Review Article - Interventional Cardiology (2015) Volume 7, Issue 2
Current strategies for bridging dual antiplatelet therapy in patients requiring surgery
- Corresponding Author:
- Michalis Hamilos
Department of Cardiolology, University Hospital of Heraklion
Crete, Greece
Tel: +30 291 037 5253
Fax: +30 281 054 2055
E-mail: xammix@hotmail.com
Abstract
Perioperative management of patients with an indication for dual antiplatelet therapy (DAPT) and, in particular, patients during the first weeks after coronary stent implantation remains a challenging task for both cardiologists and surgeons, despite the advent in clinical pharmacology and stent technology, as well as the increasing clinical experience. Precise balancing of the risk of coronary stent thrombosis after DAPT discontinuation against the hazard of life-threatening perioperative hemorrhage is paramount for decision making, but it is in many cases extremely difficult. This is reflected on the lack of universally accepted guidelines on managing such patients.
Keywords
Antiplatelet agents, bleeding, bridging, surgery, thrombosis
Perioperative management of patients with an indication for dual antiplatelet therapy (DAPT) and, in particular, patients during the first weeks after coronary stent implantation remains a challenging task for both cardiologists and surgeons, despite the advent in clinical pharmacology and stent technology, as well as the increasing clinical experience. Precise balancing of the risk of coronary stent thrombosis after DAPT discontinuation against the hazard of life-threatening perioperative hemorrhage is paramount for decision making, but it is in many cases extremely difficult. This is reflected on the lack of universally accepted guidelines [1–4] on managing such patients.
Cardiac and noncardiac surgery, apart from exposing patients to a considerable bleeding risk, is also characterized by a prothrombotic and proinflammatory response, which, coupled with sympathetic hyperactivity, could have a detrimental contribution to the risk of perioperative acute coronary syndromes (ACS) [2,5]. This risk is maximal in patients with a recent ACS and during the first weeks after stent implantation, especially if perioperative discontinuation of antiplatelet therapy (APT) is judged to be mandatory.
A bridging strategy could be defined as the temporary administration of an antithrombotic agent perioperatively in order to minimize the time period that a patient remains free from antithrombotic protection between withdrawal of oral APT and the time of surgery.
The time course of the recovery of platelet function after cessation of APT is determined by the pharmacodynamic and pharmacokinetic properties of the agent in question. Theoretically, the ideal bridging pharmaceutical agent would be a drug that provides antiplatelet efficacy and safety as close as possible to that of agents that constitute the mainstay of modern DAPT – that is, aspirin plus a platelet P2Y12-receptor inhibitor (clpopidogrel, ticagrelor or prasugrel) – but with the fastest possible offset of action after cessation of its administration and subsequent onset upon its re-introduction.
Magnitude of the problem
Approximately 5–15% of patients undergoing coronary stent implantation are estimated to undergo a surgical procedure within 2 years [6–12]. In the larg-est (n = 126,773) cohort study to date, describing the incidence and timing of noncardiac surgery after coronary stent placement, 12% of patients who received bare metal stents (BMS) and 47% of patients who received drug-eluting stents (DES) had early surgery, defined as surgical procedures occurring within 6 weeks in patients treated with BMS or within 12 months in those treated with DES [12]. If we consider all patients with an indication for prolonged DAPT, including patients treated noninvasively after an ACS, the percentage is even higher. Major surgical procedures were more likely to occur within 12 months of stent placement, in comparison with 12–24 months [13]. A decline of surgical procedures was noted after publication of the 2007 AHA Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery (13 vs 25% pre-guidelines) [13], highlighting the impact of growing evidence for the possible detrimental outcomes of DAPT discontinuation on clinical decision-making.
The population of patients requiring coronary artery bypass grafting (CABG) within the time of DAPT administration includes stabilized patients with non-ST-elevation ACS (NSTE-ACS) more suitable for CABG rather than percutaneous coronary intervention (PCI), patients with indication for emergent CABG due to failed primary PCI and ongoing ischemia or mechanical complications in the context of ST-elevation myocardial infarction (STEMI), as well as those who are suitable for specific hybrid revascularization procedures. Apart from the clearly elevated ischemic risk in all of these subpopulations, surgeryrelated bleeding increases 30-day and long-term mortality after CABG [14].
Integrated approach to the patient on DAPT reqiuring surgery
The approach to the patient on DAPT requiring surgery should involve the following sequence of actions:
• Determination of the level of surgical emergency; in emergent cases it is recommended to proceed directly to surgery, whereas elective surgery should be postponed until a full course of DAPT has been completed. The remaining steps mostly refer to the case of urgent surgery, in which waiting until the completion of full course of DAPT is considered unacceptable [1–3];
• Decision-making on continuation or discontinuation of oral antiplatelet agents, taking into account the individual ischemic and bleeding risk [1–3];
• Identification of patients in whom bridging strategies may be beneficial.
Assessment of thrombotic risk
There is evidence that unplanned or urgent surgery following coronary stenting poses patients at a higher risk for cardiac events perioperatively. Premature discontinuation of DAPT results in increased risk for stent thrombosis and other ischemic events. In a survey of 1358 consecutive patients treated with DES and discharged on aspirin and clopidogrel, surgery was identified as the second cause of early APT discontinuation within 1 year (21%) and the first cause of late discontinuation thereafter (49%) [15].
On the other hand, the investigators of the PARIS registry – a prospective observational study that enrolled more than 5000 patients undergoing PCI – found that temporary DAPT interruption for up to 14 days was not associated with an increased rate of thrombotic events, as opposed to disruption due to bleeding or noncompliance. In this latter subset, however, the association with an increased risk for major cardiovascular events (MACE) was attenuated after 30 days from DAPT discontinuation [16].
While noncardiac surgery performed early after balloon angioplasty is not associated with an increased risk for cardiac events [17], the incidence of perioperative ACSs is strikingly higher in stented patients. The reported mortality rates attributed to perioperative stent thrombosis are as high as 20% when surgery is performed within 6 weeks following coronary stenting and DAPT is discontinued [18]. Therefore, it is recommended to postpone elective surgery for a minimum of 4 weeks and ideally for up to 3 months after BMS implantation, with perioperative continuation of aspirin whenever possible [2].
While any patient within 12 months after an ACS remains at high risk for recurrent ischemic events irrespective of the type of stent implanted, growing evidence suggests that the risk of late stent thrombosis in patients with stable coronary artery disease (SCAD) treated with newer generation DES has substantially declined [19,20]. Based on those data regarding newer generation DES, the recent ESC Guidelines on Myocardial Revascularization (2014) recommend a minimum of 6 months of DAPT, as opposed to 12 months with first-generation DES [1], while recent studies suggest that even shorter durations of DAPT may be sufficient [21,22]. Newer generation stents are characterized by thin-strut metallic platforms that release limus-based antiproliferative drugs from either durable polymers with improved biocompatibility and lower polymer mass or biodegradable polymers or even polymer-free surfaces, allowing faster endothelialization than first-generation DES. Moreover, the recently introduced bioresorbable stents, combining complete platform dissolution with drug-eluting properties, seem to be an attractive option in terms of DAPT duration, but still technical improvement and large randomized trials are mandatory for their routine use to be established and for the optimal duration of DAPT after their implantation to be determined.
The risk of stent thrombosis in the perioperative period for both BMS and DES is highest in the first 4–6 weeks after stent implantation. The risk of stent thrombosis after this time period is relatively low, but still higher than without surgery, although this varies from study to study [23–26]. This risk decreases with time and appears to reach a plateau 6 months after DES implantation [27,28].
Well established risk factors for stent thrombosis include: angiographic parameters, such as bifurcation stenting, ostial stenting, small stent diameter (<3 mm), stent length >18 mm, overlapping stents, multiple stents, suboptimal result of the stenting procedure, clinical setting parameters (stenting in the context of an ACS, prior stent thrombosis) and factors referring to patient characteristics, like diabetes mellitus, renal impairment, advanced age (>80 years) and reduced left ventricular ejection fraction [4,29–31]. Moreover, the strongest risk factor for MACE following noncardiac surgery is the need for non-elective surgery, a history of myocardial infarction within 6 months of surgery and advanced cardiac disease. While timing of surgery was associated with MACE during the first 6 months after PCI, this was no longer apparent beyond 6 months [32]. Notably, stent type (BMS vs DES) was not a predictor of MACE after surgery.
Assessment of bleeding risk
Perioperative bleeding could be attributed either to the surgical procedure per se or to bleeding due perioperative antithrombotic medications administration. CABG is a special setting where bleeding involves additional mechanisms, such as full heparinization and the effects of extracorporeal circulation on platelet function and fibrinolysis, in comparison with major noncardiac surgery [33].
For hemorrhagic risk stratification, efforts have been made in the field of noncardiac and cardiac surgery, separately. Chassot et al. have classified noncardiac surgical procedures according to the associated risk of clinically important bleeding, including severe bleeding resulting in hemodynamic compromise and requiring blood transfusions, as well as intracerebral or intraocular bleeding. According to this classification, low bleeding risk procedures include biopsies, minor orthopedic and ENT procedures, general surgical procedures, endoscopies, anterior chamber ophthalmologic surgery and dental extractions. As intermediate bleeding risk procedures are regarded visceral, vascular, major orthopedic, major ENT and urologic reconstruction surgery, while high bleeding risk procedures include intracranial neurosurgery, spinal canal surgery and posterior chamber ophthalmologic surgery [34]. In other bleeding risk stratification schemes, classification is even more detailed [35].
A stratification scheme for identifying cardiac surgery patients at risk for excessive early postoperative bleeding, called the Papworth Bleeding Risk Score, has been proposed by Vuylsteke et al. [36]. The prevalence of bleeding complications was 3, 8 and 21% in the low (Papworth score = 0), intermediate (Papworth score = 1–2) and high bleeding risk group (Papworth score = 3–5), respectively. Bleeding risk factors in cardiac surgery highlighted by Rossini et al. [35] include reintervention, endocarditis, CABG after failed percutaneous coronary intervention and aortic dissection.
Perioperative use of aspirin
There is a consensus among guidelines that low dose aspirin should be continued whenever possible throughout the perioperative period in any patient requiring surgery within the first year and especially the first 3 months after stenting, provided that the associated bleeding risk is not considered unacceptable [1–4]. A large meta-analysis, including 41 studies in 49,590 patients, which compared periprocedural withdrawal versus continuation of aspirin with respect to bleeding events, found that bleeding complications with aspirin therapy were increased by 50% in incidence, but not in severity [37]. On the other hand, in patients at risk for or with known ischemic heart disease, aspirin withdrawal tripled the risk of MACE.
Perioperative use of P2Y12-receptor inhibitors
In preparation for surgical procedures with highto- very-high bleeding risk, it is currently recommended to discontinue clopidogrel and ticagrelor 5 days before surgery to reduce bleeding and the need for transfusion, while maintaining acetylsalicylic acid throughout the perioperative period. Prasugrel should be stopped 7 days before surgery, based on its prolonged and more effective platelet inhibition than clopidogrel [1–3]. On the contrary, the authors of the 2014 ESC Guidelines on Myocardial Revascularization argue against the withdrawal of P2Y12 inhibitors in high-risk cohorts, such as those with continuing ischaemia and high-risk anatomy (e.g., left main or severe proximal multivessel disease). Performing CABG in those patients while maintaining P2Y12 inhibition is encouraged, while paying particular attention to hemostasis. It is emphasized, though, that in patients with high thrombotic risk and a concomitant excessive bleeding risk it may be reasonable to withhold P2Y12 inhibitors before surgery, even among those with active ischemia, and to consider bridging strategies. DAPT should be resumed as soon as possible, including a loading dose for clopidogrel, ticagrelor, or prasugrel (if possible within 24 h of surgery), although the optimal timing for resumption of medication following CABG surgery remains uncertain [1].
Current ESC guidelines recommend continuation of DAPT in patients undergoing urgent noncardiac surgery during the first 4–6 weeks after BMS or DES implantation, if the bleeding risk does not outweigh the benefit of prevention of stent thrombosis. The authors also highlight the importance of continuation of aspirin therapy whenever possible, if discontinuation of the P2Y12-receptor inhibitor is considered mandatory (Class of indication: 1C). It should be noted that a strategy of perioperative withdrawal of aspirin and continuation of the P2Y12 inhibitor has not been evaluated in clinical trials and is thus not listed in the guidelines as an alternative, although such an approach seems reasonable. On the contrary, the recommended strategy is largely based on the data regarding the CABG-subpopulations of the CURE, TRITON-TIMI-38 and PLATO trials and simultaneously reflects the general preference of aspirin as a first choice single antiplatelet therapy (SAPT) in SCAD outside the surgical setting.
Almost every recommendation in all guidelines referring to the perioperative management of patients on DAPT includes denotations about balancing thrombotic and hemorrhagic risk without providing references to specific ways for their determination. This underscores the lack of randomized trials and the difficulties in establishing universally accepted scoring systems and algorithms [1–3].
No randomized trials have been conducted to evaluate outcomes of P2Y12-receptor inhibitors perioperative continuation in noncardiac surgery. On the contrary, there are some data from patients treated with CABG in some major trials while being on DAPT. In the CURE trial, in the 910 patients in whom clopidogrel was discontinued more than 5 days before CABG, there was no apparent excess of major bleeding within 7 days after surgery (4.4% of patients in the clopidogrel group vs 5.3% of those in the placebo group). In the 912 patients who stopped taking the medications within 5 days before CABG surgery, the rate of major bleeding was 9.6% in the clopidogrel group and 6.3% in the placebo group (RR = 1.53; p = 0.06) [38]. In the subgroup of patients of the TRITON-TIMI-38 trial treated with CABG, despite the higher rates of observed major TIMI bleeding, platelet transfusion and surgical reexploration for bleeding, prasugrel was associated with a lower rate of death than clopidogrel [39]. As for ticagrelor, in the subgroup of patients of the PLATO trial undergoing CABG within 7 days after the last study drug intake (3–5 days), ticagrelor compared with clopidogrel was associated with a substantial reduction in total and CV mortality without excess risk of CABG-related bleeding [40].
Patients suitable for bridging strategies
As noted above, management of patients on DAPT who are referred for surgical procedures requires a multidisciplinary approach to determine the patient’s risk (bleeding and thrombosis) and choose the best strategy. Current ESC guidelines state that bridging strategies should only be considered in patients at very high ischemic risk (active ischemia, high-risk coronary anatomy, surgery performed very early after stent implantation), in whom temporary discontinuation of the antiplatelets is considered inevitable due to accompanying elevated hemorrhagic risk, without further specific recommendations.
In an attempt to precisely define the temporal cut-off point, in order to select patients that are possible candidates for bridging strategies, an algorithm has been proposed by Abualsaud and Eisenberg in 2010 [41]. This algorithm suggests that the first priority of the decision-making is to determine the bleeding risk during the procedure. Bridging therapy is recommended in patients with high perioperative bleeding risk with at least one of the aforementioned factors indicating high thrombotic risk, and in patients with intermediate bleeding risk with thrombotic risk factor(s) in whom perioperative discontinuation of aspirin is considered mandatory, when surgery is carried out more than 12 months after elective stenting. On the contrary, in patients at low perioperative bleeding risk and in those with intermediate bleeding risk requiring surgery less than 12 months after elective stenting, continuation of DAPT perioperatively is encouraged.
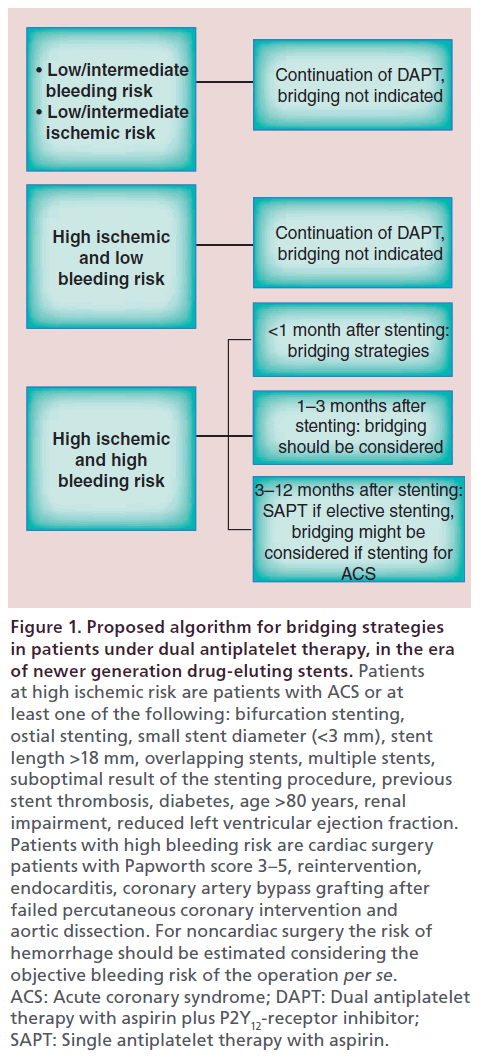
This algorithm underlines that when high bleeding risk is present, perioperative continuation of DAPT is inappropriate, whereas the concomitant presence of high thrombotic risk mandates the minimization of the total time without antithrombotic protection. On the other hand, patients at low or moderate bleeding risk can safely undergo surgery on DAPT. Moreover, if surgery is performed later than 12 months after elective old generation DES implantation and the patient is at intermediate bleeding risk with concomitant thrombotic risk factors, aspirin cessation may be considered mandatory. In this case, bridging therapy may also be considered. This algorithm was proposed before the era of the newer generation DES. Incorporating data from recent ESC guidelines [1,2], we propose an updated algorithm which could be helpful in selecting subgroups of patients suitable for APT-bridging strategies (Figure 1).
Figure 1: Proposed algorithm for bridging strategies in patients under dual antiplatelet therapy, in the era of newer generation drug-eluting stents. Patients at high ischemic risk are patients with ACS or at least one of the following: bifurcation stenting, ostial stenting, small stent diameter (<3 mm), stent length >18 mm, overlapping stents, multiple stents, suboptimal result of the stenting procedure, previous stent thrombosis, diabetes, age >80 years, renal impairment, reduced left ventricular ejection fraction. Patients with high bleeding risk are cardiac surgery patients with Papworth score 3–5, reintervention, endocarditis, coronary artery bypass grafting after failed percutaneous coronary intervention and aortic dissection. For noncardiac surgery the risk of hemorrhage should be estimated considering the objective bleeding risk of the operation per se. ACS: Acute coronary syndrome; DAPT: Dual antiplatelet therapy with aspirin plus P2Y12-receptor inhibitor; SAPT: Single antiplatelet therapy with aspirin.
The role of platelet function tests
The role of platelet function tests in the perioperative management of patients on DAPT is not well established. Main reasons for that is the absence of well defined cut off values in order to predict ischemic or hemorrhagic risk and the lack of clinical trials in order to test their ability to prevent adverse events in routine perioperative clinical practice, especially in the field of noncardiac surgery.
In fact, the TARGET-CABG study is the only trial published to date evaluating the role of point-of-care platelet function testing in reducing perioperative bleeding events. One hundred and eighty patients on background aspirin therapy with or without clopidogrel were enrolled and assessment of platelet function was performed by thromboelastography platelet-mapping assay in clopidogrel-treated patients. Surgery was scheduled within 1 day in patients with high residual platelet reactivity and in 5 days in those with low reactivity. In general, patients with high residual platelet reactivity are those that have not adequately responded to clopidogrel therapy – using different methods of testing platelet activity – and represent approximately 20–30% of the patients treated with clopidogrel. The investigators concluded that a strategy based on preoperative platelet function testing to determine the timing of CABG in clopidogrel treated patients was associated with the same amount of bleeding with that observed in clopidogrel-naive patients, while enabling an almost 50% shorter waiting time after clopidogrel discontinuation compared with the time recommended in the respective guidelines (5 days) [42].
While the 2012 update on the Society Of Thoracic Surgeons (STS) Guidelines on the Use of Antiplatelet Drugs In Patients Having Cardiac and Noncardiac operations points out the usefulness of point-of-care testing in identifying patients with high residual platelet reactivity who can possibly undergo operative procedures without elevated bleeding risk, no comparison between different assays has been made [43]. On the other hand, the recent 2014 ESC/ESA Guidelines on Cardiovascular Assessment and Management in Noncardiac Surgery stress the need for more research in this area [2].
Specific agents as possible APT-bridging strategies
Unfractionated heparin & low molecular weight heparins – patients not on oral anticoagulant
Unfractionated heparin (UFH) has a short duration of action, while short acting low molecular weight heparins (LMWHs) such as enoxaparin, despite having a longer duration of action than UFH, exhibit more pre dictable pharmacokinetics, greater bioavailability and are not significantly bound to plasma proteins. Since the primary mechanism involved in stent thrombosis is platelet accumulation and not activation of the coagulation cascade, the acting site of heparin is theoretically suboptimal for APT-bridging purposes. Moreover, heparin can also affect platelet reactivity, either positively or negatively [44].
Two prospective trials evaluating the perioperative use of UFH/LMWH in patients with coronary stents failed to show any consistent protective effect of these agents against stent thrombosis, while noting an increase in bleeding events [45,46]. These studies, however, enrolled relatively small numbers of patients (103 and 96, respectively). In the recent ESC guidelines on the perioperative management of patients undergoing noncardiac surgery, [2] the use of LMWHs for bridging purposes is discouraged due to the lack of evidence supporting the efficacy and safety of this strategy. Despite this, evidence against an UFH/LMWH-based bridging strategy is also weak.
Unfractionated heparin & low molecular weight heparins – patients on oral anticoagulant
New guidelines on antithrombotic therapy in stented patients with concomitant atrial fibrillation indirectly indicate that a reconsideration of the use of UFH and LMWH as bridging agents in some cases might be appropriate [1]. Current drug therapy of patients with an indication for oral anticoagulation such as atrial fibrillation who are treated with elective stenting for SCAD includes a triple combination of aspirin, clopidogrel and an oral anticoagulant (OAC) for an initial period of at least one month for both BMS and newer generation DES in special patient subsets. This recommendation of the Recent ESC Guidelines on Myocardial Revascularization [1] is not based on randomized trials, but on the observation that the risk of stent thrombosis between 1 and 12 months after stenting appears to be similar for both BMS and newer generation DES. The authors also emphasize on recent data on the risk of adverse events among patients who have ceased medication and patients undergoing noncardiac surgery suggesting no difference between BMS and DES [47–49]. Moreover, in those guidelines there is a recommendation (Class IIb, Level of Evidence: B) for dual initial therapy with OAC and clopidogrel as an alternative to initial triple therapy in selected patients. This recommendation is based on the results of the WOEST study [50]. The concomitant indication for an anticoagulant renders the perioperative management of such patients even more complicated, as they would probably require an anticoagulation-bridging strategy for perioperative protection from thromboembolic events, combined with decision-making for the antiplatelet medication.
Taking into account the above information and recommendations, at least a subset of such patients would possibly be treated with single APT and UFH/LMWH perioperatively (data on perioperative management of this subset of patients are not found in literature). Moreover, one could extrapolate that for patients under DAPT after stenting with a newer generation DES or a BMS, a combination of a single antiplatelet agent plus an anticoagulant might not be completely unreasonable as a perioperative bridging strategy beyond the first month or even within the first month (in selected patients) after stenting. Then there might be a role for UFH and LMWH as perioperative protection against both stent thrombosis and embolism together with single APT. Those thoughts are more ‘hypothesis generating’, as solid data are completely lacking and large randomized studies are needed.
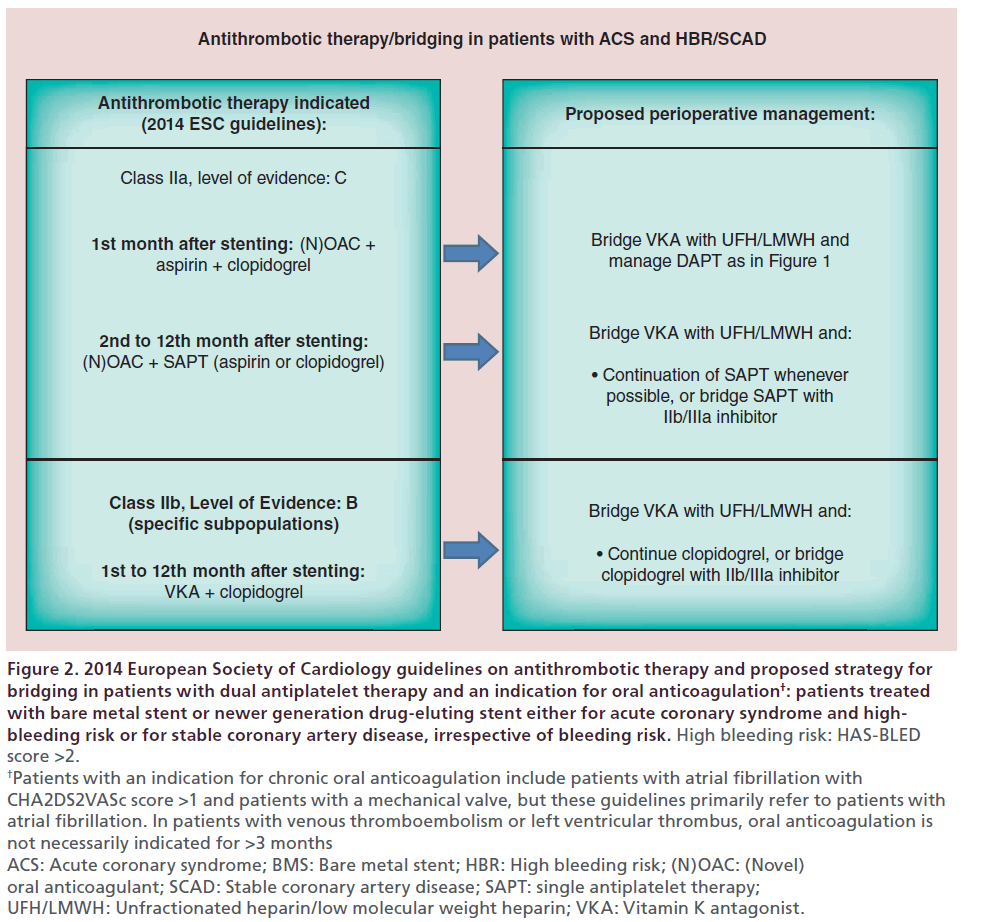
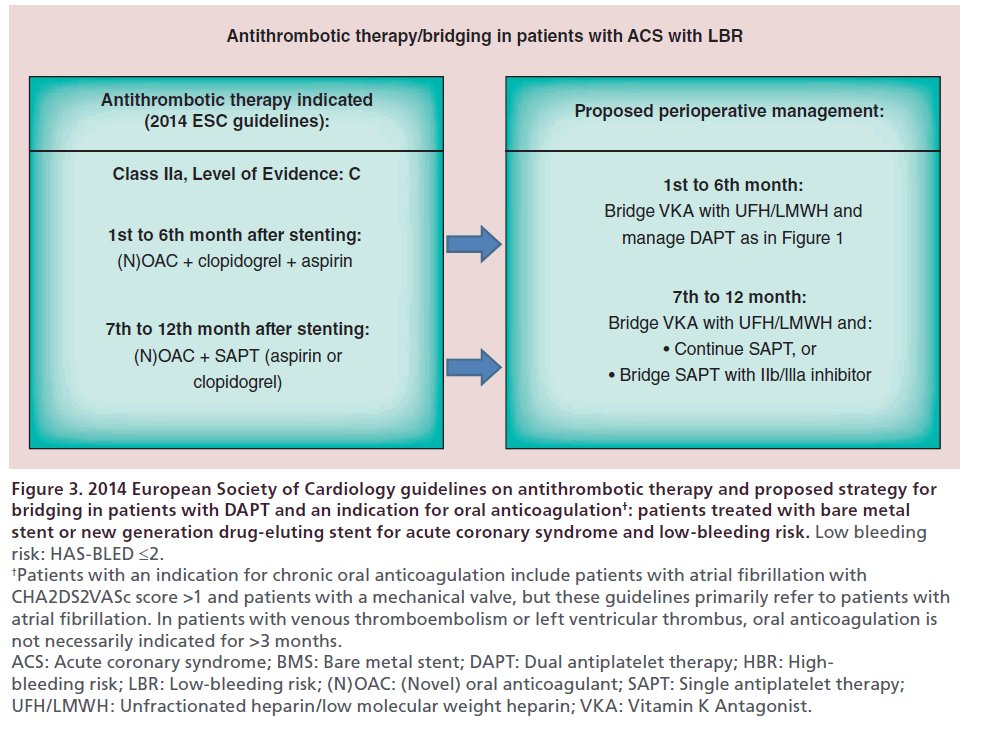
Current recommendations [1] on antithrombotic management of stented patients with an indication for oral anticoagulation, as well as the respective perioperative management that we would propose, are summarized in Figures 2 & 3.
Figure 2: 2014 European Society of Cardiology guidelines on antithrombotic therapy and proposed strategy for bridging in patients with dual antiplatelet therapy and an indication for oral anticoagulation†: patients treated with bare metal stent or newer generation drug-eluting stent either for acute coronary syndrome and highbleeding risk or for stable coronary artery disease, irrespective of bleeding risk. High bleeding risk: HAS-BLED score >2. †Patients with an indication for chronic oral anticoagulation include patients with atrial fibrillation with CHA2DS2VASc score >1 and patients with a mechanical valve, but these guidelines primarily refer to patients with atrial fibrillation. In patients with venous thromboembolism or left ventricular thrombus, oral anticoagulation is not necessarily indicated for >3 months ACS: Acute coronary syndrome; BMS: Bare metal stent; HBR: High bleeding risk; (N)OAC: (Novel) oral anticoagulant; SCAD: Stable coronary artery disease; SAPT: single antiplatelet therapy; UFH/LMWH: Unfractionated heparin/low molecular weight heparin; VKA: Vitamin K antagonist.
Figure 3: 2014 European Society of Cardiology guidelines on antithrombotic therapy and proposed strategy for
bridging in patients with DAPT and an indication for oral anticoagulation†: patients treated with bare metal
stent or new generation drug-eluting stent for acute coronary syndrome and low-bleeding risk. Low bleeding
risk: HAS-BLED ≤2. †Patients with an indication for chronic oral anticoagulation include patients with atrial fibrillation with
CHA2DS2VASc score >1 and patients with a mechanical valve, but these guidelines primarily refer to patients with
atrial fibrillation. In patients with venous thromboembolism or left ventricular thrombus, oral anticoagulation is
not necessarily indicated for >3 months.
ACS: Acute coronary syndrome; BMS: Bare metal stent; DAPT: Dual antiplatelet therapy; HBR: Highbleeding
risk; LBR: Low-bleeding risk; (N)OAC: (Novel) oral anticoagulant; SAPT: Single antiplatelet therapy;
UFH/LMWH: Unfractionated heparin/low molecular weight heparin; VKA: Vitamin K Antagonist.
Glycoprotein IIb/IIIa-receptor inhibitors
Intravenous glycoprotein IIb/IIIa inhibitors include eptifibatide, tirofiban and abciximab. Although all three agents are characterized by a short plasma halflife (30 min to 2.5 h), platelet function after abciximab discontinuation usually requires about 48 h to recover, while the drug can remain platelet-bound for up to 15 days. Thus, only tirofiban and eptifibatide exhibit rapid offset of action after cessation of administration (4–8 h) [51]. Their mechanisms of action, aiming the final common pathway of platelet aggregation, although not identical to that of P2Y12-receptor inhibitors, have made them an attractive choice in the context of DAPT-bridging strategies. Despite their theoretically suitable profile, evidence from randomized trials is totally lacking, and the results of small nonrandomized or retrospective studies have not been consistent. Recent ESC guidelines [1,2] state that the use of short acting glycoprotein IIb/IIIa inhibitors could be considered, after oral P2Y12-receptor inhibitor discontinuation, in very high-risk situations, such as in the first weeks after stent implantation, stopping the infusion 4 h before surgery. Finally, some authors propose the addition of UFH to IIb/IIIa inhibitors based on the findings of the PRISM-PLUS study in ACS [52], in which one arm was terminated early due to a noted increase in mortality at 7 days when tirofiban was given without heparin.
Cangrelor
Cangrelor is a novel non-thienopyridine intravenous antiplatelet agent with a very short plasma halflife (3–5 min) which reversibly blocks the P2Y12 receptor. These properties result in rapid offset of action, within 1 h of cessation of administration, while the onset of action is immediate. Although its characteristics theoretically approach the ideal of an APT-bridging drug, cangrelor is not yet commercially available. In the BRIDGE study, its use for bridging thienopyridine-treated patients to CABG surgery was evaluated against placebo. Oral P2Y12 inhibitors were stopped 48 h before CABG. Cangrelor resulted in a higher rate of maintenance of platelet inhibition (primary end point, P2Y12 reaction units <240; 98.8% (83/84) versus 19.0% (16/84), respectively; RR 5.2; 95% CI: 3.3–8.1; p < 0.001). Bridging with a prolonged infusion of cangrelor did not increase major bleeding before surgery [53]. The results of the BRIDGE study indicate a potent thienopyridine-like platelet inhibition produced by cangrelor, but it must be noted that the study is lacking clinical end points with respect to cardiac events. In February 2014, the US FDA advisory committee voted 9:0 against approval of the use of the agent for bridging purposes. Further studies evaluating the perioperative use of cangrelor as a bridge to cardiac and noncardiac surgery are needed.
Conclusion
It is more than evident that the optimal perioperative management of patients with an indication for DAPT, and the role and perspectives of bridging strategies in specific, are fraught with uncertainties and compromises, not only due to the lack of robust evidence from randomized trials with clinical end points, but also due to the heterogeneity of the surgical population, concerning bleeding and thrombotic risk.
At the moment, only glycoprotein IIb/IIIa inhibitors and cangrelor could theoretically fulfil the characteristics of a bridging agent after P2Y12 inhibitors discontinuation, although large randomized trials are needed to confirm this strategy. Until more evidence is available, the golden rule is individualization of management in the context of a thorough multidisciplinary discussion, between the physicians responsible for the patient’s care.
Future perspective
Despite evolving stent technology, DAPT is bound to remain the cornerstone of the medical treatment of stented patients. Although the minimal obligatory duration of DAPT in the context of elective stenting has become shorter with the use of newer generation stents, it seems highly unlikely for it to become shorter than 4–6 weeks, due to the high thrombotic risk during the first month after stenting. On the other hand, the recommended duration of DAPT in the setting of an ACS is unlikely to change. Consequently a considerable number of patients will still be candidates for APT-bridging therapy in the near future.
Further data on the efficacy and safety of cangrelor that will determine whether its use could be established in clinical practice are awaited, as is the development of novel agents with a similar mechanism of action, pharmacokinetics and pharmacodynamics. The promising results of the combination of a single antiplatelet agent with an anticoagulant even in the initial month after stenting could evoke the hypothesis that a short-acting parenteral anticoagulant might be suitable for bridging purposes. Until more evidence is available, short acting IIb/IIIa inhibitors are expected to remain the first choice in this setting.
The limitations and difficulties in conducting randomized trials regarding APT-bridging strategies render the possibility of having more evidence for their use unlikely in the upcoming years, and individualization of perioperative management will continue to be of paramount importance.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Magnitude of the problem
• Approximately 5–15% of patients undergoing coronary stent implantation are estimated to undergo a surgical procedure within 2 years.
• Major surgical procedures were more likely to occur within 12 months of stent placement, in comparison with 12–24 months.
Integrated approach to the patient on dual antiplatelet therapy requiring surgery
• The approach to the patient on dual antiplatelet therapy (DAPT) requiring surgery should involve determination of the level of surgical emergency, decision-making on continuation or discontinuation of oral antiplatelet agents and identification of patients in whom bridging strategies may be beneficial.
Assessment of thrombotic risk
• While noncardiac surgery performed early after balloon angioplasty is not associated with an increased risk for cardiac events, the incidence of perioperative acute coronary syndromes is strikingly higher in stented patients.
• The risk of stent thrombosis in the perioperative period for both bare metal stents and drug-eluting stents is highest in the first 4–6 weeks after stent implantation.
• Well-established risk factors for stent thrombosis include angiographic and clinical parameters and factors referring to patient characteristics.
Assessment of bleeding risk
• Several bleeding risk stratification schemes have been developed for noncardiac surgery, whereas the Papworth bleeding risk score has been proposed for cardiac surgery.
Perioperative use of aspirin
• There is a consensus among guidelines that low-dose aspirin should be continued whenever possible throughout the perioperative period in any patient requiring surgery within the first year and especially the first 3 months after stenting.
• Perioperative continuation of aspirin appears to increase the incidence, but not the severity of bleeding.
Perioperative use of P2Y12-receptor inhibitors
• There is consensus among guidelines that perioperative continuation of DAPT should be encouraged in patients undergoing urgent noncardiac surgery during the first 4–6 weeks after BMS or drug-eluting stent implantation, if bleeding risk permits.
• No randomized trials have been conducted to evaluate outcomes of P2Y12-receptor inhibitors perioperative continuation in noncardiac surgery.
• Data from CURE, TRITON-TIMI-38 and PLATO trials suggest that clopidogrel, prasugrel and ticagrelor should be withdrawn 5, 7 and 5 days before surgery – with concomitant continuation of aspirin, whenever possible – if bleeding risk is unacceptably high.
Patients suitable for bridging strategies
• Bridging strategies should only be considered in patients at very high ischemic risk (active ischemia, high-risk coronary anatomy, surgery performed very early after stent implantation), in whom temporary discontinuation of the antiplatelets is considered inevitable due to accompanying elevated hemorrhagic risk.
The role of platelet function tests
• The role of platelet function tests in the perioperative management of patients on DAPT is not well established.
• The TARGET-CABG study is the only trial published to date evaluating the role of point-of-care platelet function testing in reducing perioperative bleeding events. The investigators concluded that a strategy based on preoperative platelet function testing to determine the timing of coronary artery bypass grafting in clopidogrel-treated patients was associated with the same amount of bleeding with that observed in clopidogrel-naive patients, while enabling an almost 50% shorter waiting time after clopidogrel discontinuation compared with the time recommended in the respective guidelines (5 days).
Specific agents as possible APT-bridging strategies
• Unfractionated heparin and low molecular weight heparins – patients not on oral anticoagulant:
–– The use of unfractionated heparin and low molecular weight heparins for bridging purposes is discouraged due to the lack of evidence supporting the efficacy and safety of this strategy.
• Unfractionated heparin and low molecular weight heparins – patients on oral anticoagulant:
–– Data on the perioperative management of this subset of patients are not found in literature;
–– The concomitant indication for an anticoagulant renders the perioperative management of such patients even more complicated, as they would probably require an anticoagulation-bridging strategy for perioperative protection from thromboembolic events, combined with decision-making for the antiplatelet medication.
• Glycoprotein IIb/IIIa-receptor inhibitors
–– Despite their theoretically suitable profile, evidence from randomized trials is totally lacking, and the results of small nonrandomized or retrospective studies have not been consistent;
–– Recent ESC guidelines state that the use of short acting glycoprotein IIb/IIIa inhibitors could be considered, after oral P2Y12-receptor inhibitor discontinuation, in very high-risk situations.
• Cangrelor
–– Although its characteristics theoretically approach the ideal of an APT-bridging drug, cangrelor is not yet commercially available, while the US FDA advisory committee recently voted against approval of the use of the agent for bridging purposes.
Conclusion
• Until more evidence is available, the golden rule is individualization of management in the context of a thorough multidisciplinary discussion, between the physicians responsible for the patient’s care.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- Windecker S, Kolh P, Alfonso F et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 35, 2541–2619 (2014).
- Kristensen SD, Knuuti J, Saraste A et al. 2014 ESC/ ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiacsurgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur. Heart J. 35(35), 2383–2431 (2014).
- Fleisher LA, Fleischmann KE, Auerbach AD et al. 2014 ACC/ AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 64(22), e77–e137 (2014).
- Cardiac Society of Australia and New Zealand. Guidelines for the management of antiplatelet therapy in patients with coronary stents undergoing non-cardiac surgery. Heart Lung Circ. 19(1), 2–10 (2010).
- Grobben RB, van Klei WA, Grobbee DE, Nathee HM. The aetiology of myocardial injury after non-cardiac surgery. Neth. Heart J. 21(9), 380–388 (2013).
- Berger PB1, Kleiman NS, Pencina MJ et al. Frequency of major noncardiac surgery and subsequent adverse events in the year after drug-eluting stent placement results from the EVENT (Evaluation of Drug-Eluting Stents and Ischemic Events) Registry. JACC Interv. 3(9), 920–927 (2010).
- Brilakis ES, Banerjee S, Berger PB. The risk of drug-eluting stent thrombosis with noncardiac surgery. Curr. Cardiol. Rep. 9(5), 406–411 (2007).
- Conroy M1, Bolsin SN, Black SA, Orford N. Perioperative complications in patients with drug-eluting stents: a three-year audit at Geelong Hospital. Anaesth. Intensive Care 35(6), 939–944 (2007).
- Cruden NL1, Harding SA, Flapan AD et al. Scottish Coronary Revascularisation Register Steering Committee. Previous coronary stent implantation and cardiac events in patients undergoing noncardiac surgery. Circ. Cardiovasc. Interv. 3(3), 236–242 (2010).
- Gandhi NK, Abdel-Karim AR, Banerjee S, Brilakis ES. Frequency and risk of noncardiac surgery after drug-eluting stent implantation. Catheter Cardiovasc. Interv. 77, 972–976 (2011).
- Iwata Y, Kobayashi Y, Fukushima K et al. Incidence of premature discontinuation of antiplatelet therapy after sirolimus-eluting stent implantation. Circ. J. 72, 340–341 (2008).
- To AC, Armstrong G, Zeng I, Webster MW. Noncardiac surgery and bleeding after percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2, 213–221 (2009).
- Hawn MT, Graham LA, Richman JR et al. The incidence and timing of noncardiac surgery after cardiac stent implantation. J. Am. Coll. Surg. 214, 658–666 (2012).
- Hajjar LA, Vincent JL, Galas FR et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 304(14), 1559–1567 (2010).
- Rossini R, Capodanno D, Lettieri C et al. Prevalence, predictors and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent inplantation. Am. J. Cardiol. 107, 186–194 (2011).
- Mehran R1, Baber U, Steg PG et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 382(9906), 1714–1722 (2013).
- Huber KC, Evans MA, Bresnahan JF, Gibbons RJ, Holmes DR. Outcome of noncardiac operations in patients with severe coronary artery disease successfully treated pre-operatively with coronary angioplasty. Mayo Clin. Proc. 67, 15–21 (1992).
- Kaluza GL, joseph J, Lee JR, Raizner ME, Raizner AE. Catastrophic outcomes of non-cardiac surgery soon after coronary stenting. J. Am. Coll. Cardiol. 35, 1288–1294 (2000).
- Planer D, Smits PC, Kereiakes DJ et al. Comparison of everolimus- and paclitaxel-eluting stents in patients with acute and stable coronary syndromes: pooled results from the SPIRIT and COMPARE trials. JACC Cardiovasc. Interv. 4(10), 1104–1115 (2011).
- Park KW, Kang SH, Velders MA et al. Safety and efficacy of everolimus- vs sirolimus-eluting stents; a systematic review and meta-analysis of 11 randomized trials. Am. Heart J. 165(2), 241–250 (2013).
- Baber U, Mehran R, Sharma SK et al. Impact of everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J. Am. Coll. Cardiol. 58, 1569–1577 (2011).
- Feres F, Costa RA, Abizaid A et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 310, 2510–2522 (2013).
- Wilson SH, Fasseas P, Orford JL et al. Clinical outcome of patients undergoing non-cardiac surgery in the two months following coronary stenting. J. Am. Cardiol. 42, 234–240 (2003).
- Nuttal GA, Brown MJ, Staumbaugh JW et al. Time and cardiac risk of surgery after bare-metal stent percutaneous coronary intervention. Anesthesiology 109, 588–595 (2008).
- Wijeysundera DN, Wijeysundera HC, Yun L et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 126, 1355–1362 (2012).
- Van Kuijk J-P, Flu W-J, Schouten O et al. Timing of noncardiac surgery after coronary artery stenting with bare metal or drug-eluting stents. Am. J. Cardiol. 104, 1229–1234 (2009).
- Sharma AK, Ajani AE, Hamwi SM et al. Major noncardiac surgery following coronary stenting: when is it safe to operate? Catheter Cardiovasc. Interv. 63, 141–145 (2004).
- Reddy PR, Vaitkus PT. Risks of noncardiac surgery after coronary stenting. Am. J. Cardiol. 95, 755–757 (2005).
- Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293(17), 2126–2130 (2005).
- Airoldi F, Colombo A, Morici N et al. Incidence and predictors of drug-eluting stent thrombosis during and after discontinuation of thienopyridine treatment. Circulation 116(7), 745–754 (2007).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562), 667–678 (2007).
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 310(14), 1462–1472 (2013).
- Karkouti K, Mc Cluskey SA, Syed S, Pazaratz C, Poonawala H, Crowther MA. The influence of perioperative coagulation status on postoperative blood loss in complex cardiac surgery: a prospective observational study. Anesth. Analg. 110, 1533–1540 (2010).
- Chassot PG, Delabays A, Spahn DR. Perioperative antiplatelet therapy: the case for continuing therapy in patients at risk for myocardial infarction. Br. J. Anaesth. 99(3), 316–328 (2007).
- Rossini R, Bramucci E, Castiglioni B et al. Coronary stenting and surgery: a prospective observational study. Anaesth. Analg. 110, 1533–1540 (2010).
- Vuylsteke A, Pagel C, Gerrard C et al. The Papworth bleeding Risk Score: a stratification scheme for identifying cardiac surgery patients at risk of excessive early postoperative bleeding. Eur. J. Cardiothorac. Surg. 39(6), 924–930 (2011).
- Burger W, Chemnitus JM, Kneissl GD, Rucker G. Low-dose aspirin for secondary cardiovascular prevention: cardiovascular risks after its peri-operative withdrawal vs. bleeding risks with its continuation. Review and meta-analysis. J. Int. Med. 257, 399–414 (2005).
- Fox KA, Mehta SR, Peters R et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) trial. Circulation 110, 1202–1208 (2004).
- Smith PK, Goodnough LT, Levy JH et al. Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis. J. Am. Coll. Cardiol. 60(5), 388–396 (2012).
- Varenhorst C, Alstrom U, Scirica BM et al. Factors contributing to the lower mortality with ticagrelor compared with clopidogrel in patients undergoing coronary artery bypass surgery. J. Am. Coll. Cardiol. 60(17), 1623–1630 (2012).
- Abualsaud AO, Eisenberg MJ. Perioperative management of patients with drug eluting stents. JACC Cardiovasc. Interv. 3(2), 131–142 (2010).
- Mahla E, Suarez TA, Bliden KP et al. Platelet function measurement-based strategy to reduce bleeding and waiting time in clopidogrel-treated patients undergoing coronary artery bypass graft surgery: the timing based on platelet function strategy to reduce clopidogrel-associated bleeding related to CABG (TARGET-CABG) study. Circ. Cardiovasc. Interv. 5, 261–269 (2012).
- Ferraris VA, Saha SP, Oesterreich JH et al. 2012 update to the Society of Thoracic Surgeons Guideline on use of anti-platelet drugs in patients having cardiac and noncardiac operations. Ann. Thorac. Surg. 94, 1761–1781 (2012).
- Hirsh J, Warkentin TE, Shaughnessy SG et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy and safety. Chest 119, 645–945 (2001).
- Vicenzi MN, Meislitzer T, Heitzinger B, Halaj M, Fleisher LA, Metzler H. Coronary artery stenting and non-cardiac surgery – a prospective outcome study. Br. J. Anaesth. 96(6), 686–693 (2006).
- Godet G, Le Manach Y, Lesache F, Perbet S, Coriat P. Drug-eluting stent thrombosis in patients undergoing non-cardiac surgery: is it always a problem? Br. J. Anaesth. 100(4), 472–477 (2008).
- Stettler C, Wandel S, Allemann S et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370(9591), 937–948 (2007).
- Bangalore S, Kumar S, Fusaro M et al. Outcomes with various drug eluting or bare metal stents in patients with diabetes mellitus: mixed treatment comparison analysis of 22,844 patient years of follow-up from randomised trials. BMJ 345, e5170 (2012).
- Bangalore S, Kumar S, Fusaro M et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117,762 patient-years of follow-up from randomized trials. Circulation 125(23), 2873–2891 (2012).
- Dewilde WJ, Oirbans T, Verheugt FW et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 381(9872), 1107–1115 (2013).
- Khong TK, Tamargo J. A–Z of cardiac drugs – aciximab. In: Drugs in Cardiology – A Comprehensive Guide to Cardiovascular Pharmacotherapy. Kaski JC (Ed.). OxfordUniversity Press, London, UK, 331 (2010).
- Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. PRISM-PLUS Study Investigators. N. Engl. J. Med. 338(21), 1488–1497 (1998).
- Angiolillo DJ, Firstenberg MS, Price MJ et al. BRIDGE investigators. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery: a randomized controlled trial. JAMA 307, 265–274 (2012).
•• Latest guidelines on antithrombotic management of stented patients and latest data on newer generation stents.
• Guidelines for the management of antiplatelet therapy in patients with coronary stents undergoing noncardiac surgery.
• Detailed analysis of the pathophysiology of perioperative myocardial injury.
• Only study having evaluated the role of platelet function tests in determining the optimal time for cardiac surgery in clopidogrel-treated patients.