Review Article - Interventional Cardiology (2012) Volume 4, Issue 4
Current role of chimneys and sandwich grafts in complex aneurysm repair
- Corresponding Author:
- Ralf R Kolvenbach
Vascular Centre Catholic Clinics & Augusta Hospital Dusseldorf, Germany
E-mail: kolvenbach@vkkd-kliniken.de
Abstract
Keywords
aortic arch, chimneys, juxtarenal aneurysms, periscopes, sandwich technique
Background
In most cases with infrarenal abdominal aortic aneurysms, endovascular aneurysm repair is now the treatment of choice. Previously, a sufficiently long infrarenal landing zone was mandatory, but gradually, more patients with challenging anatomy, short infrarenal necks of less than 1 cm and sever angulation were treated with commercially available endografts. Despite the introduction of new grafts with more active fixation, hostile neck can be a major obstacle to long-term success. Adjuncs, such as ballon-expandable stents, can only solve a minority of problems. The optimal approach to the suprarenal or juxtarenal aortic aneurysm, often with severely compromised proximal necks, remains controversial. While open repair is often still considered the gold standard for patients with hostile neck and/or juxtarenal aneurysms, in an aging population, surgery is suboptimal in many cases due to multiple comorbidities. Fenestrated grafts, although mostly outside the USA, can now be ordered from at least three different manufacturers. Only one of these is labeled as an ‘off-the-shelf device’, and all others are still custom made with a manufacturing time of at least 2 months, even if only one scallop is ordered. There is still only one manufacturer with a branched device, although this might change as well within the next 2 years.
As an alternative to open surgery, debranching procedures involving multiple bypasses to the renal arteries, or in combination with visceral artery bypasses, have been proposed as a minimally invasive option. Basically, this means open surgery, bypasses originating from the iliac artery to the renal vessels and, subsequently, deployment of a stent graft that is anchored in the juxtarenal aorta. Although the need for crossclamping of the aorta, with all the cardiac stress involved after clamping and declamping, is avoided, debranching procedures are associated with a full-length laparotomy, and although only short ischemic intervals are required, in our experience they are not substantially less invasive compared with open surgery with prolonged intensive care unit and hospital stay due to the extensive surgical trauma.
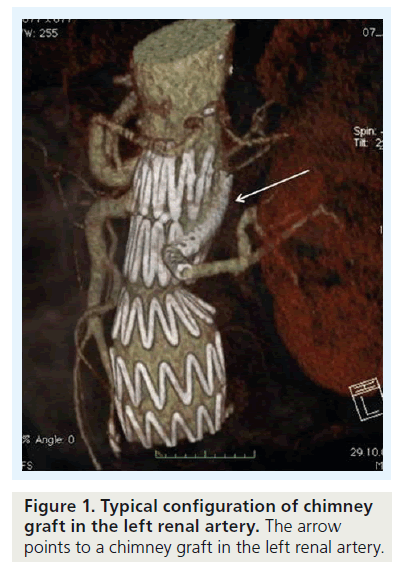
Parallel grafts have become an alternative treatment option in patients with juxtarenal or thoracoabdominal aortic aneurysms (Figure 1). Alternatively, custom-made devices or, when the patient is fit for surgery, a conventional procedure can be offered as first-line treatment. Custommade devices still require up to 2–3 months of manufacturing, which for symptomatic patients or in cases with ruptured aneurysms is too long. In these cases, parallel endografts are a readily available, off-the-shelf solution.
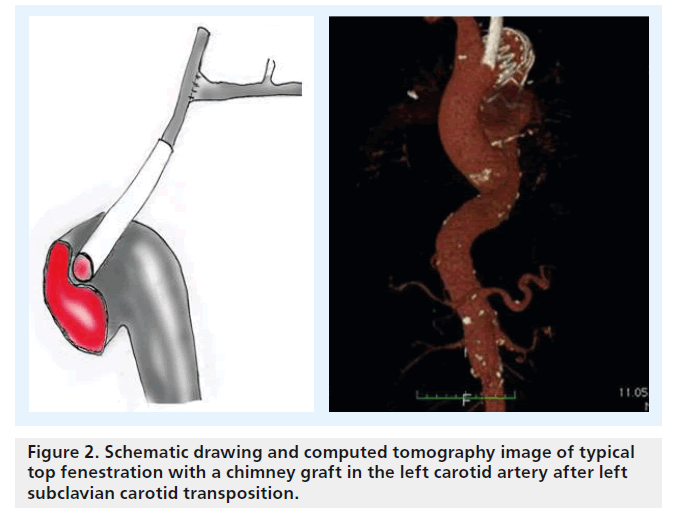
There is some confusion regarding terminology and configuration of these devices: the chimney graft, first described by Greenberg et al., is a bare-metal stent introduced from a transbrachial approach to secure inflow into the renal artery in patients with juxtarenal aneurysms (Figure 2) [1,2]. Alternatively, a covered stent can be used unilaterally or for both renal arteries for this top fenestrating technique (Figure 2), or a downward-facing chimney or periscope graft can be deployed from a transfemoral access route into the visceral and renal arteries or into the aortic arch [3]. A third technique that recently gained more popularity is the sandwich technique, in which the parallel graft runs in a gutter between two stent grafts.
Figure 2. Schematic drawing and computed tomography image of typical top fenestration with a chimney graft in the left carotid artery after left subclavian carotid transposition.
With all of the techniques discussed above, as with a custom-made fenestrated or branched graft, advanced catheter techniques are required.
Chimney technique
The chimney technique involves placement of bare-metal stents or covered grafts in parallel to the main aortic stent graft. In many cases, the space between the parallel stent, the aortic graft and the origin of the renal artery is only a few millimeters in length, resembling a top fenestration technique [4–7]. Originally advocated as a bailout procedure after accidentally covering the renal artery, these days this technique is used intentionally in juxtarenal aneurysms. Particularly in cases in which bare-metal stents have been used for selective engagement of the renal artery, the chimney technique can be very technically challenging because of wire entrapment inside the struts of the stents involved. In cases where only a top fenestration is required, a bare-metal stent can be the easiest solution for maintaining organ perfusion. In more complex cases we prefer covered balloon-expandable stents over bare-metal stents. Reinterventions should be easier when a covered stent is in place rather than a baremetal stent. The main disadvantage is the need for large-sheath placement into the brachial or subclavian artery.
Procedural details of chimney-graft deployment
Antegrade visceral access is obtained from open exposure of the subclavian, axillary or proximal brachial arteries, either separately or in combination, depending on their diameters and the number of planned chimney grafts [8,9]. In addition to the axillary access, exposure of both femoral arteries is obtained. The authors avoid placing more than one COOK flexor sheath (COOK Medical Inc. Bloomington, IN, USA) into one axillary artery. When two more chimneys are required, we either perform a cutdown of the contralateral axillary artery or use a downward-facing periscope graft that is deployed inside at least one renal artey. Alternatively, instead of performing a cutdown, percutaneous antegrade visceral/renal artery access can be obtained using a unilateral or bilateral brachial approach.
Systemic heparinization is initiated upon placement of the sheaths. Through the antegrade sheaths, the targeted renal and visceral branches are cannulated using 260-cm length hydrophilic guidewires and a 125-cm JB1 catheter (COOK Medical). Once cannulated, the sheaths are advanced coaxially into the target artery orifice. We prefer to exchange the soft hydrophilic guidewire for a 260-cm J-tip Rosen 8 wire (COOK Medical). If possible, we prefer to use ViaBahn (W. L. Gore Medical, AZ, USA) self-expanding covered stents that are advanced through the sheaths into the target branch vessel. If self-expanding covered stents are used, additional bare-metal balloon-expandable stents are placed within the ViaBahn to reinforce the snorkel stent. From the femoral access sites, the main body endograft is then advanced and positioned in the usual fashion [10,11].
Particularly in the USA, ‘home-made’ fenestrations are preferred by some surgeons due to the lack of availability of custom-made branched devices. In our experience, this can be accomplished with most aortic endografts as long as special care is taken to meticulously reload the grafts [12]. A combination of chimney grafts and a scallop can be combined in those cases where a graft with a scallop for the superior mesenteric artery and the coeliac trunk is used with two chimney grafts for the renal arteries.
Thoracoabdominal aneurysms: sandwich grafts
Mortality and morbidity of open thoraco-abdominal aortic aneurysm (TAAA) repair, in most centers, still exceeds 10%. According to the literature, there are very few centers that report mortality and morbidity rates that below the complication rates encountered in most vascular departments.
As an alternative to open surgery, or when custom-made grafts are not available, hybrid procedures can be used to avoid the cardiac stress induced by aortic cross clamping [13].
Custom-made fenestrations are primarily used in juxtarenal aneurysms or for a single visceral artery only. In most TAAAs, branched aortic endografts are used. Excellent results with fenestrated endografts have been reported. A systematic review showed perfusion of the targeted vessels in 97% of patients in the per operative period and 90% during late followup [14,15]. Branched grafts incorporating reinforced fenestrations, axial branches or helical branches have all been employed with a high rate of technical success and encouraging shortand intermediate-term results, and, moreover, can be used to treat all TAAA types. However, the same problems apply as with fenestrated grafts, limiting their applicability: they are not currently available commercially, they need to be customized to each patient, they are time consuming to manufacture, costs are more than considerable and there is a long learning curve to follow to achieve good results. Alternatively the Zurich group of Lachat et al. uses long self-expanding ViaBahn grafts, often in combination with partial debranching, to treat TAAA. When an all-chimney approach is too time consuming, easily accessible arteries are bypassed using an open technique in which ViaBahn grafts are directly inserted into the target artery in combination with chimney grafts [16,17].
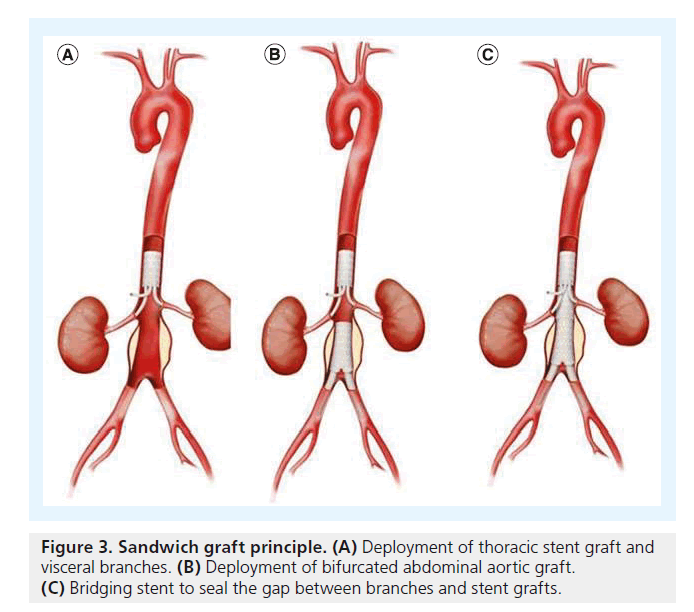
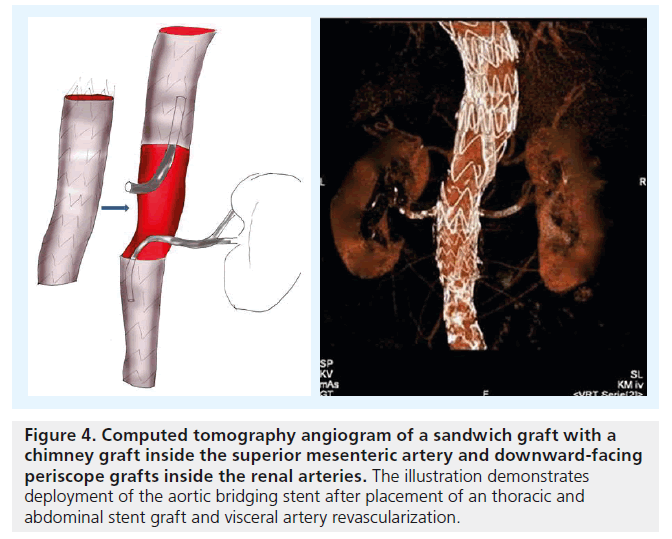
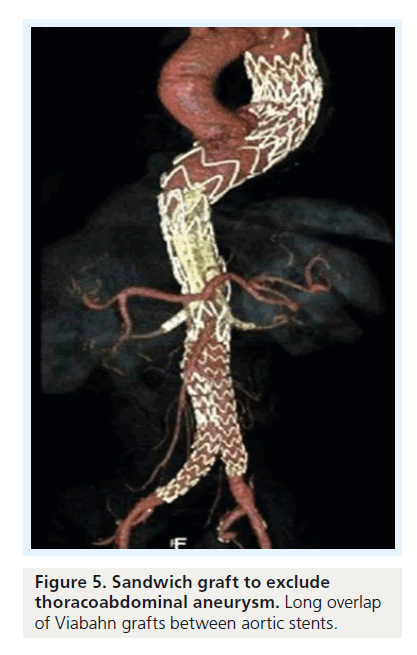
For TAAA cases, another concept has been proposed: the ‘stent-graft sandwich’ (Figure 3). This involves deploying one or more stent grafts inside a first one, which will already have been deployed [18]. Lobato et al. first described the technique to solve the short barrel chimney problem in type I, II and III TAAA, and longneck type IV TAAA, as well as long aortic dissections involving visceral arteries (Figure 4). We have now treated more than 30 patients with this technique, modifying the original by using downward-facing periscope grafts for the renal arteries (Figure 5) [19–21]. The sandwich technique can also be used in isolated iliac artery aneurysms, where a long branch is introduced into the internal iliac artery [22,23].
Figure 4. Computed tomography angiogram of a sandwich graft with a chimney graft inside the superior mesenteric artery and downward-facing periscope grafts inside the renal arteries. The illustration demonstrates deployment of the aortic bridging stent after placement of an thoracic and abdominal stent graft and visceral artery revascularization.
Only off-the-shelf stent grafts were used in these cases. The technique is similar to aneurysm exclusion with a branched stent graft. Insertion of the covered parallel grafts has to be performed using transbrachial or transaxillary access. A long overlapping zone of more than 5–7 cm between the chimney grafts and the aortic stent graft can be obtained when 10–15 cmlong parallel self-expanding grafts are used. It can be assumed that positioning the chimney graft between two ‘sandwich’ aortic stent grafts reduces any friction and movement of the grafts that would cause rapid thrombosis of the gutters between them [24].
This technique may be a valid alternative to a fenestrated stent graft in the emergency setting when a diseased segment is difficult to access owing to vessel tortuosity, or when there is no time to wait for a custom-made fenestrated or branched device. The prospect of long-term patency of these chimney and periscope grafts with a maximum length of 15 cm is unknown. Although mid- and short-term results are favorable, there is still a lack of long-term patency and durability data.
Especially in TAAA, an all-chimney approach coming from a transbrachial or axillary access is traumatic and time consuming because a cutdown for up to four visceral branches is required to accommodate an 8 French sheath. The periscope technique, at least for the renal arteries, permits a transfemoral access and, when logistically possible, a two-team approach.
The technical skills required are no less demanding than for any fenestrated or branched procedure. Complex endovascular interventions using chimney grafts and periscopes, for example, in combination with a sandwich technique, are rapidly gaining widespread popularity. In many institutions they are an alternative to custom-made branched devices, although are only reluctantly endorsed by major endograft manufacturers who, in many cases, are uncertain about the future role of these techniques. The clinical series published so far demonstrates acceptable short-term results that are not inferior to custom-made branched grafts.
Although some authors distinguish between a low-flow and high-flow type I endoleak, in our experience, in the aortic arch and in TAAA, this is directly related to the overlapping zone between aortic stent grafts and chimney grafts or periscopes. In most cases, a long self-expanding branch of 10–15 cm in length causes effective sealing of the gutters.
Sandwich grafts in combination with either chimneys or periscope grafts permit deployment of an aortic stent graft in the suprarenal aortic segment. An infrarenal landing zone of less than 5 mm does not matter anymore when the sealing zone, like in a branched or fenestrated graft, is transpositioned to the suprarenal aortic segement.
Especially in sandwich grafts, the length of the periscope graft must be carefully planned before deploying the bridging stent. So far, the maximum length available is 15 cm, where at least 3–4 cm are inside the target vessel. Due to the often curved configuration, additional shortening must be considered when choosing the length of the aortic stent.
The long-term performance of downwardfacing renal periscope grafts is still unknown. It can be argued that these function in a similar way to an ileorenal bypass in an abdominal debranching procedure, where retrograde flow is sufficient to maintain organ perfusion, but in these cases, an 8-mm polytetrafluoroethylene or Dacron graft is used in contrast to the smaller 6-mm endograft used in the periscope cases. In our experience, with more than 18 downward-facing periscope grafts having been deployed during the last 2 years, the patency rate of 90% is similar to that of chimney grafts and not significantly different to our hybrid debranching cases.
Caution with regard to indication in juxtarenal or thoracoabdominal aneurysms is still required unless these techniques are used in symptomatic cases or in ruptured aneurysms. There is still a lack of long-term performance data concerning parallel grafts. Friction causing micro- or macro-motion on the fabric of all grafts can theoretically cause tearing of the fabric and graft erosion. We do not know yet whether self-expanding covered chimney grafts should routinely be reinforced with balloon-expandable grafts to avoid kinking and vice versa.
In contrast to a branched graft where, particularly in large aneurysms, the branches ‘are free floating’ in the sac of the aneurysm, influenced by the cardiac cycle as well as respiratory movements, the sandwich technique in which the parallel grafts are in a fixed position between two aortic stent grafts should permit a more stable position of the co-axial grafts.
Intraoperative quality control of the various chimney grafts is essential: wires in the parallel self-expanding grafts stay in place until the end of the procedure. This permits selective angiography of all branches and, if necessary, pressure measurements. In those cases where there is a kink of the graft, reinforcement with a selfexpanding stent can be performed to improve long-term patency. In addition, platelet inhibition with aspirin and clopidrogel should be performed for at least 3 months.
Aortic arch
Aortic arch replacement requires cardio-pulmonary bypass and deep hypothermia. In spite of all technical improvements it is still a demanding cardiac procedure with significant morbidity and mortality The side effects of deep hypothermia, especially in elderly patients with various co-morbidities, often prolong intensive care unit and hospital stay. Alternatively lessinvasive procedures such as endovascular aneurysm exclusion with or without debranching have become viable alternatives.
Currently, endovascular treatment of aneurysms involving the aortic arch most often utilizes a hybrid approach, with surgical debranching through cervical or sternotomy exposures.
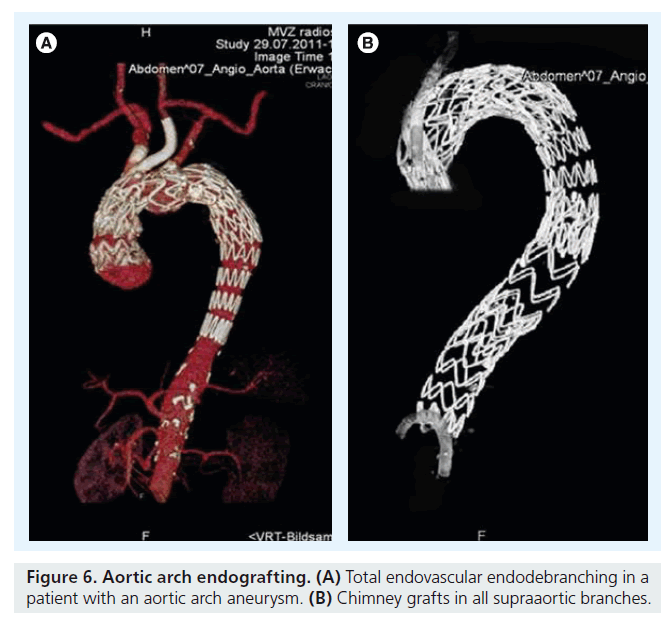
Partial or total endovascular debranching of the aortic arch using parallel grafts for all patent supra-aortic vessels may extend and simplify the application of endovascular techniques for the treatment of aneurysms involving the aortic arch. It is an alternative minimally invasive option for aortic arch ‘endodebranching’ that can replace cervical transpositions or bypasses originating from the ascending aorta to one or more branches via a median sternotomy. This endovascular technique can be combined with standard off-the-shelf stent grafts to treat lesions with inadequate fixation zones in the aortic arch and proximal ascending thoracic aneurysms. When the patient is unfit for open surgery, proximal extensions combined with parallel grafts in the left common carotid artery and, if necessary, in the innominate artery are required. In these patients, the parallel grafts extend far into zone 0 of the aortic arch (Figure 6). The more proximal grafts are deployed inside the acsending aorta in proximity to the sinu–tubular junction; catheter skills are very important that permit passage of the endograft into the left ventricle across the aortic valve [25].
Technical details of aortic arch endodebranching
ViaBahn grafts 10–15 cm in length are placed into the aortic arch until the tip of the grafts reach the ascending aorta. Depending on the extent of the aneurysm, the tip of the ViaBahn graft is placed in proximity to the sinu–tubular junction and the aortic valve following coronary angiography. If necessary, wires are placed into the coronary ostia. Repeated angiography and transesophageal echocardiography exclude obstruction of the coronary arteries or the aortic valve. Dedicated stent grafts exclusively for the ascending aorta should be used to avoid obstruction of the aortic valve by the bare springs of the endograft.
Before deployment of the parallel grafts, the aortic endograft is advanced transfemorally into the aortic arch. If necessary, the 0.035-Lunderquist guide wire is placed through the aortic valve into the left ventricle. The aortic stent graft is deployed first and, subsequently, so are the parallel grafts. Re-enforcement of the parallel grafts with self-expanding wall stents should be performed at the crossing of the parallel graft into the aortic arch. After deployment of the aortic stent graft and the chimney grafts, ballooning of the chimney grafts should be performed [26].
Challenges in the aortic arch
Single- and double-barrel chimney grafts have been used with increasing frequency, not only in our institution. Mid- and short-term results seem to be promising, although we do not have any long-term patency and durability data, and the data we do have are mostly from case reports that only include a few patients [27]. As described earlier, dislodgement of thrombus material can be a major problem in these patients with a heavily diseased aortic arch. In patients with an increased risk of cerebral embolization, clamping of the carotid artery and cerebral perfusion with femorocarotid bypass, an oxygenator and a roller pump is our preferred technique. This might change in the future with the introduction of novel filter devices, originally designed to prevent embolization during transfemoral aortic valve repair.
We know from our experience with chimney grafts in the abdominal aorta that type I endoleaks can only be avoided when the graft runs in parallel to the endoprosthesis over a long distance of at least 7 cm.
Although only off-the-shelf grafts were used, the technique described is still demanding and can only be performed under general anesthesia. Both common carotid arteries and, bilaterally, the axillary artery must be exposed.
Alternative techniques, such as in situ fenestration and deployment of fenestrated grafts or branched grafts, have been described [27]. Alignment of fenestrated or branched grafts requires, especially in patients with a gothic arch and a curved descending aorta, excellent maneuverability to get apposition of the branches or fenestrations adjacent to the origin of the cerebral vessels. The future will present new products with smaller profiles and diameter- reducing ties.
Owing to the short follow-up period, we do not have any data regarding possible side effects such as corrosion at the site of the interface between the parallel graft and the aortic endograft [28–30].
Current studies
There are only a few reports describing midterm results of parallel grafting and none with long-term results. Bruen et al. published one of the first publications with a larger series of patients – they published results in 21 patients with juxtarenal aortic aneurysms. The outcome reported, including 30-day mortality, was identical to a matched group of patients with open repair. There was one asymptomatic superior mesenteric artery stent occlusion and partial compression of a second superior mesenteric artery stent requiring balloon angioplasty. Primary patency was 84% at 12 months with no type I endoleak [11]. In another larger series, Donas et al. compared their results with balloon-expandable chimney grafts with the results of the Zurich group, who had used selfexpanding ViaBahn grafts in all their cases [31]. In the first center, 37 patients were treated in contrast to 35 in the second group. There was no difference with regard to the stent used. The success rate for target vessel preservation was 97% for the balloon-expandable and 100% for the self-expanding stent group. There were five perioperative low-flow type Ia endoleaks in the self-expanding group but only one of them was persistent after 1 year and required treatment with a cuff. Mortality was 0% in both groups. Of note is the authors’ conclusion that anything more than two chimney grafts can cause leakage and will weaken the proximal seal [31,32]. There are anecdotal reports with good results after filling the gutters between the chimneys with liquid embolization material such as Onyx® (ev3 Neurovascular, CA, USA).
Correct sizing of the aortic stent graft and the chimneys is another issue that merits discussion: Lachat suggested an elliptic model to calculate the appropriate stent-graft diameter [31]. According to this model, the stent-graft diameter for a two-chimney procedure should be equal to the circumference of an ellipse, with the major diameter (A) equal to the sum of the aortic diameter plus the chimney-grafts diameters, and the minor diameter (B) equal to the aortic diameter (A + B)/2. Another way and even simpler method is to oversize the graft by 20–30% [33].
The long-term durability of chimney grafts is unclear. We can assume that they will perform similarly to most branched or fenestrated devices.
Theoretically, the chimney graft compromises sealing of the aortic endograft, creating a gutter between the aortic graft and the vessel wall. In the long term, this could be an advantage of sandwich grafts where the chimney is in direct contact with an endograft, eliminating the gutter between the aortic wall and the abdominal or thoracic stent graft.
Any compromise in radial force of the aortic graft is avoided. The whole concept of chimney grafts, particularly to exclude juxtarenal aneurysms, depends on thrombosis of the gutters between the parallel grafts and the aortic stent graft. The importance of long gutters and chimneys must be emphasized since this is probably one of the most essential factors that promote sealing [34].
Conclusion
Endovascular repair in the setting of adverse anatomy has been the focus of much research over the past decade and is an evolving field. An off-the-shelf aortic stent graft for the exclusion of juxtarenal aneurysms is currently being tested in a clinical trial. A branched endograft that can be used in two-thirds of patients with thoracoabdominal aneurysms is on the horizon, although its introduction into the market is still unclear [35,36].
This review outlines alternative techniques for complex aneurysm repair. Although they are technically feasible with low morbidity and mortality, robust data supporting their use are lacking. Data regarding long-term durability and target-vessel patency are still to be determined. Challenges, particularly in the aortic arch and in TAAAs, currently make parallel grafts an attractive alternative to other endovascular techniques or open surgical procedures [37].
Executive summary
▪ Parallel grafts or snorkels can be used electively to enlarge the proximal landing zone in juxtarenal aneurysms. A chimney graft can either run in parallel to the aortic stent graft or as periscope configuration with retrograde perfusion of the target organ.
▪ In thoracoabdominal aortic aneurysms sandwich grafts are preferred: in these cases the chimney graft is placed in between two aortic stent grafts which enhances stabilization of the branches. This is particularly important in the arch with its high velocity and shear stress. Though the proportion of type I leaks due to the gutters in between the grafts is probably higher compared to a fenestrated or branched custom made device, in almost all cases these seal rapidly without the need for further interventions.
▪ The verdict is still open whether these techniques are only temporary or whether they will be part of the endovascular armamentarium in aortic endografting. The major problem in complex aortic endograft exclusion is still the lack of off the shelf grafts. Custom-made grafts are still associated with significant costs and prolonged manufacturing times. So far chimney techniques are the only way to treat emergency cases with currently available grafts. Off-the-shelf grafts with either fenestrations or branches are on the horizon but it will still take some time until they will be introduced into the market more generally. Until then chimney techniques are here to stay, particularly in emergency and ruptured cases.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Greenberg RK, Clair D, Srivastava S et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J. Vasc. Surg. 38, 990–996 (2003).
- Greenberg RK, Sternbergh WC III, Makaroun M et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J. Vasc. Surg. 50, 730–737 (2009).
- Greenberg R, Eagleton M, Mastracci T. Branched endografts for thoracoabdominal. aneurysms. J. Thorac. Cardiovasc. Surg. 140(Suppl. 6), S171–S178 (2010).
- Ohrlander T, Sonesson B, Ivancev K, Resch T, Dia N, Malina M. The chimney graft: a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J. Endovasc. Ther. 15, 427–432 (2008).
- Criado FJ. A percutaneous technique for preservation of arch branch patency during thoracic endovascular aortic repair (TEVAR): retrograde catheterization and stenting. J. Endovasc. Ther. 14, 54–58 (2007).
- Ricci C, Ceccherini C, Leonini S et al. Double renal chimney graft using only femoral approach. J. Cardiovasc. Surg. (Torino) 52, 93–97 (2011).
- Larzon T, Eliasson K, Gruber G. Top-fenestrating technique in stent grafting of aortic diseases with mid-term follow-up. J. Cardiovasc. Surg. (Torino) 49, 317–322 (2008).
- Allaqaband S, Jan MF, Bajwa T. The chimney graft – a simple technique for endovascular repair of complex juxtarenal abdominal aortic aneurysms in no-option patients. Cath. Cardiovasc. Interv. 75, 1111–1115 (2010).
- Hiramoto JS, Chang CK, Reilly LM, Schnieder DB, Rapp JH, Chuter TAM. Outcome of renal stenting for renal artery coverage during endovascular aneurysm repair. J. Vasc. Surg. 49, 1100–1106 (2009).
- Criado FJ, McKendrick C, Criado FR. Technical solutions for common problems in TEVAR: managing access and aortic branches. J. Endovasc. Ther. 16(Suppl. 1), I63–I79 (2009).
- Bruen KJ, Feezor JR, Daniels JM, Beck WA, Lee WA. Endovascular chimney technique versus open repair of juxtarenal and suprarenal aneurysms. J. Vasc. Surg. 53(4), 895–904 (2011).
- Oderich GS, Ricotta JJ. Modified fenestrated stent grafts: device design, modifications, implantation, and current applications. Perspect. Vasc. Surg. Endovasc. Ther. 21, 157–167 (2009).
- Quinones-Baldrich W, Jimenez JC, DeRubertis B, Moore WS. Combined endovascular and surgical approach (CESA) to thoracoabdominal aortic pathology: a 10-year experience. J. Vasc. Surg. 49, 1125–1134 (2009).
- Haulon S, Amiot S, Magnan PE et al. Association Universitaire de Recherche en Chirurgie vasculaire (AURC).
- An analysis of the French multicenter experience of fenestrated aortic endografts: medium-term outcomes. Ann. Surg. 251, 357–362 (2010).
- Verhoeven EL, Vourliotakis G, Bos WT et al. Fenestrated stent grafting for short-necked and juxtarenal abdominal aortic aneurysm: an 8-year single-centre experience. Eur. J. Vasc. Endovasc. Surg. 39, 529–536 (2010).
- Lachat M, Frauenfelder T, Mayer D et al. Complete endovascular renal and visceral artery revascularization and exclusion of a ruptured type IV thoracoabdominal aortic aneurysm. J. Endovasc. Ther. 17, 216–220 (2010).
- Pecoraro F, Pfammatter T, Mayer D et al. Multiple periscope and chimney grafts to treat ruptured thoracoabdominal and pararenal aortic aneurysms with short distal necks. J. Vasc. Surg. 51, 1293–1296 (2011).
- Lobato A. Introducing the ‘sandwich’ technique as an alternative in the treatment of TAAAs. Presented at: First International Course in Vascular Surgery. Gramado, Brazil, 12 May 2011. 19 Ketelsen D, Kalender G, Heuschmid M et al. Endovascular aneurysm repair using a reverse chimney technique in a patient with Marfan syndrome and contained ruptured chronic type B dissection. Cardiovasc. Interv. Radiol. 34, 1080–1084 (2011).
- Richardson S, Popori RK, Pichel AC, Farquharson F, Serracino-Inglott F. A ruptured thoracoabdominal aortic aneurysm managed endovascularly using the telescoping chimney technique. Ann. Vasc. Surg. 25(3), 384.e1–e4 (2011).
- Schlosser FJ, Aruny JE, Freiburg CB, Mojibian HR, Sumpio BE, Muhs BE. The chimney procedure is an emergently available endovascular solution for visceral aortic aneurysm rupture. J. Vasc. Surg. 53, 1386–1390 (2011).
- Yoshida Rde A, Yoshida WB, Kolvenbach R, Vieira PR. Modified ‘stent-graft sandwich’ technique for treatment of isolated common iliac artery aneurysm in patient with Marfan syndrome. Ann. Vasc. Surg. 26(3), 419.e7–e9 (2012).
- Yoshida Rde A, Yoshida WB, Kolvenbach R, Pinter L, Vieira PR. Retrograde endovascular treatment of internal iliac aneurysm in a patient with Marfan syndrome. Vascular 18(4), 235–241(2010).
- Kolvenbach RR, Yoshida R, Pinter L, Zhu Y, Lin F. Urgent endovascular treatment of thoraco-abdominal aneurysms using a sandwich technique and chimney grafts – a technical description. Eur. J. Vasc. Endovasc. Surg. 41(1), 54–60 (2011).
- Kolvenbach RR, Karmeli R, Pinter LS et al. Endovascular management of ascending aortic pathology. J. Vasc. Surg. 53(5), 1431–1437 (2011).
- Yoshida RA, Kolvenbach R, Yoshida WB, Wassijew S, Schwierz E, Lin F. Total endovascular debranching of the aortic arch. Eur. J. Vasc. Endovasc. Surg. 23 627–630 (2011).
- Sonesson B, Resch T, Allers M, Malina M. Endovascular total aortic arch replacement by in situ stent graft fenestration technique. J. Vasc. Surg. 49, 1589–1591 (2009).
- Gehringhoff B, Torsello G, Pitoulias GA, Austermann M, Donas KP. Performance of chimney grafts in aortic arch pathologies involving the supraaortic branches. J. Endovasc. Ther. 18(5), 650–655 (2011).
- Baldwin ZK, Chuter TAM, Hiramoto JS, Reilly LM, Schneider DB. Double-barrel technique for endovascular exclusion of an aortic arch aneurysm without sternotomy. J. Endovasc. Ther. 15, 161–165 (2008).
- Criado FJ. A percutaneous technique for preservation of arch branch patency during thoracic endovascular aortic repair (TEVAR): retrograde catheterization and stenting. J. Endovasc. Ther. 14, 54–58 (2007).
- Donas KP, Pecoraro F, Torsello G et al. Use of covered chimney stents for pararenal aortic pathologies is safe and feasible with excellent patency and low incidence of endoleaks. J. Vasc. Surg. 55, 659–665 (2012).
- Donas KP, Torsello G, Austermann M et al. Use of abdominal chimney-grafts is feasible and safe: short-term results. J. Endovasc. Ther. 17, 589–593 (2010).
- Coscas R, Kobeiter H, Desgranges P, Becquemin JP. Technical aspects, current indications, and results of chimney graft for juxtarenal aortic aneurysms. J. Vasc. Surg. 53, 1520–1527 (2011).
- Bakoyiannis CN, Economopoulos KP, Georgopoulos S et al. Fenestrated and branched endografts for the treatment of thoracoabdominal aortic aneurysms: a systematic review. J. Endovasc. Ther. 17, 201–209 (2010).
- Nordon IM, Hinchliffe RJ, Manning B et al. Toward an ‘off-the-shelf ’ fenestrated endograft for management of short-necked abdominal aortic aneurysms: an analysis of current graft morphological diversity. J. Endovasc. Ther. 17, 78–85 (2010).
- Park KH, Hiramoto JS, Reilly LM, Sweet M, Chuter TA. Variation in the shape and length of the branches of a thoracoabdominal aortic stent graft: implications for the role of standard off-the-shelf components. J. Vasc. Surg. 51, 572–576 (2010).
- Kolvenbach R. The role of periscopes and chimneys in complex aneurysm cases. J. Endovasc. Ther. 18(5), 661–665 (2011).