Research Article - International Journal of Clinical Rheumatology (2019) Volume 14, Issue 5
Cyclophosphamide in a cohort of biologic naïve rheumatoid arthritis patients resistant to conventional DMARDs
- Corresponding Author:
- Noran O El-Azizi
Department of Internal Medicine and Faculty of Medicine
Ain Shams University Cairo
Egypt Tel: 09008740965
Fax: 416-603-6919
E-mail: nowara2005@yahoo.com
Abstract
Background: Rheumatoid Arthritis (RA) poses a significant economic burden in health services worldwide. The most important factor influences poor outcome in RA patients is the lack of drug adherence that is mainly due to financial cost and poor patient education. There is a significant number of RA patients fail to respond or show poor response to conventional DMARDs and they can’t afford the high cost of biologic therapy. Cyclophosphamide (CYC) is an immunosuppressive drug that inhibit the actively dividing inflammatory cells, suppressing the inflammatory cytokines and stops inflammation induced joint erosions in RA patients. To evaluate the efficacy of IV pulse methylprednisolone (MP) and Cyclophosphamide (CYC) in induction of remission in a cohort biologic naïve aggressive RA patients resistant to different combinations of conventional Disease-Modifying Anti Rheumatic Drugs (DMARDs). Methods and Findings: A prospective observational study performed on 30 RA patients diagnosed according to American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria, who fail to respond to different combinations of conventional DMARDs for at least two years. All patients subjected to history taking, rheumatological examination, assessment of RA disease activity, laboratory investigations; Complete Blood Count (CBC), Erythrocyte Sedimentation Rate (ESR), C-Reactive Protein (CRP), Rheumatoid Factor (RF) and anti-cyclic citrullinated peptide antibody (ACCP) recorded at baseline and after 6 months (study endpoint). All RA patients have been treated with monthly IV pulse 1 g MP and CYC at a dose 500 mg/m2 surface area for 6 months. On comparing baseline to endpoint data there was a significant reduction in all disease activity parameters: number of tender and swollen joints, disease activity scores 28 (DAS28), Health Assessment Questionnaire (HAQ), Visual Analog Scale (VAS), ESR, CRP and platelet (PLT) level, with increase in haemoglobin in all treated patients at the study endpoint. No significant difference between RF, ACCP seropositivity and different disease activity markers, except for increased haemoglobin in RF positive and reduction of PLT count in positive ACCP patients’ post-treatment. High CRP at baseline indicates poor prognosis, response to pulse CYC therapy and high VAS at the study endpoint. Conclusions: Pulse MP and CYC monthly for 6 months significantly benefit in induction of remission in a biologic naïve aggressive RA patients resistant to different combinations of conventional DMARDs.
Keywords
resistant aggressive RA patients • naïve Biologic RA patients • cyclophosphamide • pulse methylprednisolone
Abbreviations:
RA: Rheumatoid Arthritis; DMARDs: Disease Modified Anti Rheumatic Drugs; MP: Methylprednisolone; CYC: Cyclophosphamide
Introduction
Rheumatoid Arthritis (RA) is characterized by abnormal proliferation of synoviocytes, leukocyte infiltration, and angiogenesis [1]. Epidemiological studies show that RA affects 1% of the population worldwide [2]. The prevalence of RA is relatively constant in many populations at 0.5-1%. The prevalence of RA in rural Egypt is 0.29% similar to other oriental rural populations but lower than western populations [3]. The most important factor influences poor outcome in RA patients is the lack of drug adherence that is mainly due to financial coast and poor patient education [4,5].
RA poses a significant economic burden in health services worldwide. The therapeutic array of RA includes several categories of medicinal products, of varying potential [6]. There are several criteria for the classification medicine used in RA therapeutic protocol into: Symptommodifying anti-rheumatic drugs (non-steroidal anti-inflammatory drugs and corticosteroids), Disease Modifying Anti-Rheumatic Drugs (DMARDs), (antimalarial, sulfasalazine, methotrexate, and leflunomide) and the era of biological therapy [7]. The economic burden and the costs of biologics in treating RA patients are remarkably high beyond the financial capacity of many developing countries, keeping in consideration the large number of RA patients deserve this therapy- this makes biologics are important target for economic evaluations [8]. There is a significant number of RA patients fail to respond or show poor response to conventional DMARDs and they can’t afford for the high cost of biologic therapy and are not covered by any health insurance [9].
Cyclophosphamide (CYC) is a potent immunosuppressive cytotoxic drug that can inhibit the actively dividing inflammatory cells mainly of T and B lymphocytes populations, suppressing the inflammatory cytokines and stops inflammation in the rheumatoid synovium which induce joint damage and erosions in RA patients [10], it has been found to be effective in treating serious complications of rheumatoid arthritis such as vasculitis and Interstitial Pulmonary Fibrosis (IPF) [11]. However, by reviewing the literature there were very limited studies about the use of cyclophosphamide as a disease modifying anti-rheumatic drug in aggressive RA patients not responding to different combinations of conventional DMARDs.
Aim of the study was to evaluate the efficacy of pulse methylprednisolone (MP) and cyclophosphamide (CYC) in induction of remission in a cohort of biologic naïve aggressive RA patient’s resistant to different combinations of conventional DMARDs for at least two years with persistently high disease activity despite regular DMARDs therapy and they can’t afford for biological therapy.
Patients and methods
This is a prospective observational study that carried out at Ain Shams University hospital in the period from May 2016 to August 2017 on 30 RA patients diagnosed according to American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria of RA [12]; they were selected from rheumatology department, Ain Shams University hospital, who fail to respond to treatment by different combinations of two/ three conventional DMARDs with low dose corticosteroids for at least two years. After informed consent approved by Ain Shams University ethical committee all patients were subjected to full history taking, rheumatological examination with assessment of RA disease activity using disease activity score 28 (DAS28) [13], Health Assessment Questionnaire (HAQ) [14], and Visual Analog Scale (VAS) [15] scores. Laboratory investigations including Complete Blood Count (CBC) using coulter [16], Erythrocyte Sedimentation Rate (ESR) [17], C-Reactive Protein (CRP) with titer [18], Rheumatoid Factor (RF) using latex agglutination test and Anti-Cyclic Citrullinated Peptide antibody (ACCP) titre using ELISA technique at the study entry and at end point after 6 months. Pulse IV 1 g methylprednisolone (MP) (diluted in 250 cc glucose 5%) to be given slowly over 2 hours+CYC therapy at a dose 500 mg/m2 surface area (diluted on 500 cc glucose 5% to be given slowly over 2 hours).
Statistical analysis
Results were analysed using SPSS17. Chi-square test was used to compare qualitative variables. T-test was used to compare two independent quantitative variables. Pearson’s correlation coefficient test was used to rank different variables against each other's positively or inversely.
Results
Twenty-three (76.7%) RA patients were females, and 7(23.3%) were males. Their ages ranged from 18-68 years (mean ± SD) 39.77 ± 10.41 years. Disease duration ranged from 0.8-30 years (mean ± SD) 6.76 ± 5.91 years. 23 (76.7%) RA patients had positive RF and 20 (66.7%) patients had positive ACCP. On comparing the clinical and laboratory data at the study entry and at end point after 6 months: there are a highly statistically significant improvement in RA patients as regard: fever. As there was 25/30 (83%) patients having fever at baseline and only 3/28 (10.7%) patients are still having fever at endpoint (p<0.005). There is a highly statistically significant improvement in RA patient regarding the Swollen Joint Count (SJC) and Tender Joint Count (TJC), (11.04 ± 2.83) vs. (1.07 ± 1.18) SJC and (16.61 ± 3.01) vs. (3.43 ± 1.2) TJC respectively, (p<0.001). Also, there is a highly statistically significant improvement in all RA disease activity parameters assessed by DAS- 28, HAQ and VAS scores: DAS-28 (6.94 ± 0.43) vs. (2.90 ±0.57), HAQ (2.52 ± 0.23) vs. (0.32 ± 0.11), VAS (81.67 ± 8.07) vs. (9.58 ± 4.98) respectively (p<0.001). Moreover, there is significantly reduction in acute phase reactant: ESR, CRP and platelets count (PLT) between baseline and endpoint. While there is a highly statistically significant increase in haemoglobin level in RA patients after successful induction of remission and controlling disease activity at study endpoint compared to the baseline 9.36 ± 1.94 vs. 12.02 ± 0.90 g% (p<0.001) (Table 1).
Variables |
RA patients (n=30) | p | |
|---|---|---|---|
| Baseline (n=30) mean ± SD (Minimum-Maximum)/no. (%) | Endpoint (n=28) mean ± SD (Minimum-Maximum)/no. (%) | ||
| Fever | 25 (83.3) | 3 (10.7%) | <0.001 |
| SJC | 11.04 ± 2.83 (5-18) | 1.07 ± 1.18 (0-4) | <0.001 |
| TJC | 16.61 ± 3.01 (9-22) | 3.43 ± 1.20 (2-6) | <0.001 |
| DAS-28 | 6.94 ± 0.43 (5.8-7.78) | 2.90 ± 0.57 (1.75-3.9) | <0.001 |
| HAQ | 2.52 ± 0.23 (2.1-2.8) | 0.32 ± 0.11 (0.2-0.6) | <0.001 |
| VAS | 81.67 ± 8.07 (70-95) | 9.58 ± 4.98 (5-20) | <0.001 |
| ESR (mm/1st h) | 86.21 ± 15.67 (60-120) | 26.96 ± 9.40 (7-48) | <0.001 |
| CRP | 48.36 ± 26.81 (6-96) | 7.12 ± 4.09 (2.7-25) | <0.001 |
| Haemoglobin (g/dl) | 9.36 ± 1.94 (6.5-14) | 12.02 ± 0.9 (9.5-13.6) | <0.001 |
| Platelets (x103/mm3) | 329.14 ± 92.53 (134-480) | 237.39 ± 61.82 (120-409) | <0.001 |
SJC: Swollen Joint Count; TJC: Tender Joint Count; DAS-28: Disease Activity Score -28; HAQ: Health Assessment Questionnaire; VAS: Visual Analogue Scale; ESR: erythrocyte sedimentation rate; CRP: C-Reactive Protein.
Table 1. Comparison between RA patients at baseline and endpoint regarding clinical and laboratory data.
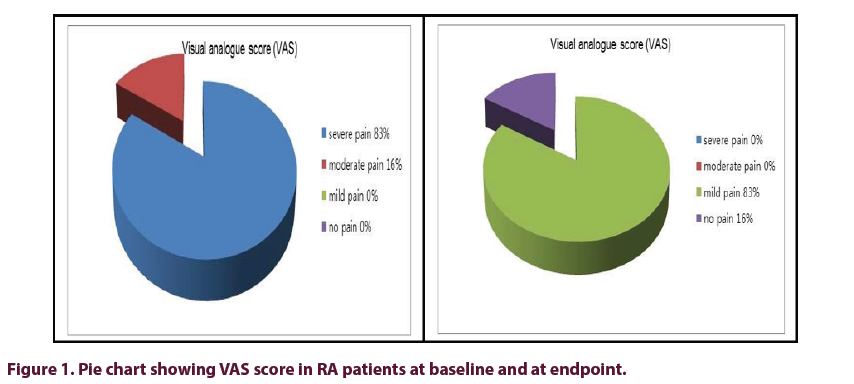
At baseline 28/30 (93%) of RA patients had severe disease activity by DAS-28, while at endpoint 19/28 (67.9%) patients were in remission or low disease activity and only 9/28 (32.1%) patients still had moderate disease activity; indicating significant improvement in DAS-28 score with treatment. At baseline 83% of patients have high pain score (VAS), while significant improvement in pain score occur at study endpoint (Figure 1).
In this study there is no significant difference between RF positive and RF negative patients regarding the disease activity parameters post treatment (P>0.05), except in haemoglobin level which is significantly increased with treatment in seropositive RF RA patients (p<0.05) (Table 2).
Variables |
RF patients (n=28) | ||
|---|---|---|---|
| Negative (n=7) mean ± SD (Minimum-Maximum) | Positive (n=21) mean ± SD (Minimum-Maximum) | p | |
| SJC | 1.43 ± 1.40 (0–4) | 0.95 ± 1.12 (0-3) | 0.367 |
| TJC | 3.86 ± 1.35 (2–6) | 3.29 ± 1.15 (2-6) | 0.283 |
| DAS-28 | 3.01 ± 0.66 (1.8–3.9) | 2.86 ± 0.55 (1.75-3.82) | 0.562 |
| HAQ | 0.25 ± 0.07 (0.2–0.35) | 0.35 ± 0.12 (0.25-0.6) | 0.159 |
| VAS | 11.25 ± 6.29 (5–20) | 8.75 ± 4.43 (5-15) | 0.439 |
| ESR (mm/1st h) | 26.86 ± 10.92 (7–40) | 27.00 ± 9.14 (10-48) | 0.973 |
| CRP | 9.67 ± 7.16 (2.7–25) | 6.27 ± 2.05 (2.7-10) | 0.055 |
| Hemoglobin (g/dl) | 11.43 ± 1.17 (9.5–13.1) | 12.22 ± 0.73 (10.9-13.6) | 0.042 |
| Platelets (x103/mm3) | 260.43 ± 70.70 (168–380) | 229.71 ± 58.42 (120-409) | 0.263 |
SJC: Swollen Joint Count; TJC: Tender Joint Count; DAS-28: Disease Activity Score -28; HAQ: Health Assessment Questionnaire; VAS: Visual Analogue Scale; ESR: erythrocyte sedimentation rate; CRP: C-Reactive Protein
Table 2. Comparison between RF Negative and Positive RA patient regarding clinical and laboratory data at study endpoint.
Also, there is no significant difference between ACCP positive and ACCP negative patients regarding the disease activity parameters post treatment (P>0.05), except in platelets count which is significantly reduced with treatment in seropositive ACCP RA patients (p<0.05) (Table 3).
Variables |
ACPA patients (n=28) | ||
|---|---|---|---|
| Negative (n=9) mean ± SD (Minimum-Maximum) | Positive (n=19) mean ± SD (Minimum-Maximum) | p | |
| SJC | 1.44 ± 1.33 (0–4) | 0.89 ± 1.10 (0-3) | 0.259 |
| TJC | 3.78 ± 1.56 (2–6) | 3.26 ± 0.99 (2-5) | 0.298 |
| DAS-28 | 3.09 ± 0.63 (2.2–3.9) | 2.81 ± 0.54 (1.75-3.82) | 0.241 |
| HAQ | 0.30 ± 0.08 (0.2–0.4) | 0.33 ± 0.15 (0.2-0.6) | 0.634 |
| VAS | 10 ± 6.32 (5–20) | 9.17 ± 3.76 (5-15) | 0.787 |
| ESR (mm/1st h) | 29.56 ± 10.39 (16–48) | 25.74 ± 8.92 (7-40) | 0.325 |
| CRP | 7.11 ± 1.54 (6–10) | 7.13 ± 4.91 (2.7-25) | 0.993 |
| Hemoglobin (g/dl) | 11.93 ± 1.28 (9.5–13.6) | 12.06 ± 0.70 (10.9-13) | 0.729 |
| Platelets (x103/mm3) | 272.89 ± 79. 9 (188–409) | 220.58 ± 44.36 (120-291) | 0.034 |
SJC: Swollen Joint Count; TJC: Tender Joint Count; DAS-28: Disease Activity Score -28; HAQ: Health Assessment Questionnaire; VAS: Visual Analogue Scale; ESR: erythrocyte sedimentation rate; CRP: C-Reactive Protein
Table 3. Comparison between ACCP Negative and ACCP Positive RA patients clinical and laboratory data at study endpoint.
By Pearson’s correlation test, there is significant positive correlation between CRP at base line and VAS score at study endpoint, as the higher the CRP at baseline the higher the VAS score at endpoint i.e. poor response to therapy (R=0.738, P- value=0.006). However, there was nonsignificant statistical correlation between ESR at the baseline and each of: DAS-28, HAQ, VAS scores at the study endpoint (Table 4).
| CRP baseline | r | p-value | |
|---|---|---|---|
| DAS-28 endpoint | 0.184 | 0.35 | |
| HAQ endpoint | 0.166 | 0.607 | |
| VAS endpoint | 0.738 | 0.006 S | |
| ESR baseline | DAS-28 endpoint | 0.265 | 0.173 |
| HAQ endpoint | 0.092 | 0.777 | |
| VAS endpoint | 0.318 | 0.314 |
CRP: C-Reactive Protein, ESR: erythrocyte sedimentation rate, DAS-28: Disease Activity Score -28, HAQ: Health Assessment Questionnaire, VAS: Visual Analogue Scale.
Table 4. Correlation between CRP and ESR with different disease activity parameters DAS-28, HAQ and VAS score at study endpoint.
Discussion
RA causes considerable burden upon the society and the governments in terms of morbidity and mortality, long- term disability and economic cost [19]. Treatment of RA imposes a significant economic burden worldwide; the economic burden of RA is thought to be substantial for both patients and the national health services. In Egypt the average yearly cost of RA patient is statistically undetermined, however, the Quality of Life (QoL) is significantly impaired [20]. The poor socioeconomic status in developing countries makes it very difficult to cover all RA patients under the umbrella of the national health insurance. In the era of biologics, this is especially true when we speak about the high financial coast of biological therapy [8].
Cyclophosphamide is one of the alkylating agent and a well-known potent immunosuppressive, inhibiting the actively dividing inflammatory cells mainly T and B lymphocytes, suppress inflammatory cytokines, interrupts the ongoing inflammatory autoimmune process, reduces inflammation, hence inhibits joint damage by the actively dividing synovial inflammatory cells [10]. Cyclophosphamide has been found to be effective in treating serious complications of RA such as vasculitis. Various types of small and medium sized vessels vasculitis. Because of its serious side effects, cyclophosphamide is most often reserved for use in people with severe RA that has not responding to other DMARDs [21].
We studied 30 patients with RA who satisfied the ACR/ EULAR classification criteria of RA [12]. Patients data was collected at baseline, history taking and clinical examination with special emphasis on musculoskeletal examination, assessment of DAS-28, VAS and HAQ scores were done at baseline and at study endpoint. Laboratory investigations including; CBC, ESR, CRP, RF and ACCP were done. All patients received monthly pulse MP and CYC 500 mg/ m2 IV drip for 6 consecutive months, follow up data was collected and reassessment of different disease activity scores: DAS-28, VAS, HAQ and laboratory investigations CBC, ESR, CRP were done again at the study endpoint (after six months’ induction therapy).
At baseline 93% of RA patients had severe disease activity mean DAS-28 (6.94±0.43) while at endpoint 67% of RA patients enter into remission or low disease activity DAS- 28 (2.90 ± 0.57), (p<0.001); this indicating marked improvement in all RA disease activity parameters. Interestingly, this improvement has no relation to baseline RF or ACCP sero positivity as there was no significant difference regarding all disease activity scores (DAS -28, HAQ or VAS) at the study endpoint, between sero positive and sero negative RA patients, indicating that baseline RF/ACCP sero positivity has no relation to the patient’s response to this therapy in this RA cohort. While Hb level was significantly improved on induction of remission in patients with positive RF than in patients with negative RF (p<0.05). This agreed with Walters and Cawley [22], who stated that IV pulses of methylprednisolone (MP) produce relief of symptoms and improvement in laboratory tests lasting for up to three months. Oral cyclophosphamide has been shown to be of benefit in the treatment of active RA over an eight-month period. When given for RA vasculitis as an intermittent IV bolus combined with MP, the side effects of CYC are reduced without loss of the therapeutic effect and suggested that MP given alone or combined with CYC may induce a clinical remission in severe active RA.
Williams et al. study [23], had used CYC orally in treatment of RA patients, they stated that oral CYC has been of benefit in severe intractable RA, but the possibility of inducing serious longterm side effects, such as haemorrhagic cystitis, carcinoma of the bladder, leukaemia or lymphoma has caused concern. There are few published data on the use of intermittent IV pulses of CYC in active RA, although when combined with MP improves rheumatoid vasculitis, with a low incidence of side effects.
Wallace and Sherry study [24], had reported that pulse CYC with MP has been proposed for induction of remission of severe systemic-onset juvenile RA that failed to respond to conventional DMARDs. Four children (two males, two females) with systemic- onset juvenile RA, joint destruction and polyarthritis that remained active despite maximal therapy with different combination of DMARDs, they were given intravenous CYC (500-1000 mg/m2) and MP 30 mg/kg/day (1g maximum) monthly. Patients received six to ten monthly treatments followed by two to thirteen subsequent treatments every two to three months. All patients showed clinical improvement with 12-20 intravenous pulses. Three patients achieved disease remission despite the discontinuation of CYC.
Additionally, Suarez-Almazor et al. [10] stated that CYC appears to have a clinically statistically significant benefit in controlling disease activity of patients with RA, similar to some DMARDs; such as anti-malarial or sulfasalazine, but in a disagreement with our results - they said- CYC has a lower efficacy than methotrexate in controlling RA disease activity.
In agreement with our results, Townes et al. [25] in a double-blind study compared high dose CYC and placebo on 24 RA patients they noted that CYC group showed significant improvement in all measures of disease activity including number of tender joints, swollen joints, morning stiffness and grip strength.
In this study there was significant reduction in RA induced thrombocytosis as a successful response to pulse CYC and MP therapy, this response was more observed in RA patients with positive ACCP than in patients with negative ACCP (P=0.034).
Importantly, the clinical and laboratory improvement observed in our RA patients after this induction regimen was not correlated with any of the patient’s baseline data: DAS-28, SJC, TJC or ESR. However, this was not true regarding the baseline CRP, the response to this treatment was negatively correlated with the baseline CRP level, the higher CRP at baseline the higher will be the VAS score at the study end point, indicating poor prognosis and poor response to this treatment protocol [26], stated that RA patients with severe arthritis, high ESR and CRP indicate poor prognosis, joint damage and functional disability.
However, Lidsky et al. [27] disagree and reported that, RA patients treated with 50-75 mg of CYC daily were not significantly different from RA patients received placebo. A possible explanation for this difference is the lack of control for concomitant treatment and the CYC was given by oral root and absence of concomitant MP pulse therapy. Meanwhile, Gaffney and Scott [28], stated that CYC is effective in treatment of severe extra-articular manifestations of RA but is ineffective in treatment for rheumatoid synovitis and may even results in mild flare of arthritis which disagrees with our results. This difference may be due to different patient characteristics or different ethnicity. The limitation of this study was that there were no control group used.
Conclusion
IV pulse MP and CYC monthly for 6 months is of a significant benefit in induction of remission in a cohort of biologic naïve RA patients with aggressive, resistant disease, failed to respond to conventional DMARDs. However, further multicentre wide scale studies are much needed for better assessment of this simple effective and cheap treatment protocol.
References
- Park YJ, Yoo SA, Kim WU. Role of Endoplasmic Reticulum in pathogenesis of Rheumatoid Arthritis. Korean. Med. Sci. 29(1), 2-11 (2014).
- Rojas-Villarraga A, Bayona J, Zuluagaet N et al. The impact of rheumatoid foot on disability in Colombian patients with rheumatoid arthritis. BMC. Musculoskelet. Disord. 10(1), 67 (2009).
- El-Labban AS, Omar HAA, El-Shereif RR et al. Pattern of young and old onset rheumatoid arthritis (YORA and EORA) among a group of Egyptian patients with rheumatoid arthritis. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders. 3, 25 (2010).
- Alhefny AE, Abd El-Rahman MA, Abd El-Moteleb S et al. Evaluation of Adherence to Drug Treatment in Patients with Rheumatoid Arthritis. Egypt. J. Rheumatol. Clin. Immunol. 4(1), 81-92 (2016).
- Uhlig T, Moe R, Kvien K. The burden of disease in rheumatoid arthritis. Pharmacoeconomics. 32(9), 841-851 (2014).
- Fazal SA , Khan M, Nishi SE et al. A Clinical Update and Global Economic Burden of Rheumatoid Arthritis. Endocrine Metabolic & Immune Disorders - Drug Targets. 18(2), 98-109 (2018).
- Negrei C, Bojinca V, Balanescu A et al. Management of rheumatoid arthritis: Impact and risks of various therapeutic approaches. Exp. Ther. Med. 11(4): 1177-1183 (2016).
- Joensuu JT, Huoponen S, Aaltonen KJ et al. The coast effectiveness of biologics for the treatment of Rheumatoid arthritis. A Systemic Review. Plos One. 10(3), e0119683 (2015).
- SA
- SE
- Longo DL, Fauci AS, Kasper DL. Harrison's principles of internal medicine. 18th edn The McGraw- Hill Companies. 2, 2738-2752 (2012).
- Aletaha D, Neogi T, Silman AJ et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis. Rheum. 62(9), 2569-2581 (2010).
- Prevoo MLL, Hof van't MA, Kuper HH et al. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis. Rheum. 38(1), 44-48 (1995).
- Pincus T, Sokka T. Can Multi-Dimensional Health Assessment Questionnaire (MDHAQ) and Routine Assessment of Patient Index Data (RAPID) scores be informative in patients with all rheumatic diseases? Best. Pract. Res. Clin. Rheumatol. 21(4), 733–753(2007).
- Hawker G, Mian S, Kendzerska T et al. Measures of adult pain: Visual analog scale for pain, numeric rating scale for pain, pain questionnaire, short-form pain questionnaire, chronic pain grade scale, short form-36 bodily pain scale and measure of intermittent and constant osteoarthritis pain. Arthritis. Care. Res. 63(S11), S240-S252 (2011).
- Britten E, Brecher G, Johnson C. Evaluation of the coulter counter model S. Am. J. Clin. Pathol. 52(6), 679-689 (1969).
- Farheen K, Agarwal S.Assessment of Disease Activity and Treatment Outcomes in Rheumatoid Arthritis. J. Manag. Care. Pharm. 17(9), S09-S13 (2011).
- Meyer O, Labarre C, Dougados M et al. Anticitrullinated protein/ peptide antibody assays in early rheumatoid arthritis for predicting five-year radiographic damage. Ann. Rheum. Dis. 62(2), 120-126 (2011).
- Cooper NJ. Economic burden of rheumatoid arthritis, a systematic review. Rheumatology. 39(1), 28-33 (2009).
- Gamal RM, Mahran SA, El Fetoh NA et al. Quality of life assessment in Egyptian rheumatoid arthritis patients: Relation to clinical features and disease activity. Egypt. Rheumatologist. 38(2), 65-70 (2016).
- Watts RA, Ntatsaki E. Refractory rheumatoid vasculitis- a therapeutic dilemma. Oxford. Med. Case. Reports. 2016(11), omw081 (2016).
- Walters MT, Cawley M. Combined suppressive drug treatment in severe refractory rheumatoid disease: an analysis of the relative effects of parenteral methylprednisolone cyclophosphamide, and sodium aurothiomalate. Ann. Rheum. Dis. 47(11), 924-929 (1988).
- Williams HJ, James CR, John RW et al. Comparison of high and low dose cyclophosphamide therapy in rheumatoid arthritis. Arthritis. Rheumatol. 23(5): 521-527 (1980).
- Wallace CA, Sherry DD. Trial of intravenous pulse cyclophosphamide and methylprednisolone in the treatment of severe systemic-onset juvenile rheumatoid arthritis. Arthritis. Rheum. 40(10), 1852-1855 (1997).
- Townes AS, Sowa JM, Shulman LE. Controlled trial of cyclophosphamide in rheumatoid arthritis. Arthritis. Rheum. 19563-19573 (1976).
- Scott DL. Prognostic factors in early Rheumatoid arthritis. Rheumatology. 39(1): 24-29 (2000).
- Lidsky MD, Sharp JT, Billings S. Double-blind study of cyclophosphamide in rheumatoid arthritis. Arthritis. Rheum. 16(2), 148-153 (1973).
- Gaffney K, Scott DGI. Azathioprine and cyclophosphamide in the treatment of rheumatoid arthritis. British. J. Rheumatol. 37(8), 824-836 (1998).