Review Article - Interventional Cardiology (2022) Volume 14, Issue 0
Current status and future directions of Robotic PCI: A review
- Corresponding Author:
- Eric Wyffels
Cardiovascular Center Aalst, OLV-Clinic, Aalst, Belgium, E-mail: eric.wyffels@olvz-aalst.be
Received date: 30-Nov-2022, Manuscript No. FMIC-22- 81803; Editor assigned: 02-Dec-2022, PreQC No. FMIC-22- 81803 (PQ); Reviewed date: 16-Dec-2022, QC No. FMIC-22- 81803;Revised date: 23-Dec-2022, Manuscript No. FMIC-22- 81803 (R);Published date: 03-Jan-2023, DOI: 10.37532/1755-5310.2022.14(S14). 319
Abstract
The last 35 years robotic systems were introduced in the medical field with an increasing recognition of their possible benefits for both patients and physicians. Robotic Percutaneous Coronary Intervention (R-PCI) is an approach where the operator manipulates guidewires and catheter devices remotely from a radiation-shielded cockpit. Trials evaluating R-PCI demonstrated high technical and procedural success with low complication rate.
R-PCI provides several advantages over conventional manually-performed PCI, in terms of reduction in radiation exposure and occupational hazards, enhanced procedural precision and the possibility of performing remote tele-stenting procedures.
While significant improvements have been done in the technical capabilities of R-PCI during the last years there are still several limitations that we have to overcome as this technology continues to evolve.
This review examines the current role and applications of R-PCI in the catheterization laboratory and the potential to impact future practice.
Keywords
Percutaneous Coronary Interventions (PCI) • Robotics • Artificial intelligence • Automations • Training
Abbreviations
NASA: National Aeronautics and Space Administration; DARPA: Defense Advanced Research Projects Agency; CABG: Coronary Artery Bypass Grafting; R-PCI: Robotic Assisted Percutaneous Coronary Intervention; AI: Artificial Intelligence; RNS: Remote Navigation System; PRECISE study: Percutaneous Robotically-Enhanced Coronary Intervention study; CORA-PCI trial: Complex Robotically Assisted Percutaneous Coronary Intervention trial; LGM: Longitudinal Geographic Miss
Introduction
The last 35 years robotic systems were introduced in the medical field with an increasing recognition of their possible benefits for both patients and physicians. The application of robots in medical procedures originates from the need that mainly two goals are fulfilled, the telepresence of the operator and the performance of repetitive and accurate tasks. Interestingly, the first surgical robot commercially available was born from a joint of the Defense Advanced Research Projects Agency (DARPA) and the National Aeronautics and Space Administration (NASA) project in 1980s as an effort to develop a “telesurgical” robot capable of performing surgery remotely on the battlefield or in other remote environments [1]. The first robotic assisted procedure in medicine was performed in 1985 when the PUMA 200 robotic device performed what is considered to be the first application of a robot in surgery at Memorial Medical Center of Long Beach, precisely guiding the needle to its destination in a stereotactic brain biopsy [2]. Since then, robotic technologies have become popular in a range of surgical specialties [3,4]. The surgeon, located outside the operating room, manipulates the endoscopic system via the robot arms. Comparative studies of robotic and laparoscopic surgical procedures in general surgery have shown similar results with regard to perioperative, oncological, and functional outcomes [5,6]. Despite the lack of comparative studies with a head to head randomized set up this new technology has gained wide spread adoption in the surgical community. Since the introduction of minimally invasive cardiac surgery in 1995, the use of robotic systems has gained popularity [7,8]. The most common applications for robotics have been single and double vessel Coronary Artery Bypass Grafting (CABG), Mitral Valve (MV) replacement [9,10]. For the interventional cardiology community however we had to wait until 2006 when Beyar et al reported the first in human Robotic assisted Percutaneous Coronary Intervention (R-PCI). A novel approach where the operator manipulates guidewires and catheter devices remotely from a radiation-shielded cockpit [11]. Today R-PCI could represent the next paradigm shift in contemporary PCI practice enabling increased procedural accuracy, improving safety and ergonomics for the operator. The application of Artificial Intelligence (AI) technology, integrated multimodality imaging and roboticals could have a profound impact on procedural outcomes and patient journey. In this review we aim to give an overview of the history, current applications, limitations and future directions of roboticals in interventional cardiology.
Literature Review
History and available devices
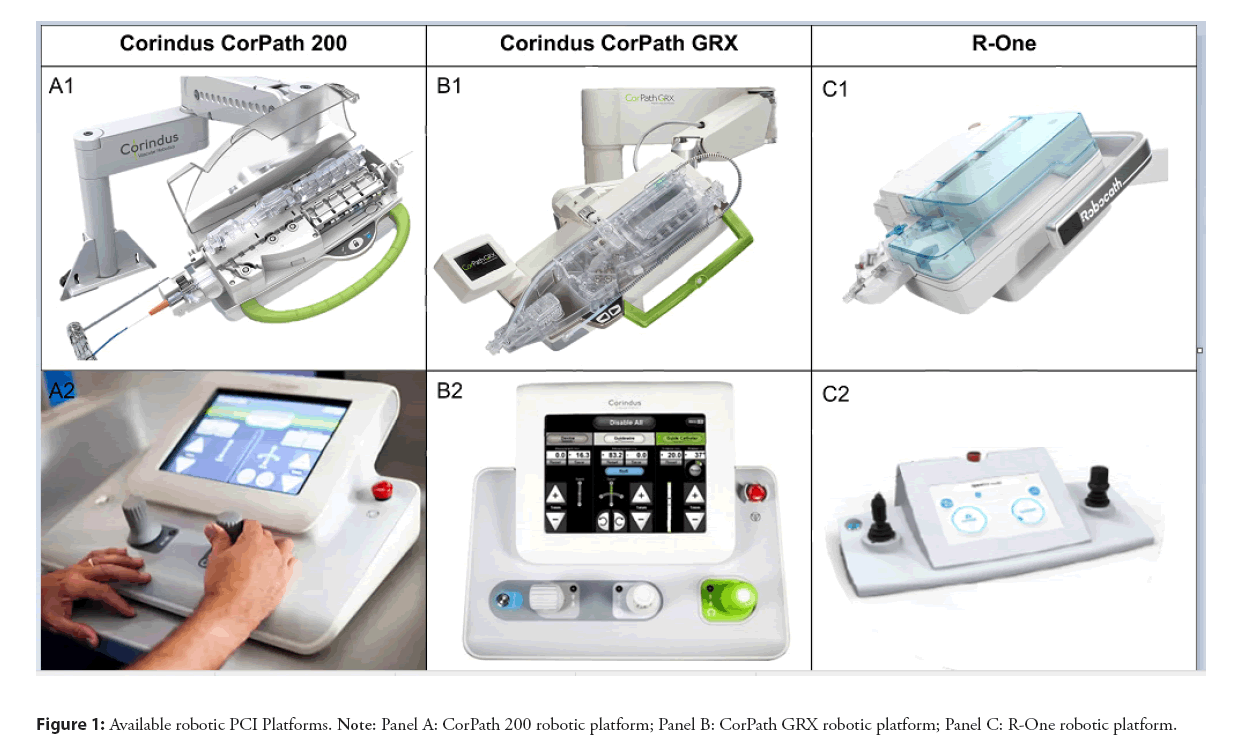
Since the first in human application of the method in 2006 there was a tremendous interest and advancements in R-PCI. The attention and exposure during major scientific meetings is only exemplary of this interest. The first device, the Remote Navigation System (RNS, NaviCath, Haifa, Israel) consisted of two main parts, the bedside unit and the operator control unit. The bedside unit included the motor base and the detachable wire and device navigators. The operator control unit was located away from the patient bed; the operator using a fully computerized system could remotely navigate angioplasty guidewires, balloons, and stents [11]. Based on this prototype different robotic platforms became commercially available and have now received regulatory approval and are available for use in clinical practice. First device that received FDA approval in 2012 and became commercially available was CorPath 200 system (Corindus Inc, Waltham, MA, USA). This device consisted of an articulating arm with a robotic drive and single-use cassette attached to the patient table. The physician was able to control the guidewire and PCI devices (eg balloons and stents) using joysticks from a radiation shielded Interventional Cockpit (Figure 1, A1 and A2). In January 2017, a second-generation model, CorPath GRX (Corindus) system was released. This device increased procedural control providing extra features. With the CorPath GRX, a third joystick was added to the operator control unit, enabling the robotic-assisted control of the guide catheters (Figure 1, B1 and B2). The second disruptive feature that the CorPath GRX provided is the technIQ Smart Procedural Automation. This a set of automated robotic movements designed especially for the CorPath GRX System. The automations mimic the manual techniques of highly skilled interventionalists to provide predictable and consistent movements that aid in advanced navigation, lesion crossing, and device manipulation during complex coronary. In the following years the CorPath GRX was used in peripheral interventions (FDA clearance). In 2020 FDA clearance was granted for neurovascular interventions. Today more than 10.000 robotic PCIs were performed and there are 150 programs worldwide. A second company (Robocath Inc., Rouen, France), introduced R-One in 2019 (received CE marking in February) as a device for robotic assisted coronary interventions (Figures 1, C1 and C2). This device is based on the same “philosophy” consisting of two main parts, a bedside unit and a control console but with similar features to the first model of the Corindus, the CorPath 200. The vast majority of robotical platforms that are being used are either the first or the second generation of Corindus and in this review we mainly refer on those devices shown in the Figure 1.
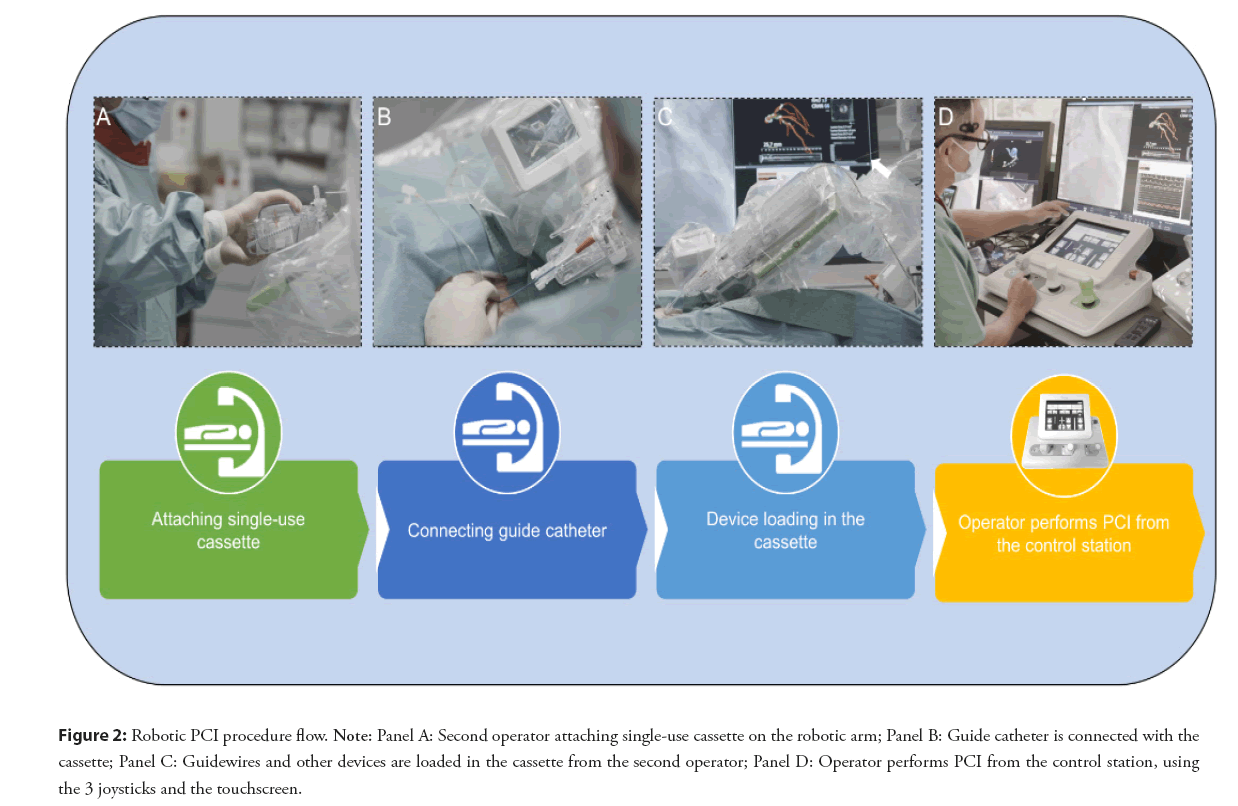
Figure 2: Robotic PCI procedure flow. Note: Panel A: Second operator attaching single-use cassette on the robotic arm; Panel B: Guide catheter is connected with the cassette; Panel C: Guidewires and other devices are loaded in the cassette from the second operator; Panel D: Operator performs PCI from the control station, using the 3 joysticks and the touchscreen.
Catheterization laboratory setup and procedural description
The installation of a robotic platform is feasible without important adjustments of the existing equipment in a catheterization laboratory. The robotic console consists of two parts, a bedside unit and a control station (the cockpit). The bedside unit consists of an articulating arm and a robotic drive shown in the Figure 2, which is operated by the interventionalist. Classically arterial access, diagnostic coronary angiography, and engagement of the guiding catheter are still performed by the traditional manual method. Some operators already perform the diagnostic part and engagement of the coronaries with robotic assistance. After the activation of the robotic platform a sterile single use cassette is attached on the robotic arm (Figures 2A-2C). This cassette is a device translating the Joystick movements to the wires, balloons and stents. The control station or cockpit is usually outside the cathlab, the operator has a visual contact with the tech or nursing team responsible for loading all equipment in the cassette. Headsets are used to communicate. Sometimes, usually in a transitory learning phase the cockpit can be placed inside the cathroom (radiation shielding is provided via an additional radioprotected cockpit). The control station allows the operator to control the guidewire and other devices remotely by using a touchscreen and different joysticks.
Establishing a R-PCI program and R-PCI training
Despite the fact that the learning curve for the use of the robot is previously reported to be relatively short, establishing a new R-PCI program can be a challenging process for both the interventional cardiologists and the nurses [12-14]. The role and responsibilities of both the operator and technician or assisting nurse do have to change. This necessitates training and good communication before and during the procedure. The interventional cardiologists will have to adopt to a change in their daily routine. Performing the procedure away from the patient, without close proximity is a big change for the physician. The loss of the tactile feedback during wiring and instrumentation is another important change. The operator has to learn to rely completely on the visual sign of interaction of the devices with the vessel wall. The pace and speed during a RPCI is set by the second operator, an important role change for most of first operators. The second operator (assisting nurse or technician) will have to emancipate in his role in the cathlab. The second operator during a R-PCI procedure has a key role, not only being alone in the room observing the vital signs and communicating with the patient but also being responsible to manipulate and load all the materials into the cassette. In this setting different team dynamics will occur. This new procedural flow in the catheterization laboratory can potentially discourage operators and nurses from participating in a newly established R-PCI program.
The training of a dedicated team (interventional cardiologists, nurses or fellows in training) is very important for the successful introduction of this new technology. Several training possibilities are now available. Dedicated workshops, simulators or onsite visits to centers of excellence in R-PCI are all possibilities. Robotic PCI suits perfectly to simulator training. The incorporation of simulator training with the use of special devices, 3D models or even animal labs seems a promising training possibility that can improve performance during clinical interventional cases.
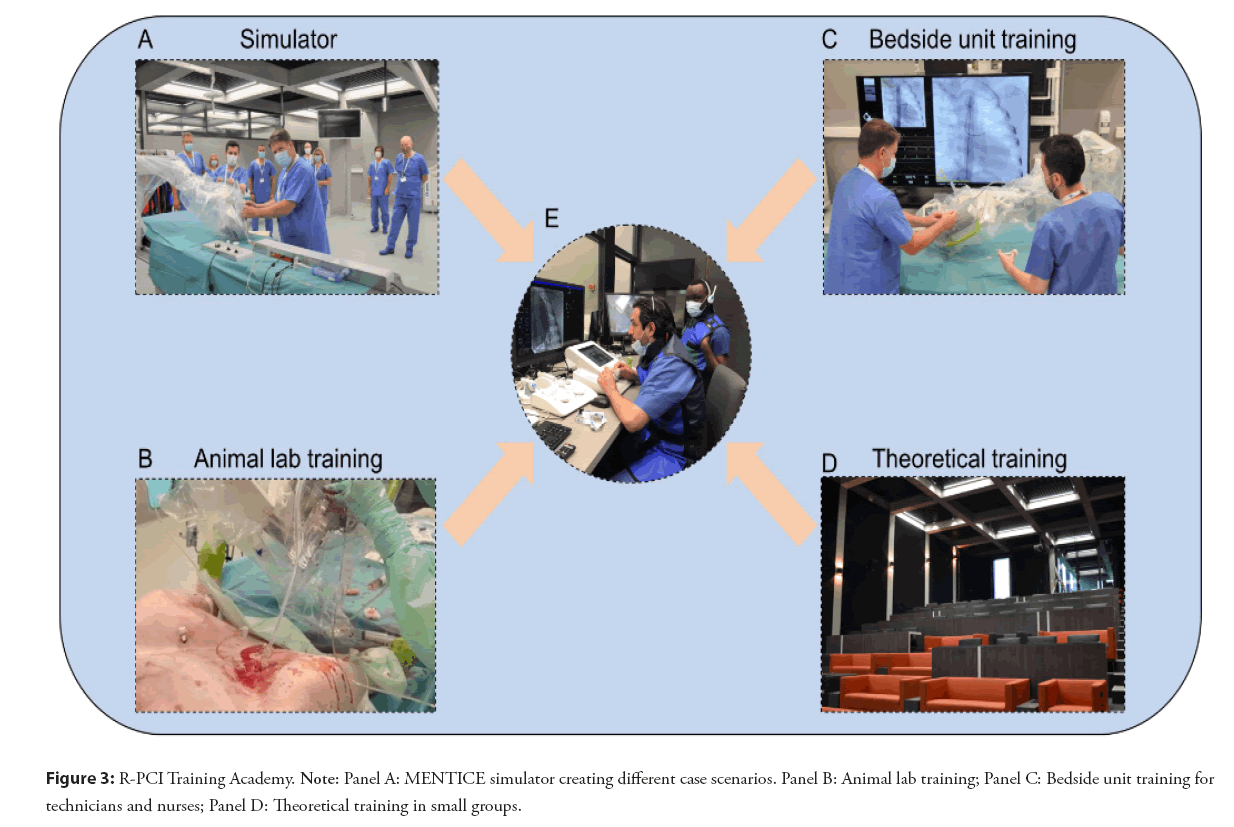
In 2021 Corindus made an important step in robotic PCI training by installing a robot in Orsi’s robotics center (Belgium) [15]. In this robotic academy one is trained using an ex vivo simulator (Mentice) and “in an animal” lab-experience. Both can be perfectly used for educational training in R-PCI creating different case scenarios and shortening the learning curve for the team. In that way small groups of physicians and other catheterization laboratory staff can gain hands-on experience (Figure 3), with CorPath GRX and technIQ™ Smart Procedural Automations.
Clinical evidence
Several studies performed the last 15 years confirmed the safety and efficacy of robotic assisted coronary interventions shown in the Table 1. Beyar et all published the first-in-human clinical trial where 17 of the 18 patients enrolled were successfully treated with a fully robotically RNS [16]. The CorePath 200 system was first used in 8 patients, where the feasibily of R-PCI was tested successfully [17]. The first large-scale multicenter study that evaluated safety, efficacy, and clinical effectiveness of this robotic system was the PRECISE (Percutaneous Robotically-Enhanced Coronary Intervention) study. This trial included 164 patients with low to moderate-risk lesions (68.2% of lesions were type A or B according to the ACC/AHA classification). The results showed a procedural success (device technical success) rate of 98.8%, with a conversion to Manual PCI (M-PCI) that occurred in only 2 patients. The clinical success rate was 97.6% with 4 reports of periprocedural MI, however without clinical consequences, stating the safety and efficacy of the CorePath 200 platform for the treatment of low to moderate-complexity lesions [18]. Following the PRECISE study, the first registry concerning R-PCI (PRECISION Registry), in which 273 patients and 344 lesions were included, reported a procedural success rate of 93.7 and 85.7% respectively for both radial and femoral access procedures [19]. An important step towards more complex lesions was the Complex Robotically Assisted Percutaneous Coronary Intervention (CORA-PCI) trial. CORA-PCI evaluated the use of R-PCI for the treatment of complex CAD. In this trial 108 patients undergoing R-PCI were compared to 226 undergoing M-PCI; all patients with moderate to severe-risk lesions (78,3% of lesions were ACC/AHA type B2/C in the R-PCI arm compared with 68,6% in the M-PCI). The procedural success rate for R-PCI was 91,7% with a total manual conversion required in 7,4%. The clinical success rate was comparable in both groups (99,1% for R-PCI vs. 99,1% for M-PCI; p=1.00) with similar 12 months clinical outcomes [20,21].
| Study | Study Design | R-PCI system | Patients | Primary Endpoints | Groups | Technical success | Clinical success |
|---|---|---|---|---|---|---|---|
| Beyar, et al. 2006 | Pilot Clinical Study | Remote Navigation System (RNS) | 18 | To evaluate safety and feasibility of a novel RNS | 94% Guide wire navigation 83% Overall procedure | 100% | |
| Granada, et al. 2011 | Single-arm, open-label, prospective | CorePath 200™ | 8 | Device clinical success (< 30% residual stenosis) without in-hospital MACE | 97.9% The robotic-system completed 47 of 48 planned steps |
100% | |
| PRECISE 2013 | Prospective, single-arm, multicenter, open-label, non-randomized study | CorePath 200™ | 164 | Clinical procedural success device technical success | 98.8% Conversion to M-PCI (n = 2) |
97.9% Periprocedural MI (n = 4) | |
| PRECISION Registry | Registry | CorePath 200™ | 273, 344 lesions | 93.70% | 85.70% | ||
| CORA-PCI-2017 | Non-randomized, single-center, comparison study | CorePath 200™ | 315 | Clinical success (successful PCI without MACE) | 108 R-PCI, 226 M-PCI | 91.7% R-PCI Manual assistance (11.1%) Manual conversion (7.4%) | 91.1% R-PCI 91.1% M-PCI |
| Smitson et al. 2018 | Prospective, single-arm, multicenter, open-label, non-randomized study | CorPath GRX | 40 | Clinical procedural success (<30% residual stenosis without in-hospital MACE) Device technical success (robotic procedural success without the need for unplanned manual conversion) | 90% Unable to advance overlapping stent (n = 1) Unable to cross lesion with guide wire (n = 1) Unable to cross lesion with balloon (n = 1) | 97.50% | |
| Hirai et al. 2020 | Retrospective | CorPath GRX | 95 | Procedure time Cockpit time | 46 Manual CTO-PCI 49 Robotic CTO-PCI | No difference in procedure time or MACE Higher cockpit time in robotic PCI | |
Note: RNS: Remote Navigation System; R-PCI: Robotic-Percutaneous Coronary Intervention; M-PCI: Manual-Percutaneous Coronary Intervention; MACE: Major Adverse Cardiovascular Events; CTO: Chronic Total Occlusion
Table 1: Studies reporting clinical and safety outcomes for robotic percutaneous coronary interventions.
Following the PRECISE and CORA-PCI trials, several cases reports showed the feasibility of R-PCI in complex scenarios, like unprotected LM lesions [22], (including cases requiring hemodynamic support as intra-aortic balloon pump or Impella 2.5 Heart Pump), patients with multivessel disease, saphenous vein grafts, and ST Elevation MI (STEMI) [23,24].
The introduction on the market of the second generation of Corindus robotic platform further enhanced R-PCI by incorporating new features like the guide catheter joystick and the available automations. In a cohort of 40 patients, this novel CorPath GRX system demonstrated procedural and clinical success rates of 90% and 97.5% respectively [25].
Since then several reports were published about the successful use of CorPath GRX with different devices that enable operators to deal with complex lesions and scenarios like laser atherectomy, intravascular lithotripsy and IVUS [26]. An interesting concept about the use of CCTA for planning (combining physiology and plaque characterization) and guiding (stent sizing and positioning) of robotic-assisted procedures was reported. This concept is perfect for R-PCI as it can eliminates the use of extra material that are not compatible with the existing robotic consoles (FFR wires, OCT catheters) [27,28]. Favorable results were also reported by Hirai et al and Walters et al with R-PCI for Chronic Total Occlusion (CTO) [29,30]. Recently, a new concept was described with the first report of totally robotically-assisted hybrid coronary artery revascularization combining RE-MIDCAB and R-PCI, highlighting how this hybrid technique, combining the advantages of surgical and percutaneous revascularization, both guided by robotic techniques, could lead to additional benefits in optimizing patient outcomes [31]. Most recently the report of the first in human Robotic-Assisted Renal Denervation proved the compatibility between the two devices (robotic platform and denervation catheter) and the feasibility of this procedure [32].
Advantages of R-PCI
The safety and efficacy of R-PCI has been demonstrated in several studies and case reports. During a typical career in interventional cardiology, operators are subject to the adverse consequences of cumulative radiation exposure and an increased prevalence of orthopedic injuries [33,34]. Reduced radiation, ergonomic benefits and a better procedural precision are mostly cited. The proof of clear clinical relevant benefits for both the patients and the operators is a precondition that has to be confirmed in the upcoming years. The PRECISE study was the first registry that showed a drastic reduction in radiation exposure for the operator compared to manual PCI, without any increase in radiation expose for the patients [18]. Additional studies with head to head randomized comparison with follow up of radiation exposure for the second operator and outcomes are needed before wide spread implementation. One speculative benefit of R-PCI is that by allowing operators to perform procedures whilst seated in ergonomically designed stations the incidence of orthopedic injuries may be reduced.
R-PCI offers increased precision in positioning stents and consequentially secures a reduction in the incidence of Longitudinal Geographic Miss (LGM). The latter being the failure to fully cover a coronary artery disease segment. The occurrence of LGM can have a significant negative impact on patient long-term clinical outcomes. In a retrospective analysis it was found that the incidence of LGM was significantly reduced in patients undergoing R-PCI (PRECISE study) compared with a cohort of patient who underwent M-PCI (STLLR trial); 12.2% vs. 43.1%, p <0.0001 [35].
Robotics and artificial intelligence
Conventionally, intra-coronary wiring with a robotic platform is performed by advancing, retracting or rotating a joystick which translates the operators movements into axial or rotational forces upon an intra-coronary wire or device. Although the speed and amplitude of the movements can be controlled, the actual wiring technique or patterns of rotation cannot be altered using just the joystick or touchscreen controls. In the latest-generation robotic platform, CorPath GRX, artificial intelligence technology has been used to combine the conventional axial and rotational joystick movements with different patterns of wiring techniques or device manipulations. Different techniques, termed TechnIQ have been designed to replicate many of the common movements and manipulations performed by operators. Wiring techniques; spin, wiggle, rotate on retract and constant speed, are applied to any wire (0.014” or 0.018”) placed within the active wire drive whilst device manipulation techniques include dotter and constant speed can be applied to any rapid-exchange device (balloon, stent, intra-coronary imaging catheter) placed within the active device drive.
In the spin technique, as the operator advances the joystick, the wire rotates 360 degrees clockwise three times, followed by 360 degrees anti-clockwise three times. This feature is particularly useful for routine wiring and navigation particularly through vessels with larger lumens and tortuosity. The wiggle technique, involves rapidly alternating clockwise and anti-clockwise rotations being applied to the wire as its advanced. These faster yet lower amplitude rotations are particularly helpful when navigating through a tight lesion or stenosis inside smaller lumen vessels. Rotate on Retract works when an intra-coronary wire is being pulled back and an automatic 270 degree rotation is applied to change the direction of the tip of the wire. This technique is particularly effective when wiring a vessel and multiple side-branches are encountered. If the wire enters into a side-branch, the Rotate on Retract feature will automatically rotate the wire as the operator pulls the wire out of the side-branch to re-direct the wiring trajectory towards the distal segment of the vessel.
The dotter technique applies a rapid alternating ‘back and forth’ movement to the intra-coronary device as its advanced and replicates a common technique applied by operators to facilitate crossing of difficult lesions, tight stenosis, calcific segments or stent struts.
The different TechnIQ movements can be simultaneously combined with the joystick and touchscreen controls, and additionally, the operator can easily and instantly switch between different TechnIQ manipulations in order to select the most appropriate wiring and device strategy to tackle the intra-coronary challenge. A key advantage of these artificial intelligence derived movements is their impact on an operator’s learning curve and initial experience with R-PCI. Experienced operators can replicate common techniques and movements using the robotic platform, whilst early-career interventional cardiologists, can learn to safely and effectively wire vessels and lesions during R-PCI. Further data on the safety and procedural effectiveness of these TechnIQ movements will be evaluated in the NAVIGATE-GRX randomized clinical trial (ClinicalTrials.gov: NCT04883008).
The combination of robotic technology with artificial intelligence for PCI procedures provides operators with a powerful combination of consistent and reliable mechanical stability and precision, which is free from human factors with the technical skill and creativity required to successfully perform complex manipulations and movements. Further combinations and applications of artificial intelligence to robotic technologies are eagerly anticipated to further extend the range and complexity of coronary artery disease that can be treated robotically.
Limitations and Future Perspectives
Technical and procedural limitations
During the last 15 years several milestones were achieved that allowed R-PCI to be performed in complicated cases and in selected centers the implementation of a robot in the cathlab almost became an all comers strategy. There are however still limitations that have to be exceeded as this technology continues to evolve.
A major limitation is the incompatibility of the robotic platform with a number of devices. Several over-the-wire plaque modification equipment (eg rotational or orbital atherectomy), microcatheters and guiding extension catheters lack the compatibility with the available robotic platforms. Intravascular Lithotripsy (IVL) as a plaque modification device is fully compatible with the current robot platforms and can be effectively used in calcified lesions. Most of the intravascular imaging catheters are not compatible with R-PCI (with the exception of certain IVUS catheter) so planned use of intracoronary imaging would require manual assistance [36]. Although R-PCI is found to be efficient both in simple and complex cases, there was a decrease in technical success when complex cases were performed [20]. Even with the second generation device, only 1 coronary guidewire at a time and only 1 balloon or stent can be manipulated at a certain time. This can make complex interventions with an upfront two stent strategy (eg Culotte, DK Crush) challenging but not impossible. A hybrid approach by which certain aspects of the procedure are delt with in a “manual” way and others by means of the robot can offer an answer to this challenge. Although the CorPath GRX system allows robotically controlled guide catheter manipulation, this still may provide insufficient support for crossing lesions and delivering angioplasty balloons and stents in more complex cases. Heavily calcified lesions can thus be the Achilles tendon for R‐PCI, given the incompatibility with some plaque modification devices and difficulties with guide catheter support. Further adjustments on the robotic consoles are clearly needed. Additional active ports for advancing more catheters and wires and catheters simultaneously will make the approach in complex bifurcations lesions easier. Ensuring compatibility of future devices with the robot will prove useful in further adoption in complex cases.
The benefit in avoiding LGM (Longitudinal Geographic Miss) was already discussed earlier in this review. This results from improved measurement accuracy with the robotic software and improved visualization given closer screen proximity for the operator. The fixed device position during inflation and stent deployment is cited as an additional benefit. The CorPath GRX indeed allows measurements of the lesion length during pullback of any intracoronary device, leading to more accurate stent length selection. Regarding positioning of the stent, one has to realize that accuracy in positioning depends on 1:1 joystick input to movement. Depending solely on the measurements depicted on the console in guiding the stent delivery and positioning can be inaccurate when a device stucks and when the guiding is moving.
In terms of safety, the lack of tactile feedback is somehow balanced with the safety features that the Corindus platforms includes. In case of much calcified lesions with resistance to device crossing the safety feature of the robot warns the operator and will (after three times) shut down the system in order to prevent robot induced vessel complications. On first sight this is a safety feature but in the real world several operators remark that this feature is too “sensitive” blocking the procedure too easily. A possible solution for the future generation platforms could be either the reduction of the safety features or some extra feature where the level of safety is upon user discretion.
Telestenting and teleproctorship
Telestenting and teleproctoring are applications of R-PCI with significant potential to impact future practice. Patel and colleagues successfully performed remote R-PCIs with the operator located 20 miles away [37]. The potential application of “tele-stenting” will need to be explored, as it may open the possibility for remote treatment in rural areas. A trained catheterization laboratory team would still be required to be in attendance but it may mean less experienced interventional cardiologists are required to cover a certain rural area which may encompass a large geographical area. The possibility of teleproctoring could be an attractive feature in complex or rare cases. The feasibility and safety of a remote telerobotical primary PCI program is being explored in an ongoing study.
CT guided R-PCI and patient journey
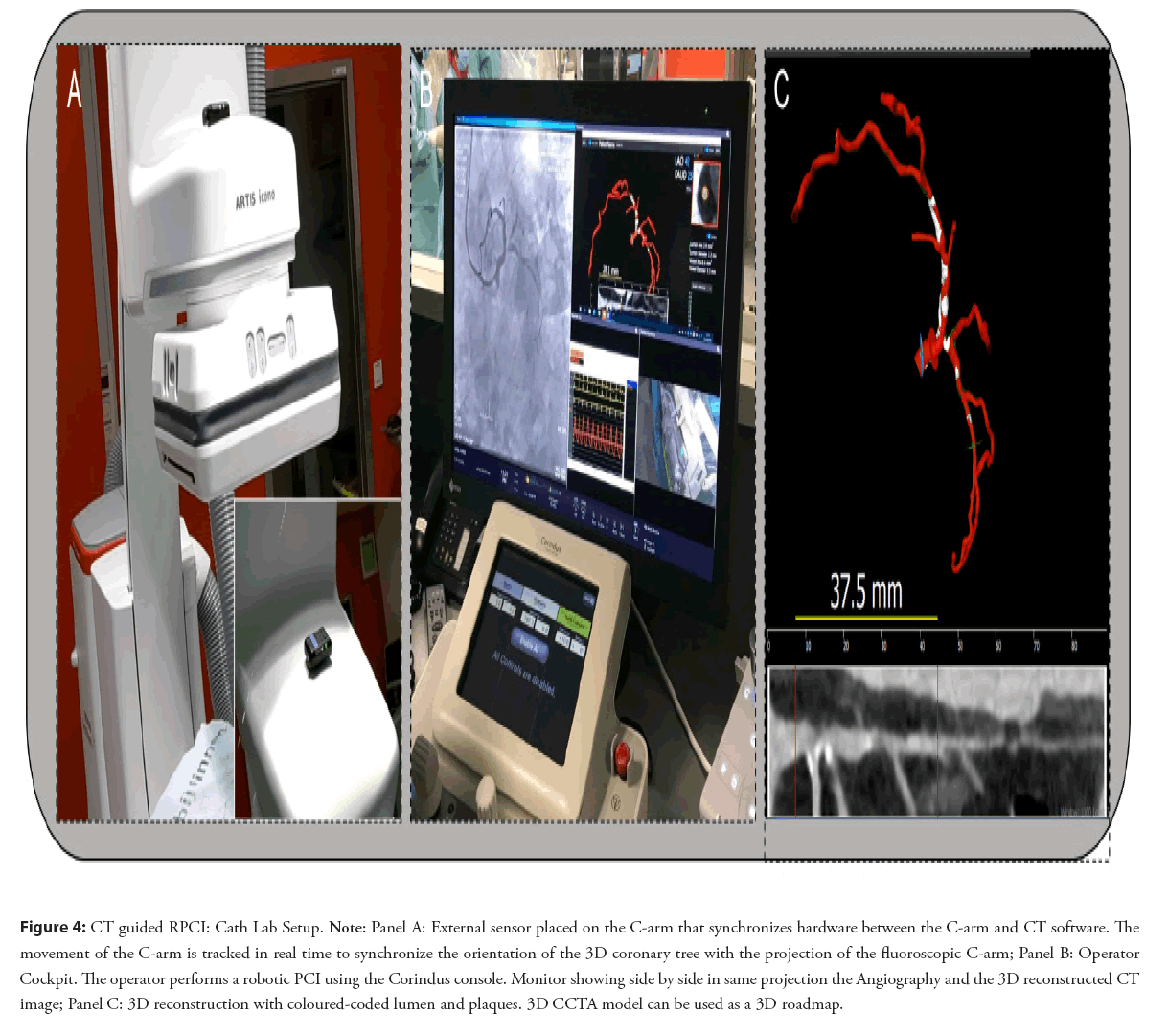
Combining robotics with pre procedural planning could stratify patients into low, medium and high risk patients journeys and delineate upfront the necessary bill of materials and need for hospitalization for a given procedure. The combination of better procedural planning and perprocedural guidance with procedural precision could change the patient journey into a same day discharge procedure. This approach has been shown to have a meaningful impact on hospital economics offering added value for the patient at a lower cost [38]. The combination of computed tomography based 3D reconstruction and CT based computational Fractional Flow Reserve (FFRCT), assessing anatomy and physiology in one study, can play an important role both in risk stratification and procedural planning. CT-guided revascularization is the synchronization of a CCTA 3D reconstruction with the C-arm shown in the Figure 4.
Figure 4: CT guided RPCI: Cath Lab Setup. Note: Panel A: External sensor placed on the C-arm that synchronizes hardware between the C-arm and CT software. The movement of the C-arm is tracked in real time to synchronize the orientation of the 3D coronary tree with the projection of the fluoroscopic C-arm; Panel B: Operator Cockpit. The operator performs a robotic PCI using the Corindus console. Monitor showing side by side in same projection the Angiography and the 3D reconstructed CT image; Panel C: 3D reconstruction with coloured-coded lumen and plaques. 3D CCTA model can be used as a 3D roadmap.
Conclusion
The use of a preprocedural CCTA-based strategy formulation could influence the choice of materials and the overall patient journey and related costs. Pre and per-procedural CT guidance could also enhance the accuracy and technical success of a robotic assisted revascularization. R-PCI has significant potential for expanding our capabilities in the catheterization laboratory. Standardization of the procedure, improved ergonomics and radiation exposure for the operator and the prospect of telestenting or teleproctoring are the major benefits of the technology. While significant improvements have been done in the technical capabilities of R-PCI during the last years there are still several limitations that we have to overcome. Future studies are clearly needed.
Fundings and Disclosures
Dr Bertolone, Dr Paolisso and Dr. Leone are supported by a research grant from the CardioPaTh PhD Program. University of Naples Federico II, Naples, Italy, Dr Wyffels and Dr Bermpeis are speakers for Siemens.
References
- Alexander AD. Impacts of telemation on modern society. On theory and practice of robots and manipulators. 121-136 (1972).
- Kwoh YS, Hou J, Jonckheere EA, et al. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng. 35(2): 153-160 (1988).
[CrossRef] [Google Scholar] [PubMed]
- Leow JJ, Chang SL, Meyer CP, et al. Robot-assisted versus open radical prostatectomy: A contemporary analysis of an all-payer discharge database. Eur Urol. 70(5): 837-845 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Hemli JM, Patel NC. Robotic cardiac surgery. Surg Clin North Am. 100(2): 219-236 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Ficarra V, Cavalleri S, Novara G, et al. Evidence from robot-assisted laparoscopic radical prostatectomy: A systematic review. Eur Urol. 51(1): 45-55 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Rosero EB, Kho KA, Joshi GP, et al. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 122(4): 778-786 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Carpentier A, Loulmet D, Carpentier A, et al. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C R Acad Sci III. 319(3):219-223 (1996).
- Falk V, Walther T, Autschbach R, et al. Robot-assisted minimally invasive solo mitral valve operation. J Thorac Cardiovasc Surg. 115(2): 470-471 (1998).
[CrossRef] [Google Scholar] [PubMed]
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med. 374(4): 344-353 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Loulmet D, Carpentier A, d'Attellis N, et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg. 118(1): 4-10 (1999).
[CrossRef] [Google Scholar] [PubMed]
- Beyar R, Gruberg L, Deleanu D, et al. Remote-control percutaneous coronary interventions: Concept, validation, and first-in-humans pilot clinical trial. J Am Coll Cardiol. 47(2): 296-300 (2006).
[CrossRef] [Google Scholar] [PubMed]
- Ragosta M, Singh KP. Robotic-assisted percutaneous coronary intervention: Rationale, implementation, case selection and limitations of current technology. J Clin Med. 7(2): (2018).
[CrossRef] [Google Scholar] [PubMed]
- Patel TM, Shah SC, Patel AT, et al. Learning curve of robotic percutaneous coronary intervention: A single-center experience. J Geriatr Cardiol. 1(6): 100508 (2022).
- Grüntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: Percutaneous transluminal coronary angioplasty. N Engl J Med. 301(2): 61-68 (1979).
[CrossRef] [Google Scholar] [PubMed]
- Vanlander AE, Mazzone E, Collins JW, et al. Orsi Consensus meeting on european robotic training (ocert): Results from the first multispecialty consensus meeting on training in robot-assisted surgery. Eur Urol. 78(5): 713-716 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Beyar R, Wenderow T, Lindner D, et al. Concept, design and pre-clinical studies for remote control percutaneous coronary interventions. EuroIntervention. 1(3): 340-345 (2005).
- Granada JF, Delgado JA, Uribe MP, et al. First-in-human evaluation of a novel robotic-assisted coronary angioplasty system. JACC Cardiovasc Interv. 4(4): 460-465 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Weisz G, Metzger DC, Caputo RP, et al. Safety and feasibility of robotic percutaneous coronary intervention: PRECISE (Percutaneous Robotically-Enhanced Coronary Intervention) Study. J Am Coll Cardiol. 61(15): 1596-1600 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Madder RD, Campbell PT, Caputo R, et al. TCT-435 Feasibility and success of radial-access robotic percutaneous coronary intervention: Insights from the precision registry. J Am College Cardiol. 66(15): B177-B178 (2015).
- Mahmud E, Naghi J, Ang L, et al. Demonstration of the safety and feasibility of robotically assisted percutaneous coronary intervention in complex coronary lesions: Results of the CORA-PCI study (complex robotically assisted percutaneous coronary intervention). JACC Cardiovasc Interv. 10(13): 1320-1327 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Walters D, Reeves RR, Patel M, et al. Complex robotic compared to manual coronary interventions: 6- and 12-month outcomes. Catheter Cardiovasc Interv. 93(4): 613-617 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Mahmud E, Dominguez A, Bahadorani J. First-in-human robotic percutaneous coronary intervention for unprotected left main stenosis. Catheter Cardiovasc Interv. 88(4): 565-570 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Kapur V, Smilowitz NR, Weisz G. Complex robotic-enhanced percutaneous coronary intervention. Catheter Cardiovasc Interv. 83(6): 915-921 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Cimmino G, Gallinoro E, Serafino LD, et al. Antiplatelet therapy in acute coronary syndromes. Lights and shadows of platelet function tests to guide the best therapeutic approach. Curr Vasc Pharmacol. 18(3): 262-272 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Smitson CC, Ang L, Pourdjabbar A, et al. Safety and feasibility of a novel, second-generation robotic-assisted system for percutaneous coronary intervention: First-in-human report. J Invasive Cardiol. 30(4): 152-156 (2018).
[Google Scholar] [PubMed]
- Almasoud A, Walters D, Mahmud E. Robotically performed excimer laser coronary atherectomy: Proof of feasibility. Catheter Cardiovasc Interv. 92(4): 713-716 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Wyffels E, Bermpeis K, Mileva N, et al. CT-guided robotic-assisted revascularisation in multi-vessel coronary artery disease. (2021).
- Mizukami T, Sonck J, Gallinoro E, et al. Duration of hyperemia with intracoronary administration of papaverine. J Am Heart Assoc. 10(3): e018562 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Hirai T, Kearney K, Kataruka A, et al. Initial report of safety and procedure duration of robotic-assisted chronic total occlusion coronary intervention. Catheter Cardiovasc Interv. 95(1): 165-169 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Walters D, Patel M, Reeves R, et al. Planned robotic chronic total occlusion percutaneous coronary intervention: Feasibility report. J Invasive Cardiol. 32(6): 201-205 (2020).
[Google Scholar] [PubMed]
- Bertolone DT, Bermpeis K, Gallinoro E, et al. First report of totally robotically assisted hybrid coronary artery revascularization combining RE-MIDCAB and R-PCI: Case report. J Card Surg. 37(9): 2907-2911 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Bermpeis K, Ohashi H, Bertolone DT, et al. First-in-man robotic-assisted renal denervation. JACC Case Rep. 101669 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Goldstein JA, Balter S, Cowley M, et al. Occupational hazards of interventional cardiologists: Prevalence of orthopedic health problems in contemporary practice. Catheter Cardiovasc Interv. 63(4): 407-411 (2004).
0[CrossRef] [Google Scholar] [PubMed]
- Klein LW, Tra Y, Garratt KN, et al. Occupational health hazards of interventional cardiologists in the current decade: Results of the 2014 SCAI membership survey. Catheter Cardiovasc Interv. 86(5): 913-924 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Bezerra HG, Mehanna E, Weisz G, et al. Longitudinal geographic miss (lgm) in robotic assisted versus manual percutaneous coronary interventions. J Interv Cardiol. 28(5): 449-455 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Khokhar A, Zelias A, Zlahoda-Huzior A, et al. Advancements in robotic PCI technology: Time to tackle the complex lesions: Technological advancements in robotic PCI. AsiaIntervention. 8(1): 50-51 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Patel TM, Shah SC, Pancholy SB. Long distance tele-robotic-assisted percutaneous coronary intervention: A report of first-in-human experience. EClinicalMedicine. 14: 53-58 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Koshy SKG, George LK, Das P. Cost-effectiveness and outcomes with early or same-day discharge after elective percutaneous coronary intervention. Curr Cardiol Rep. 22(6): 42 (2020).
[CrossRef] [Google Scholar] [PubMed]