Review Article - Interventional Cardiology (2011) Volume 3, Issue 5
Current management approach for left renal vein entrapment syndrome: the so-called âÃâ¬ÃËNutcrackerâÃâ¬Ã⢠syndrome
- Corresponding Author:
- Matthew T Menard
Brigham & Womens Hospital, Division of Vascular and Endovascular Surgery,75 Francis St., Boston, MA, USA
E-mail: mmenard@partners.org
Abstract
Keywords
endovenous stenting,hematuria,nutcracker syndrome,pelvic congestion syndrome,renal vein entrapment
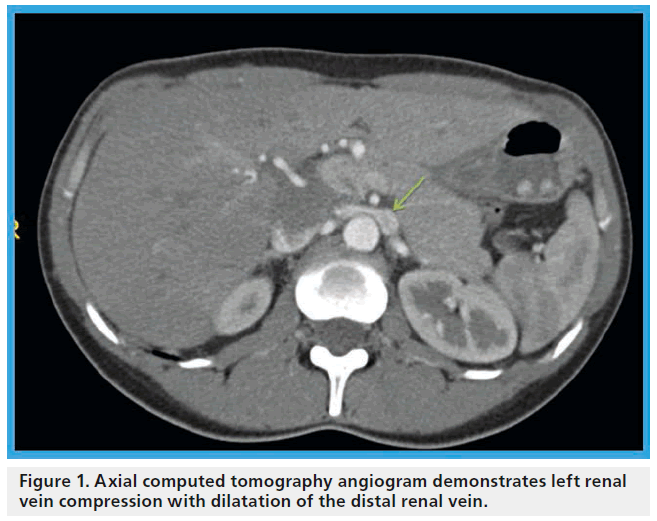
James T McPhee1 & Matthew T Menard1 1Brigham & Womens Hospital, Division of Vascular and Endovascular Surgery, 75 Francis St., Bost (SMA) and the abdominal aorta, as it traverses between these two structures toward the inferior vena cava was first described in 1937 [1]. In certain instances, compression of the vein may lead to clinically relevant dilatation of its distal aspect (Figure 1). The important features associated with this unique anatomic relationship were recognized and ultimately given the moniker the ‘nutcracker’ phenomenon in the subsequent decades [2–4]. An analogous syndrome has been described in patients with an anomalous retroaortic LRV, whereby the vein is compressed between the abdominal aorta and the vertebral column [3]. With this new description, it has become necessary to describe the SMA compression of an ante-aortic LRV as ‘anterior nutcracker syndrome’ and the compression of a retroaortic LRV as ‘posterior nutcracker syndrome’. With just ten known cases of the latter syndrom, first described in 1986 [5], the anterior configuration remains far more commonly observed [6].
Figure 1: Axial computed tomography angiogram demonstrates left renal vein compression with dilatation of the distal renal vein.
The first description of surgical repair of the venous ouflow obstruction emerged in 1974 [7], and since that time, a logical approach to the evaluation and diagnosis of suspected LRV entrapment has evolved. In part given its relatively rare incidence, what remains unexplained is the variable clinical penetrance of a somewhat common radiographic abnormality. As up to 50–75% of asymptomatic children and adults may demonstrate some degree of LRV entrapment and dilatation on axial imaging surveys without clinical sequelae, clearly an unknown variable exists that predisposes some patients to manifest symptoms of renal venous hypertension while others do not [8,9]. Further to this point, it is well-known that elective ligation of the LRV during abdominal aortic aneurysm repair is well-tolerated in most circumstances [10], as is the elective procurement of the LRV for use as a bypass conduit in abdominal vascular reconstructions [11,12].
In addition to the evolution of diagnostic maneuvers that have helped to clarify the clinical relevance of radiographic findings in a given patient, the surgical management of LRV entrapment has similarly evolved. Approaches have ranged from a creative array of major open abdominal vascular reconstructions to, more recently, less invasive approaches such as external stenting, SMA elevation techniques and laparoscopic decompression. Concurrent advancements in endoluminal therapy in general and endovenous interventions for left iliac vein compression and venous thoracic outlet obstruction in particular have paved the way for percutaneous treatment of this anatomically analogous external mechanical compression of the LRV.
Clinical presentation
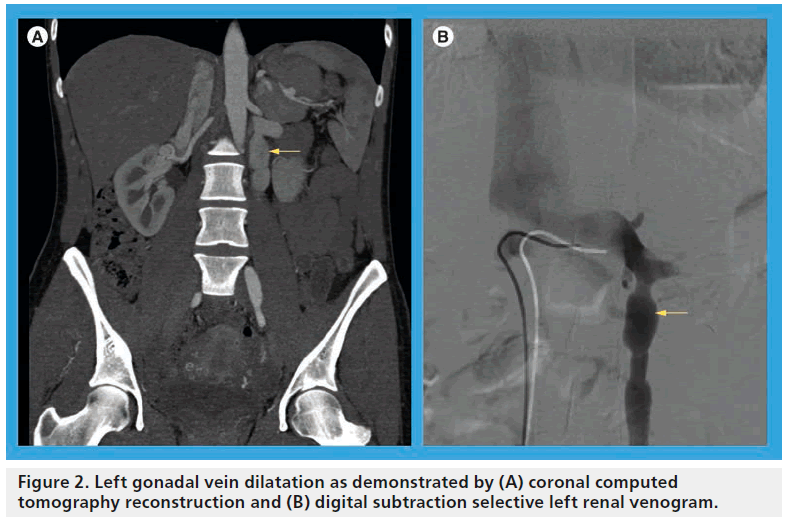
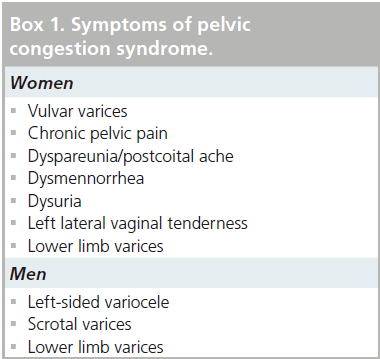
The symptom complex associated with nutcracker syndrome relates to relative venous hypertension of the LRV. The syndrome is most commonly observed in thin young men and middle-aged women. It typically manifests as left-sided flank pain and hematuria, which may range in severity from a microscopic finding observed on urinalysis to gross hematuria with an ongoing transfusion requirement [13]. The hematuria is likely due to microvascular rupture of thin-walled renal pelvic and periureteral varices [14,15] and may be intermittent in nature, perhaps related to a relative increase of the venous hypertension, which may worsen symptoms after periods of physical exertion or while in an upright position [16], which has been shown radiographically [17]. Additional symptoms that have an underlying anatomic explanation relate to collateral drainage venous hypertension, but are not universally present in patients with LRV compression. Most notably, elevated pressure in the gonadal venous circuit may ultimately lead to reflux, dilatation (Figure 2), collateralization and variceal formation. The presence of gonadal vein involvement is a hallmark feature of the so-called ‘pelvic congestion syndrome’ (PCS), which manifests as left-sided varicocele in young men and chronic lower abdominal or pelvic pain and dyspareunia in middle-aged women (Box 1) [18,19]. A minority of patients with PCS will have overlapping symptoms attributable to LRV compression. A study by Scultetus et al. reviewed a series of 51 female patients with symptoms of PCS (gluteal, vulvar and lower extremity varices), nine of which (17%) had additional symptoms of hematuria and left flank pain [19]. They concluded that patients that have PCS in addition to hematuria may benefit from a diagnostic evaluation of possible LRV compression.
LRV entrapment syndrome is also welldescribed in children, although it is typically self-limited in nature in this population. Based on reports of long-term success when early intervention has been deferred, management is usually expectant [20]. Etiologic explanations for this phenomenon in developing children relate to a relative paucity of retroperitoneal fat; this more posterior position of the left kidney leads to ‘bow-stringing’ of the LRV across the aorto-mesenteric angle. Subsequent development resulting in increased height and body mass index serve to create a more favorable hemodynamic situation for the LRV and typically lead to symptom resolution [21].
Diagnosis
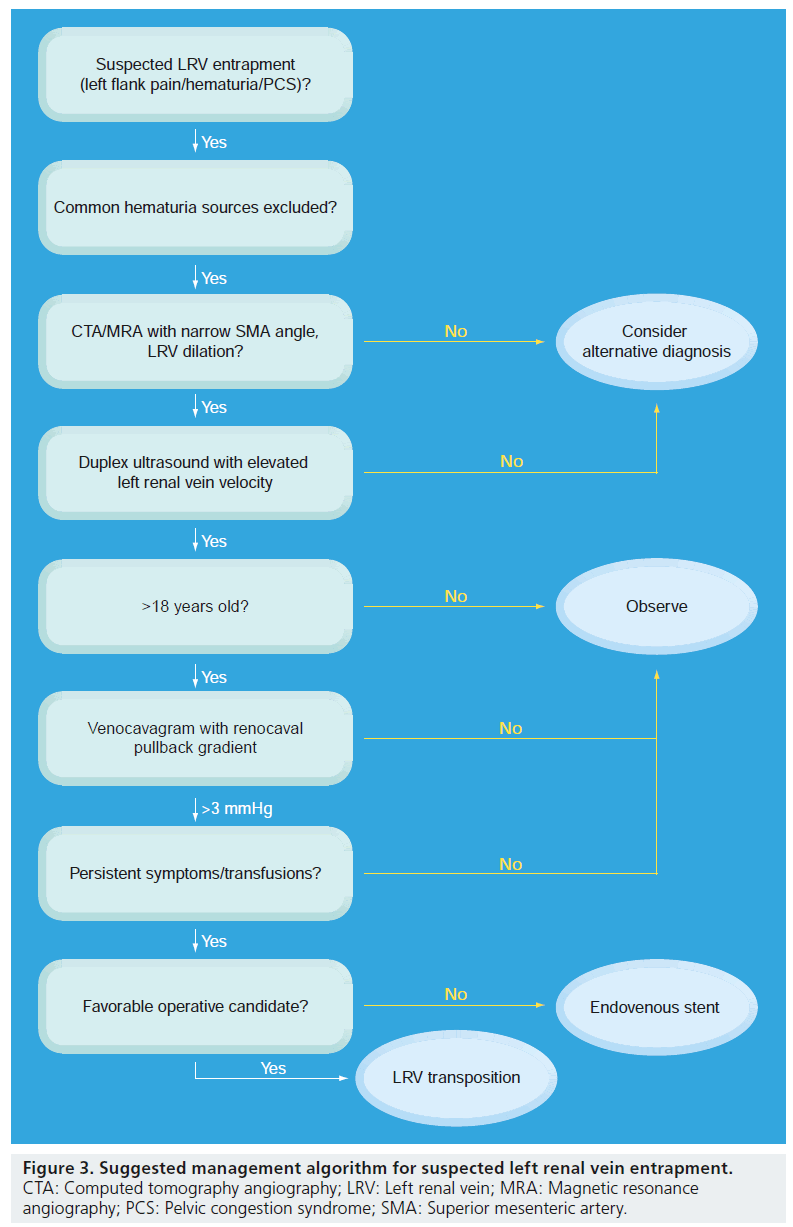
A reliable diagnosis of LRV compression is predicated on a comprehensive evaluation of patients with suggestive symptoms. A suggested management algorithm is provided in Figure 3. When a young man or middle-aged woman presents with symptoms concerning LRV entrapment, a directed evaluation aimed at excluding multiple other diagnostic possibilities is paramount. It should be noted that the successful identification of LRV entrapment, as with other uncommon intra-abdominal compression disease entities such as median arcuate ligament syndrome or vascular compression of the duodenum, is often preceded by protracted periods of diagnostic confusion; not infrequently, the correct diagnosis is not considered until months or years after initial symptom development.
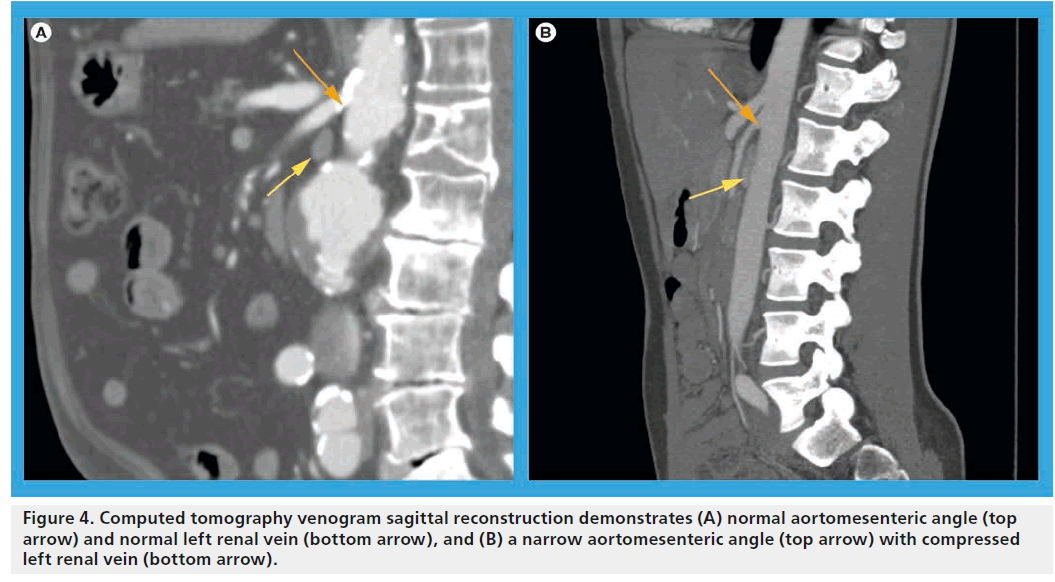
If hematuria is present, a full urologic workup to rule out other possible causative factors is indicated. Cystoscopic confirmation of isolated left ureteral hematuria suggests left-sided venous hypertension, but is an insensitive test due to the intermittent and potentially microscopic nature of the hematuria. Axial imaging, including computed tomography angiography or magnetic resonance angiography, serve both to reveal the necessary inclusion criteria of LRV compression/ dilatation and exclude other nephrogenic or abdominal etiologies (Figure 4). In addition, evaluation of the aortomesenteric angle may be discerned via sagittal reconstructions of axial imaging. Typically, this angle should be >35° in normal subjects, but has been shown to be as narrow as 16° or less in affected individuals [22]. As previously alluded to, cross-sectional imaging alone by no means secures the diagnosis, as renal vein compression may be a normal variant in a substantial portion of the adult and pediatric population [8]. Duplex ultrasonography has recently emerged as a sensitive and accurate means of identifying elevated left renal venous velocities across the aorto-mesenteric angle [22,23]. A ratio of venous peak systolic velocity (PSV) at the narrowed segment as compared with the noncompressed hilar segment has been used to characterize the syndrome. While varying PSV ratio data are reported in the literature, in general a venous PSV ratio >4.7 is very sensitive and specific (>90% for both) to the diagnosis of the venous compression syndrome [24]. The main limitation of duplex imaging remains its lack of universal reproducibility secondary to significant inter-institutional and operator variance. Its primary appeal relates to its safety and non-invasive nature and its utility in serially tracking patients in an expectant fashion or pre- and post-intervention.
Currently, a definitive diagnosis of LRV entrapment in symptomatic individuals relies on venocavography and demonstration of a LRV pull-back pressure gradient. Demonstration of such a gradient in the right clinical setting suggests a causative relative hypertensive state in the LRV. It has been conclusively shown that normal individuals will have a renocaval pressure gradient of <3 mmHg, and that in fact, the vast majority of individuals will have a gradient <1 mmHg [25,26]. These historical studies form the underpinning for the declarative statements throughout the nutcracker syndrome literature that the demonstration of an elevated gradient of ≥3 mmHg in symptomatic individuals is imperative to confirming the suspected diagnosis. Of note, variability in pressure measurements with patient positioning has been demonstrated by some groups [17].
Treatment options
Treatment options for LRV entrapment syndrome consist of expectant management, open surgical reconstruction, minimally invasive surgical reconstruction or endovascular stenting.The decision to intervene and by which method should be tailored to the individual patient and their particular demographic circumstances. As previously mentioned, in the pediatric population, symptoms are often self-limited and normal development will usually obviate the need for intervention [27]. As such, in the absence of severe, persistent symptoms or transfusiondependent hematuria in a developing child, these authors would advocate for an observational approach. Moreover, the slender build of pediatric patients with LRV entrapment syndrome render them good candidates for serial monitoring with advanced duplex imaging, obviating the need for more invasive imaging that would be unlikely to alter treatment recommendations.
Some adult patients may also experience spontaneous symptom resolution if managed conservatively, as was shown in one series with intermediated follow-up [24]. Certainly, for patients with prohibitive anatomic or prohibitive co-morbid risk factors, observation likely remains the safest approach. However, for adult patients with persistently debilitating symptoms and a demonstrated elevation in the renocaval pressure gradient, early intervention has the potential to be curative. For better risk patients, the decision becomes surgical reconstruction versus endovascular repair. It should be noted that a discussion of welldescribed endovenous techniques for isolated symptoms of pelvic congestion in the absence of flank pain and hematuria was considered beyond the scope of this review [28–30].
▪ Open surgical treatment options
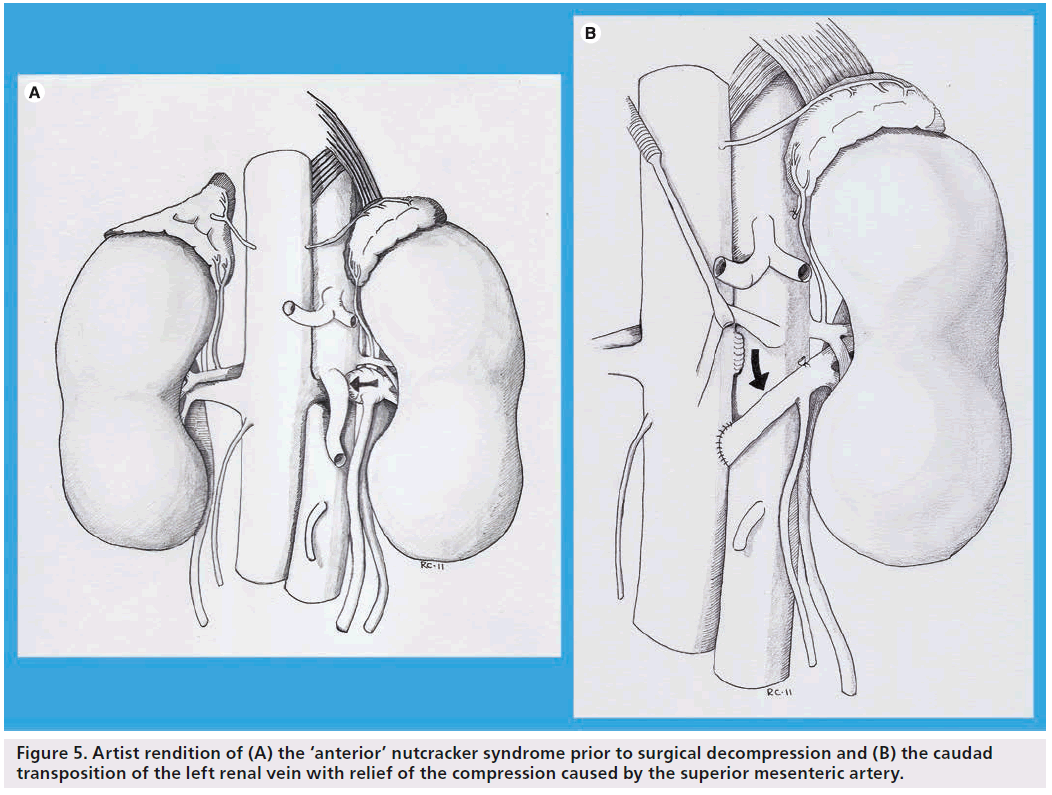
The earliest report of surgical intervention to relieve LRV entrapment (‘anterior nutcracker syndrome’) was in 1974 and described extensive venolysis to successfully free a constricted LRV [7]. Since that initial description, a variety of approaches have been utilized and modified over time to address different aspects of this obstructive process. Early approaches included direct attempts at addressing the narrowed SMA angulation by way of transposing the SMA to an alternative origin [31]. Similarly, attempts were made at increasing the aortomesenteric angle by elevating the SMA with a prosthetic wedge to relieve the compression [32]. While these procedures have largely been abandoned due to attendant morbidity, the concept was later modified in the form of an external stent structure (ringed PTFE graft) placed around the LRV to alleviate the venous hypertension. This was initially described as an open surgical technique [33], but has more recently been accomplished in a laparoscopic fashion [18]. More radical open surgical options have included autotransplantation of the left kidney to the pelvis [34] and even a left nephrectomy [35]. Techniques more directly targeting surgical decompression of the hypertensive venous circuit include left renocaval [18] and gonadocaval bypass [36] and, more recently, a laparoscopic splenorenal bypass [37]. Although no prospectively randomized studies have been performed, the aggregate cumulative experience represented by case reports and retrospective series suggests that transposition of the LRV remains the most common approach and has very good long-term results [24,34,38]. Technically, transposition involves a laparotomy and division of the LRV at its junction with the inferior vena cava (IVC). The renal vein is then transposed several centimeters caudad to create a neo-renocaval junction, thereby relieving compression by the SMA (Figure 5). This approach has been performed with low rates of morbidity even when performed through shorter incisions of <15 cm in length [24,34,37,39]. In a recent report by Reed et al. from the Mayo Clinic, symptoms of flank pain and hematuria resolved in >80% of patients treated with LRV transposition. Of note, the longest follow-up in this series was 12 years [24].
Figure 5: Artist rendition of (A) the ‘anterior’ nutcracker syndrome prior to surgical decompression and (B) the caudad transposition of the left renal vein with relief of the compression caused by the superior mesenteric artery.
Recently, reports have emerged detailing surgical management of retroaortic LRV compression (‘posterior nutcracker syndrome’). While the published operative experience to date is limited to <ten cases, the most common approach remains LRV transposition and creation of a neocaval junction in an ante-aortic location [6,34].
▪ Endovenous stenting
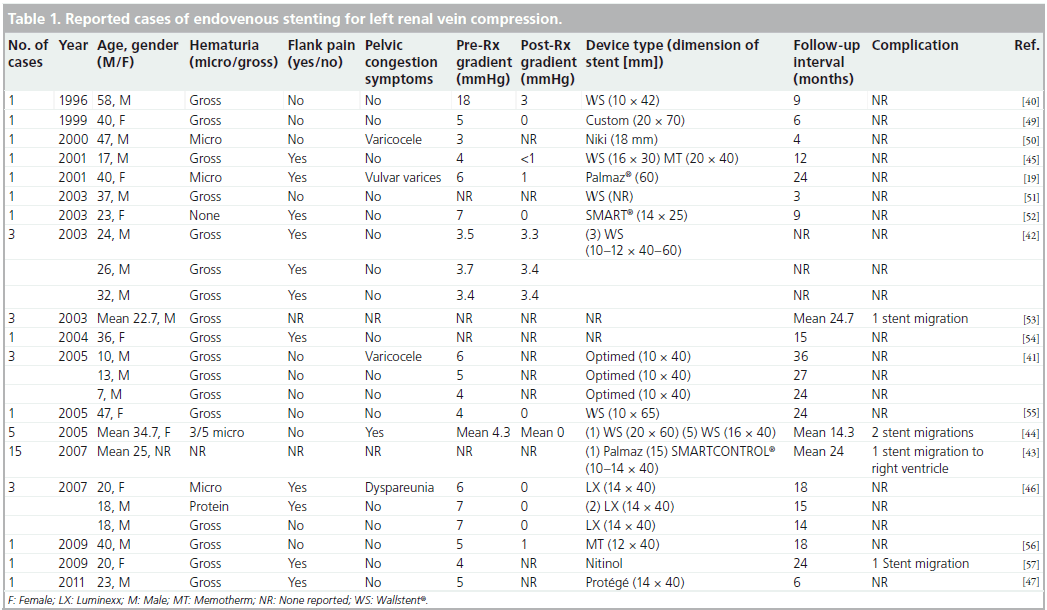
The first description of placement of an intravascular stent for LRV compression emerged in 1996 [40]. In a recent survey of the literature, 44 cases were identified, including three in young children [41]. The clinical details of all reported cases are included in Table 1. Overall, the most common presenting symptoms were hematuria and flank pain. Patient ages ranged from 7 to 58 years and the longest available follow-up period was 3 years. Most reports include detailed pre- and post-procedural renocaval gradient information, with normalization of the gradient after intervention seen in the majority. An exception to this pattern was a series reported by Zhang et al. in which symptom resolution was achieved despite the presence of a persistent gradient following stent deployment in all three patients treated [42].
A total of five cases of stent migration have been reported, including one traveling to the right ventricle and necessitating a thoracotomy [43]. Four others became lodged within the IVC at various levels, leading to re-stenting [42,44]. While symptom resolution was reported in the vast majority of patients, microhematuria did persist in some patients despite an adequate radiologic result and normalization of the pressure gradient [42].
▪ Technical considerations
General anesthesia may be the preferred approach in selected cases, as on occasion considerable patient discomfort may be experienced upon dilation of the renal vein [43]. Some authors, on the other hand, have indicated that use of newer nitinol stents may obviate the need for post-dilation of the stent, theoretically lowering the risk of stent migration and rendering the use of local anesthesia a more appealing option. The laterality of venous access appears less critical as successful reports exist with both left and right sided femoral vein access [41–43]. Chiesa and colleagues described a subclavian vein approach after failure of the device to track appropriately with femoral venous access [45]. On rare occasion, angulation of the LRV may preclude easy cannulation from a femoral approach and an upper extremity access may prove necessary. Concurrent arterial access with a catheter in the SMA has been described, but is not considered necessary [44]. Both pre-operative antiplatelet therapy administration and intra-procedural systemic heparinization are recommended.
As with most endovascular procedures, stent selection and sizing are important to achieving technical success. Zhang et al. report the largest series of endovenous stent placement for the LRV entrapment syndrome [43]. In their early experience, they used the stiff balloon-expandable Palmaz® stent (Cordis, Johnson & Johnson, USA), but subsequently favored the self expanding SMART® CONTROL® stent (Cordis, Johnson & Johnson, USA). They found the less rigid stent conformed well to the renal vein anatomy and that delivery proved more reliable. They did note that post-deployment shortening may limit stent visibility, which may be mitigated by using a slightly longer device (>40mm) [42]. Other authors have avoided the self-expanding metal Wallstent® (Boston Scientific, Natick MA, USA) for similar reasons [43,44].
In the absence of established stent sizing guidelines, sizing should be individualized to the encountered anatomy. Zhang et al. advocate 20% stent over-sizing relative to renal vein diameter on initial venography, and most commonly used a 14 mm diameter stent in their series [42]. In instances of stent migration, reporting authors typically upsized the subsequent replacement stent [42,43]. Some authors suggest that optimal stent placement will leave it flush with the ostium rather than protruding across the renocaval junction [46]. An exception to this was a recent report by Baril et al. in which an endovenous stent was utilized to treat a symptomatic recurrence following prior open LRV transposition. In that instance, the authors were specifically treating an anastomotic stenosis at the neo-renocaval junction and intentionally allowed the stent to protrude into the IVC to adequately traverse the stenotic segment. They also employed intravascular ultrasound to enhance localization of the stenosis [47]. As a final technical consideration to consider, if ovarian vein embolization is being considered, this may be best undertaken prior to renal vein stenting; doing so avoids the unnecessary potential challenge associated with crossing of the stent when accessing the ovarian vein.
While post-procedural intravenous heparin is sometimes utilized, most investigators believe antiplatelet therapy should be instituted prior to and following discharge [45]. No clear consensus exists on class and duration of a post-procedure medication regimen. Whether duel aspirin and plavix antiplatelet therapy versus solo treatment with either aspirin or plavix alone is preferable remains unclear. Notably, low rates of in-stent thrombosis exist in the reported series to date. This has been attributed by some to physiologic levels of urokinase present in the renal vein, which may confer intrinsic protection [48]. Post operatively, patients may be followed with interval duplex scanning to survey for in-stent stenosis that may potentially require secondary therapy.
Future perspective
The rarity of renal vein entrapment syndrome as it relates to symptomatic individuals is such that a prospectively designed trial evaluating treatment options will likely not be undertaken in the foreseeable future. Most agree that clear diagnostic criteria with regard to symptoms and appropriate radiographic findings should be present, in order to reliably make the diagnosis. Left renal venography demonstrating a pullback pressure gradient across the compressed segment of vein is a further key feature of the diagnostic standard. Venous duplex serves as an important adjunct as it represents a non-invasive way to serially monitor patients that forego intervention; at present it remains institution- and operator-dependent and may be adversely affected by individual patient body habitus.
In terms of treatment, experiential reports and small case series reflect a wide array of historical techniques, ranging from the potentially dangerous and now rarely utilized manipulations of the SMA to a growing experience with LRV transposition. Newer minimally invasive surgical techniques, such as laparoscopic extravascular stenting or laparoscopic bypass techniques, may have a future role, but remain largely untested. Most recently, endovascular stenting has been shown to be technically feasible and has emerged as an enticing option with acceptable short and mid-term results. The available literature on this therapeutic strategy, however, is currently limited to case reports and small series with limited long-term follow-up. The aggregate experience reviewed in this summary demonstrated that up to 11% of stents ultimately migrated from the LRV into the IVC and/or heart. To be fair, this figure presumably reflects the learning curve for clinicians exploring alternative treatment options for this rare condition.
In summary, while primary endovascular stenting of the LRV in an otherwise healthy young person with LRV entrapment syndrome is an acceptable treatment option, it remains of questionable long-term durability. It may be the preferred therapy, on the other hand, for patients with prohibitively hostile anatomic constraints (e.g., those with an abdominal stoma or a history of prior abdominal operations or intra-abdominal radiation) or in the setting of a symptomatic recurrence following prior open surgical repair.
Acknowledgements
The authors would like to acknowledge the artistic contributions by Ryan Cauley.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Clinical presentation
▪▪ The hallmark symptoms of nutcracker syndrome include left flank pain and hematuria (microscopic or gross). The possibility of concomitant pelvic congestion syndrome exists.
Diagnosis
▪▪ Axial imaging will demonstrate left renal vein compression/dilatation with a narrowed aortomesenteric angle.
▪▪ Venography will demonstrate a renocaval pullback gradient ≥3 mmHg and represents the current gold standard for diagnosis.
Treatment options
▪▪ Children and developing minors should be treated conservatively as it is usually a self-limited process.
▪▪ Adult patients with persistent symptoms may be candidates for repair.
▪▪ High-risk surgical patients are best treated expectantly.
Open versus endovascular management
▪▪ Open surgical reconstruction with left renal vein transposition is the standard open surgical approach based on available favorable 12-year follow-up data.
▪▪ Endovenous stenting remains the preferred approach for recurrent disease or patients at higher risk for open abdominal operations.
▪▪ Stent migration rates remain >10% in the available reported literature.
Endovenous stent technical considerations
▪▪ The anesthetic approach will be dictated by the clinical scenario and physician preference.
▪▪ Left or right femoral venous access is acceptable. Occasionally central or upper extremity venous access may be necessary for left renal vein cannulation.
▪▪ A self-expanding stent should be used, with stent postdilation only in necessary cases.
▪▪ A 14–16 mm stent diameter is the most commonly reported with desired 10–20% oversizing to the renal vein.
▪▪ Antiplatelet therapy is the mainstay of pharmacotherapy. Routine peri-procedure systemic heparinization is also recommended.
References
Papers of special note have been highlighted as:
▪ of interest
- Grant JCB. Methods of Anatomy. (Ed.). Williams & Wilkins. MD, USA (1937).
- El-Sadr AR, Mina E. Anatomical and surgical aspects in the operative management of varicocele. Urol. Cutaneous. Rev. 54, 257–262 (1950).
- Chait A, Matasar KW, Fabian CE et al. Vascular impressions on the ureters. Am. J. Roentgenol. Radiat. Ther. 111, 729–749 (1971).
- De Schepper A. ‘Nutcracker’ fenomeen van de vena renalis en veneuze pathologie van de linkder nier [in Belgian]. J. Belge. Radiol. 55,507–511 (1972).
- Lau JL, Lo R, Chan FL, Wong KK. The posterior “nutcracker”: hematuria secondary to Lau JL, Lo R, Chan FL, Wong KK. The posterior “nutcracker”: hematuria secondary to retroaortic left renal vein. Urology 28, 437–439 (1986).
- Marone EM, Psacharopulo D, Kahlberg A et al. Surgical treatment of posteriornutcracker syndrome. J. Vasc. Surg. 54(3), 844–847 (2011).
- Pastershank SP. Left renal vein obstruction by a superior mesenteric artery. J. Can. Assoc. Radiol. 25, 52–54 (1974).
- Buschi AJ, Harrison RB, Brenbridge ANAG et al. Distended left renal vein: CT/sonographic normal variant. AJR Am. J. Roentgenol. 135, 339–342 (1980).
- Zerin JM, Hernandez RJ, Sedman AB et al. ‘Dilatation’ of the left renal vein on computed tomography in children: a normal variant. Pediatr. Radiol. 21, 267–269 (1991).
- Mehta T, Wade RG, Clarke JMF. Is it safe to ligate the left renal vein during open abdominal aortic aneurysm repair? Ann. Vasc. Surg. 24, 758–761 (2010).
- Choi SH, Hwang HK, Kang CM et al. Potential use of left renal vein graft in pancreaticoduodenectomy combined with long segmental resection of the superior mesenteric–splenic–portal vein confluence. JOP 12, 234–240 (2011).
- Ohwada S, Hamada K, Kawate S et al. Left renal vein graft for vascular reconstruction in abdominal malignancy. World J. Surg. 31, 1215–1220 (2007).
- Venkatachalam S, Bumpus K, Kapadia SR et al. The nutcracker syndrome. Ann. Vasc. Surg. DOI: 10.1016/j.avsg.2011.01.002(2011) (Epub ahead of print).
- Hayashi M, Kume T, Nihira H. Abnormalities of renal venous system and unexplained renal hematuria. J. Urol. 124, 12–16 (1980).
- Beinart C, Sniderman KW, Saddekni S et al. Left renal vein hypertension: a cause of occult hematuria. Radiology 145, 647–650 (1982).
- Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo. Clin. Proc. 85, 552–559 (2010).
- Fitoz S, Ekim M, Ozcakar ZB et al. Nutcracker syndrome in children: the role of upright position examination and superior mesenteric artery angle measurement in the diagnosis. J. Ultrasound Med. 26, 573–580 (2007).
- Maleux G, Stckx L, Wilms G et al. Ovarian vein embolization for the treatment of pelvic congestion syndrome: long-term technical and clinical results. JVIR 11, 859–864 (2000).
- Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvic venous disorders. J. Vasc. Surg. 34, 812–819 (2001).
- Tanaka H, Waga S. Spontaneous remission of persistent severe hematuria in an adolescent with nutcracker syndrome: seven years’ observation. Clin. Exp. Nephrol. 8, 68–70 (2004).
- Shin JI, Park JM, Lee SM. Factors affecting spontaneous resolution of hematuria in childhood nutcracker syndrome. Pediatr. Nephrol. 20, 609–613 (2005).
- Arima M, Hosokawa S, Ogino T et al. Ultrasonographically demonstrated nutcracker phenomenon: alternative to angiography. Int. Urol. Nephrol. 22, 3–6 (1990).
- Cheon JE, Kim WS, Kim IO et al. Nutcracker syndrome in children with gross haematuria: Doppler sonographic evaluation of the left renal vein. Pediatr. Radiol. 36, 682–686 (2006).
- Reed NR, Kalra M, Bower TC et al. Left renal vein transposition for nutcracker syndrome. J. Vasc. Surg. 49, 386–393 (2009).
- Zerhouni EA, Siegelman SS, Walsh PC et al. Elevated pressure in the left renal vein in patients with varicocele: preliminary observations. J. Urol. 123, 512–513 (1980).
- Beinart C, Sniderman KW, Tamura S et al. Left renal vein to inferior vena cava pressure relationship in humans. J. Urol. 127, 1070–1071 (1982).
- Genc G, Ozkaya O, Bek K et al. A rare cause of recurrent hematuria in children: Nutcracker syndrome. J. Trop. Pediatr. 56, 275–277 (2010).
- Kim HS, Malhotra AD, Rowe PC et al. Embolotherapy for pelvic congestion syndrome: long-term results. JVIR 17, 289–297 (2006).
- Venbrux AC, Chang AH, Kim HS et al. Pelvic congestion syndrome (Pelvic Venous Incompetence): impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic pain. JVIR 13, 171–178 (2002).
- d’Archambeau O, Maes M, De Schepper AM. The pelvic congestion syndrome: role of the’nutcracker phenomenon’ and results of endovascular treatment. JBR-BTR 87, 1–8 (2004).
- Thompson PN, Darling RC, Chang BB et al. A case of nutcracker syndrome: treatment by mesoaortic transposition. J. Vasc. Surg. 16, 663–665 (1992).
- Ariyoshi A, Nagase K. Renal hematuria caused by ‘nutcracker’ phenomenon: a more logical surgical management. Urology 35, 168–170 (1990).
- Barnes RW, Fleisher HL, Redman JF et al. Mesoarotic compression of the left renal vein (the so-called nutcracker syndrome): repair by a new stenting procedure. J. Vasc. Surg. 8, 415–421 (1988).
- Ali-El-Dein B, Osman Y, Shebab El-Din AB et al. Anterior and posterior nutcrackersyndrome: a report on 11 cases. Transplant. Proc. 35, 851–853 (2003).
- Hohenfellner M, D’Ellia G, Hampel C et al. Transposition of the left renal vein for treatment of the nutcracker phenomenon: long-term follow-up. Urology 59, 354–357 (2002).
- Shaper KR, Jackson JE, Williams G. The nutcracker syndrome: an uncommon cause of haematuria. Br. J. Urol. 74, 144–146 (1994).
- Chung BK, Gill IS. Laparoscopic splenorenal venous bypass for nutcracker syndrome. J. Vasc. Surg. 49, 1319–1323 (2009).
- Stewart BH, Reiman G. Left renal venous hypertension ‘nutcracker’ syndrome. Managed by direct renocaval reimplantation. Urology 20, 365–369 (1982).
- Salehipour M, Khexri A, Rasekhi A et al. Left renal vein transposition for treatment of the nutcracker syndrome. Arch. Iran. Med. 9, 161–166 (2006).
- Neste MG, Narasimham DL, Belcher KK. Endovascular stent placement as a treatment for renal venous hypertension. J. Vasc. Interv. Radiol. 7, 859–861 (1996).
- Chen W, Chu J, Yang J et al. Endovascular stent placement for the treatment of nutcracker phenomenon in three pediatric patients. JVIR 16, 1529–1533 (2005).
- Zhang H, Shang N, Li M. Treatment of six cases of left renal nutcracker phenomenon: surgery and endografting. Chin. Med. J. 116, 1782–1784 (2003).
- Zhang H, Li M, Jin W, San P, Xu P, Pan S. The left renal entrapment syndrome: diagnosis and treatment. Ann. Vasc. Surg. 21, 198–203 (2007).
- Hartung O, Grisoli D, Boufi M et al. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. J. Vasc. Surg. 42, 275–280 (2005).
- Chiesa R, Anzuini A, Marone EM et al. Endovascular stenting for the nutcracker phenomenon. J. Endo. Ther. 8, 652–655 (2001).
- Basile A, Tsetis D, Calcara G et al. Percutaneous nitinol stent implantation in the treatment of nutcracker syndrome in young adults. JVIR 18, 1042–1046 (2007).
- Baril DT, Polanco P, Makaroun MS, Chaer RA. Endovascular management of recurrent stenosis following left renal vein transposition for the treatment of Nutcracker syndrome. J. Vasc. Surg. 53, 1100–1103 (2011).
- Hong SY, Yang DH, Him N. Urokinase concentration in renal artery and vein. Nephron 61, 176–180 (1992).
- Segawa N, Haruhito A, Iwamoto Y. Expandable metallic stent placement for nutcracker phenomenon. Urology 53, 631–633 (1999).
- Park YB, Lim SH, Ahn JH et al. Nutcracker syndrome: intravascular stenting approach. Nephrol. Dial. Transplant. 15, 99–101 (2000).
- Wei SM, Chen ZD, Zhou M. Intravenous stent placement for treatment of the nutcracker syndrome. J. Urol. 170, 1934–1935 (2003).
- Hosotani Y, Kiyomoto H, Fujioka N, Hohno M. The nutcracker phenomenon accompanied by rennin-dependent hypertension. Am. J. Med. 114, 617–618 (2003).
- Lin WQ, Huang HF, Li M et al. Diagnosis and therapy of the nutcracker phenomenon: long-term follow-up. Zhonghua Wai Ke Za Zi 41, 889–892 (2003).
- Van der Laan L, Vos JA, de Boer E, van der Berg JC, Moll FL. The central-venous compression syndrome: rare, but adequately treated with endovascular stenting. Ned. Tijdschr. Geneeskd. 148, 433–437 (2004).
- Kim SJ, Kim CW, Lee TH et al. Long-term follow-up after endovascular stent placement for treatment of nutcracker syndrome. JVIR 16, 428–431 (2005).
- Cohen F, Amabile P, Varoquaux A et al. Endovascular treatment of circumaortic nutcracker syndrome. JVIR 20, 1255–1257 (2009).
- Gagnon LO, Ponsot Y, Benko A, Tu le M. Nutcracker syndrome in a 20‑year-old patient treated with intravascular stent placement: a case report. Can. J. Urol. 16, 4765–4769 (2009).
▪ The term ‘nutcracker’ first used to describe anatomical relationship of left renal vein (LRV) compressed at the aortomesenteric angle.
▪ First description of surgical repair of LRV compression.
▪ Important study lending support that the radiographic finding of LRV compression is far more common.
▪ The most comprehensive report of left renal vein transposition with excellent technical results over a follow-up period of up to 12 years.
▪ The first reported case of endovenous stent placement for the LRV compression syndrome.
▪ The first reported case of endovenous stenting for recurrent disease following prior open surgical venous reconstruction.