium base. There are current studies on the frontomaxillary facial angle and maxillary mandibular angle, for fetuses carrying trisomy 18 and frontomaxillary angle for trisomy 21 (Figure 10) [20–22].
The use of 3D/4D ultrasound technology has become an integral part of fetal ultrasonography, both in routine and complex cases. Through detailed scans of the fetal face, it is possible to examine the variables of circadian uterine life, such as in the sleep–awake mode, yawning, automatic reactions and functions, pleasures and nonpleasures [23,24]. This observation can be performed continuously, following all stages of pregnancy until birth. Therefore, for craniofacial anomalies, it is becoming increasingly more important to make use of these 3D/4D ultrasound reconstructions since they can give a greater accuracy of data analysis, as well as offering the capability for offline analysis with software dedicated to such methods [25].
Such 3D/4D ultrasound application is essential in volumetric studies and is, therefore, critical for the definitive prenatal diagnosis. The multiplanar modality, in which the images are displayed in three orthogonal planes, allows one to study the volumes acquired in detail, just like working in real time, yet being offline. This technique allows you to completely navigate inside the volume acquired, through the intersection of the three planes represented by orthogonal 1 caliper on each image shown on the monitor. The caliper can be moved and modified at any time on each image represented. The points may provide a precise relationship of the cranial structures and their possible ‘deviations’ from the normal.
As was the case with Down’s syndrome and other anomalies, where 2D ultrasound markers may indicate the possibility of these underlying diseases, the 3D ultrasound knowledge of the normal craniofacial anthropometric relationships may be supportive to the 3D diagnosis of syndromic cases [26].
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Kjær I: Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta Odontol. Scand. 53, 135–143 (1995).
- Noden DM: Cell movements and control of patterned tissue assembly during craniofacial development. J. Craniofac. Genet. Dev. Biol. 11, 192–213 (1991).
- Moss ML: The functional matrix. In: Vistas in Orthodontics. Kraus BS, Riedel R (Eds). Lea & Febiger, PA, USA 85–98 (1962).
- Moss ML: The functional matrix hypothesis revisited. 1. The role of mechanotransduction. Am. J. Orthod. Dentofac. Orthop. 112(1), 8–11 (1997).
- Moss ML: The functional matrix hypothesis revisited. 2. The role of an osseous connected cellular network. Am. J. Orthod. Dentofac. Orthop. 112(2), 221–226 (1997).
- Moss ML: The functional matrix hypothesis revisited. 3. The genomic thesis. Am. J. Orthod. Dentofac. Orthop. 112(3), 338–342 (1997).
- Moss ML: The functional matrix hypothesis revisited. 4. The epigenetic antithesis and the resolving synthesis. Am. J. Orthod. Dentofac. Orthop. 112(4), 410–477 (1997).
- Enlow DH: Facial Growth (3rd Edition). WB Saunders, PA, USA (1990).
- Larsen WJ: Human Embryology (2nd Edition). Churchill Livingstone Inc., NY, USA (1997).
- Sardi ML, Ventrice F, Ramìrez Rozzi F: Allometries throughout the late prenatal and early postnatal human craniofacial ontogeny. Anat. Rec. 290, 1112–1120 (2007).
- Esser T, Rogalla P, Bamberg C, Kalache K: Application of the three-dimensional maximum mode in prenatal diagnosis of Apert syndrome. Am. J. Obstet. Gynecol. 193, 1743–1745 (2005).
- Faro C, Chaoui R, Wegrzyn P, Levaillant JM, Benoit B, Nicolaides KH: Metopic suture in fetuses with Apert syndrome at 22–27 weeks of gestation. Ultrasound Obstet. Gynecol. 27(1), 28–33 (2006).
- Ward ER, Jamison PL, Allason JE: Quantitative approach to identifying abnormal variation in the human face exemplified by a study of 278 individuals with five craniofacial syndromes. Am. J. Med. Genet. 91, 8–17 (2000).
- Miller C, Losken HW, Towbin R et al.: Ultrasound diagnosis of craniosynostosis. Cleft Palate Craniofac. J. 39(1), 73–80 (2007).
- Hansen L, Skovgaard LT, Nolting D, Hansen BF, Kjaer I: Human prenatal nasal bone lengths: normal standards and length values in fetuses with cleft lip and cleft palate. Cleft Palate Craniofac. J. 42, 165–170 (2005).
- Farkas LG: Anthropometry of the Head and Face (2nd Edition). Raven Press, NY, USA (1994).
- Ricketts RM: A foundation for cephalometric communication. Am. J. Orthodontics 46, 330–357 (1960).
- Benda CE: Down’s Syndrome: Mongolism and its Management. Grune & Stratton, NY, USA (1969).
- Dagklis T, Borenstein M, Peralta CF, Faro C, Nicolaides KH: Three-dimensional evaluation of mid-facial hypoplasia in fetuses with trisomy 21 at 11 + 0 to 13 + 6 weeks of gestation. Ultrasound Obstet. Gynecol. 28, 261–265 (2006).
- Lauridsen H, Hansen BF, Reintoft I, Keeling JW, Skovgaard LT, Kjaer I: Short hard palate in prenatal trisomy 21. Orthod. Craniofac. Res. 8, 91–95 (2005).
- Borenstein M, Persico N, Strobl I, Sonek J, Nicolaides KH: Frontomaxillary and mandibulomaxillary facial angles at 11 + 0 to 13 + 6 weeks in fetuses with trisomy 18 Ultrasound Obstet. Gynecol. 30(7), 928–933 (2007).
- Rembouskos G, Cicero S, Longo D, Vandecruys H, Nicolaides KH: Assessment of the fetal nasal bone at 11–14 weeks of gestation by three-dimensional ultrasound. Ultrasound Obstet. Gynecol. 23(3), 232-236 (2004).
- Sforza C, Dellavia C, Dolci C, Donetti E, Ferrario VF: A quantitative three-dimensional assessment of abnormal variations in the facial soft tissues of individuals with Down syndrome. Cleft Palate Craniofac. J. 42, 410–416 (2005).
- Morimoto N, Ogihara N, Katayama K, Shiota K: Three-dimensional ontogenetic shape changes in the human cranium during the fetal period. J. Anat. 212, 627–635 (2008).
- Roelfsema NM, Hop WC, van Adrichem LN, Wladimiroff JW: Craniofacial variability index in utero: a three-dimensional ultrasound study. Ultrasound Obstet. Gynecol. 29, 258–264 (2007).
- Hartmun SL, Dagmar W, Rolf PW, von der Malsburg C, Horsthemke B: Computer-based recognition of dysmorfic faces. Eur. J. Hum. Genet. 11, 555–560 (2003).