Research Article - Imaging in Medicine (2010) Volume 2, Issue 6
Day-to-day variation in Doppler activity in patients on stable etanercept treatment: an exploratory cohort study
Lene Terslev1, Klaus Müller2,3, Robin Christensen1, Karen Ellegaard1, Søren Torp-Pedersen1, Sif Gudbrandsdottir2 and Henning Bliddal†1
1The Parker Institute, Department of Rheumatology, Frederiksberg Hospital, DK 2000, Frederiksberg, Denmark
2Institute of Inflammatory Research, Department of Rheumatology, Rigshospitalet, Copenhagen, Denmark
3Paediatric Clinic II, Rigshospitalet, Copenhagen, Denmark
- *Corresponding Author:
- Henning Bliddal
The Parker Institute, Department of Rheumatology
Frederiksberg Hospital, DK 2000, Frederiksberg, Denmark
Tel: +45 3816 4155
Fax:+45 3816 4159
E-mail: henning.bliddal@frh.regionh.dk
Abstract
Aim: Doppler ultrasound was used to evaluate the day-to-day variation in inflammatory activity in rheumatoid arthritis patients on stable TNF alpha blocker treatment.
Materials & methods: A total of 16 patients with rheumatoid arthritis were included in the study, according to the American College of Rheumatology criteria of unaltered etanercept dosage for the last 3 months. All patients had a stable total TNF-binding capacity during the examination period. The most swollen joint was chosen as the target joint (11 wrist joints and five metacarpophalangeal joints) and was examined by ultrasound the morning before injection of 25 mg etanercept at day 0, and the same time on days 1, 2 and 3 after the injection. Color Doppler and spectral Doppler were used to estimate the inflammation. The C reactive protein was measured at each visit.
Results: The disease activity was assessed only at baseline. Mean disease activity score 28 was 3.97. Only slight changes in ultrasound variables were seen over the 4 day period. Overall, there was no statistically significant effect of time, and all pairwise comparisons were nonsignificant (p > 0.14). No changes were found for C reactive protein and TNF-binding capacity between the individual visits (time effect: p = 0.85 and p = 0.23, respectively).
Conclusion: There was no significant day-to-day variation in Doppler parameters in patients on stable etanercept treatment. Ultrasound may therefore be performed at any time between two etanercept injections and significant alterations in Doppler activity may be a potential indicator of change in disease activity.
Keywords
Doppler; rheumatoid arthritis; TNF‑a; ultrasound
TNF is a proinflammatory cytokine that plays a central role in the inflammatory process in rheumatoid arthritis (RA). The levels of TNF have been shown to be elevated in both serum and synovial fluid in patients with active disease. Currently, there are several therapeutic agents, which neutralize TNF, thereby inducing a swift response on the level of disease activity and even remission. These TNF inhibitors are administered at varying intervals from twice weekly (etanercept) over every other week (adalimumab) to every eighth week (infliximab).
It must be assumed that serum levels of the drug would be highest immediately after injection, and lowest immediately before the next administration per se. For etanercept, it has been shown that by administering the drug twice weekly, subcutaneously, a stable blood concentration of the drug is obtained [1]. In a previously published study, it has also been demonstrated that, in patients on stable treatment, TNF-binding capacity in the serum is also stable. This indicates stable levels of biologically active drug between two administrations of the drug [2]. It remains, however, to be demonstrated whether or not the drug administration itself affects the inflammatory activity in the joints, thereby causing fluctuation in Doppler between drug administrations. The effect on disease activity may be estimated by ultrasound using Doppler measurements of hyperemia (color fraction) and vascular resistance (resistive index [RI]). These variables have previously been validated and were demonstrated to be sensitive to changes when initiating both local and systemic treatment [3,4]. Possible alterations in disease activity before or after drug administration would be of importance for choosing when to evaluate the patient by ultrasound.
Therefore, Doppler ultrasound was used to evaluate the day-to-day variation in inflammatory activity in RA patients on stable TNF‑a blocker treatment.
Materials & methods
In a previous study the level of TNF was determined in 16 RA patients on stable etanercept treatment (14 patients received 25 mg subcutaneously twice weekly and two received 50 mg of the drug subcutaneously once weekly) day-to-day over a 4‑day period [2]. These patients were considered the cohort in this exploratory study. The patients had been on stable therapy for a minimum of 11 months and on unaltered dosage for at least 3 months. No individual variation in the level of TNF-binding capacity was found during the observation period but a fairly high degree of interindividual variation was found, which to some extent was related to differences in levels of C‑reactive protein (CRP) [2].
Subjects with RA & measurements
The patients (three men and 13 women; mean age 63 [38–76] years) fulfilled the American College of Rheumatology criteria with a mean duration of etanercept treatment of 27 (11–47) months [5]. Disease activity was assessed at baseline with the disease activity score (DAS)‑28 using CRP. All patients had a stable total TNF-binding capacity during the examination period, as previously described [2]. The joint on the hand with the most pronounced Doppler activity was chosen as the target joint (11 wrist joints and five metacarpophalangeal joints) and was examined by ultrasound in the morning before the injection of etanercept (day 0) and the same time on days 1, 2 and 3 after the injection. The CRP was measured at each visit.
The study was approved by The Committees on Biomedical Research Ethics for the Capital region of Denmark and informed consent from all patients was obtained.
Ultrasound
The joints of the hands were examined with an Acuson Sequoia® (CA, USA) equipped with a 15 MHz linear array probe. The patient was examined in an upright position with the hand of interest placed on a cushion, palm down and in a neutral relaxed position.
The target joint was only examined on the dorsal side. The wrist was scanned from side-to-side in the longitudinal plane and from superior to inferior in the transverse plane. The metacarpophalangeal joints were scanned on the longitudinal plane and dorsal aspect only.
On the Acuson Sequoia ultrasound machine the color Doppler is more sensitive than the power Doppler when using optimal settings for slow flow. The color Doppler was therefore used in the present study in order to obtain the most information on inflammatory activity in the investigated joints. The color Doppler settings for the examinations were the same for all joints (gain setting just below the noise level, Nyquist limit (±0.014 m/s) and 7 MHz Doppler frequency).
The synovial vascularization in the joints was visualized by color Doppler and the flow pattern of the synovium in the target joints was evaluated using spectral Doppler with calculation of the RI. The mean RI was estimated using three measurements, if possible, at three different places in the synovial hypertrophy, and a mean value was used as an estimate of the synovial inflammation.
Image evaluation
Quantitative estimation of the vascularization in the synovial membrane was performed using the color Doppler image with maximum color activity selected for analysis. The digitally stored color Doppler image in the Digital Imaging and Communications in Medicine (DICOM) format was transferred to a processing program (Corel Photo-paint 7®). The synovium inside the color box was traced, thereby defining a region of interest. The amount of color pixels was then expressed in relation to the total amount of pixels in the marked region of interest (i.e., the color fraction) [6].
Statistical analysis
The experimental design leads to a natural hierarchy of the random effects as it includes repeated measures on the same patient. All analyses were done using SAS 9.1.3 (SAS Institute Inc., NC, USA). We used a linear mixed effects model, handling patients as a random factor (with 16 levels). This was fitted in SAS via a restricted maximum likelihood method using PROC MIXED. We applied a square-root-transformation for RI and color fraction, and a log-transformation for the CRP. A 95% CI was obtained on the transformed scale. Such an interval is not symmetric, reflecting the asymmetric distribution on the original scale.
Results
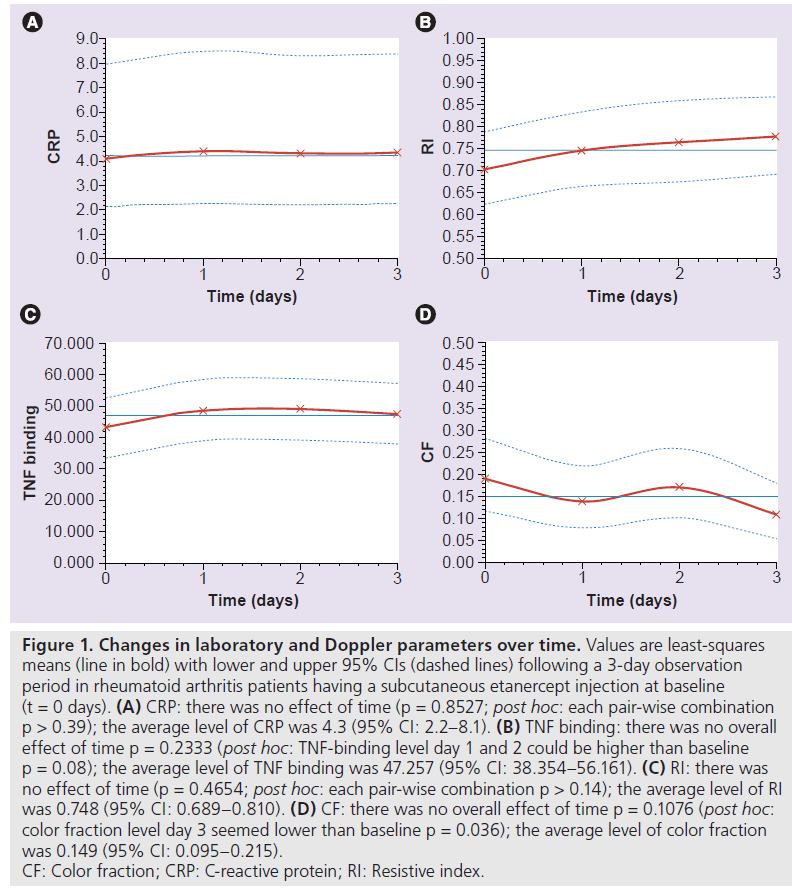
As presented in Table 1 the disease activity at the time of investigation – assessed only at baseline – showed a mean DAS‑28 score of 3.97. As illustrated in Figure 1, there were only slight changes in ultrasound variables over the 4‑day period. RI changed from 0.71 (95%CI: 0.63; 0.79) to 0.78 (95%CI: 0.69; 0.87); overall there was no statistical significant effect of time, and all pair-wise comparisons were nonsignificant (p > 0.14). Color fraction decreased from 0.19 (0.12; 0.28) to 0.11 (0.05; 0.18), with a tendency towards a time effect; however, there was a significant difference between baseline and day 3 (p = 0.036). No changes were found for CRP and TNF-binding capacity between the individual visits (time effect: p = 0.85 and p = 0.23, respectively).
Figure 1. Changes in laboratory and Doppler parameters over time. Values are least-squares means (line in bold) with lower and upper 95% CIs (dashed lines) following a 3-day observation period in rheumatoid arthritis patients having a subcutaneous etanercept injection at baseline (t = 0 days). (A) CRP: there was no effect of time (p = 0.8527; post hoc: each pair-wise combination p > 0.39); the average level of CRP was 4.3 (95% CI: 2.2–8.1). (B) TNF binding: there was no overall effect of time p = 0.2333 (post hoc: TNF-binding level day 1 and 2 could be higher than baseline p = 0.08); the average level of TNF binding was 47.257 (95% CI: 38.354–56.161). (C) RI: there was no effect of time (p = 0.4654; post hoc: each pair-wise combination p > 0.14); the average level of RI was 0.748 (95% CI: 0.689–0.810). (D) CF: there was no overall effect of time p = 0.1076 (post hoc: color fraction level day 3 seemed lower than baseline p = 0.036); the average level of color fraction was 0.149 (95% CI: 0.095–0.215). CF: Color fraction; CRP: C-reactive protein; RI: Resistive index.
Discussion
In the 16 patients on stable TNF‑a blocker treatment, no signs of day-to-day variation in Doppler activity were found. All the patients in the present study had measurable Doppler activity in the chosen target joint. The study demonstrates that on stable TNF-binding capacity there were no statistical significant differences in color fraction or RI before and between two injections with eternacept, indicating no variability in inflammatory activity in patients on stable treatment even when administering a drug with a short half-life [2]. Whether this is the case for other anti-TNFs given at much longer administration intervals remains to be clarified.
The borderline decrease in color fraction from baseline to day 3 did not correlate to any clinical parameters.
The use of one target joint instead of multiple joint analyses for the evaluation of disease activity was validated in a recent study [7]. Single joint analysis correlates well with other disease activity markers, although further studies are warranted in this area. The present study includes a smaller cohort of patients and the results will need to be validated in a larger cohort to eliminate a type 2 error (accepting no difference although there actually is one).
The present study has indicated that Doppler measurements may be performed at any time during treatment and with no specific relation to the time the drug is administered. The lack of significant changes in color fraction and RI indicate that alterations in Doppler activity are a potential marker of changes in disease activity, at least on group level. Our results will need to be confirmed in a larger cohort of patients on stable treatment.
To some extent the findings support the ongoing validation of the use of Doppler, as no apparent fluctuations of significant importance were found from day-to-day in patients on stable treatment. The variables used in the present study have already been validated in previous studies [3,4,8].
Conclusion
No significant day-to-day variation in color fraction or mean RI was found in this exploratory cohort of patients on stable etanercept treatment. Ultrasound may, therefore, be performed at any time between two etanercept injections and significant alterations in Doppler activity may possibly be regarded as a sign of change in disease activity.
Financial & competing interests disclosure
L Terslev, K Ellegaard and S Torp-Pedersen have received speaker’s honorarium from Wyeth. S Gudbrandsdottir has received educational grants from Amgen. R Christensen and H Bliddal have received research or institutional support, educational grants, equipment, services or expenses from Abbott, Amgen, Astellas Pharma, Axellus, Bristol-Myers Squibb, Cambridge Nutritional Foods, Dansk Droge, DSM Nutritional Products, Expanscience, Hyben Vital, HypoSafe A/S, MundiPharma, NorPharma, NutriCare, Pharmavie, Pfizer, Roche, Sanofi-Aventis, Scandinavian Clinical Nutrition and Wyeth. This study was supported by the Oak Foundation and Helsefonden. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Papers of special note have been highlighted as:
* of interest
References
- Jarvis B, Faulds D: Etanercept: a review of its use in rheumatoid arthritis. Drugs 57, 945–966 (1999).
- Gudbrandsdottir S, Bliddal H, Petri A et al.: Plasma TNF binding capacity profiles during treatment with etanercept in rheumatoid arthritis. Scand. J. Rheumatol. 33, 385–388 (2004). & Describes earlier findings on TNF-binding capacity in patients on stable etanercept treatment.
- Terslev L, Torp-Pedersen S, Qvistgaard E, Danneskiold-Samsoe B, Bliddal H: Estimation of inflammation by Doppler ultrasound: quantitative changes after intra-articular treatment in rheumatoid arthritis. Ann. Rheum. Dis. 62(11), 1049–1053 (2003). & Applied Doppler parameters for evaluating inflammation and changes.
- Terslev L, Torp-Pedersen S, Qvistgaard E et al.: Effects of treatment with etanercept (Enbrel, TNRF:Fc) on rheumatoid arthritis evaluated by Doppler ultrasonography. Ann. Rheum. Dis. 62(2), 178–181 (2003). & Applied Doppler parameters for evaluating inflammation and changes.
- Arnett FC, Edworthy SM, Bloch DA et al.: The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31(3), 315–324 (1988).
- Qvistgaard E, Røgind H, Torp-Pedersen S, Terslev L, Danneskiold-Samsøe B, Bliddal H: Quantitative ultrasonography in rheumatoid arthritis: evaluation of inflammation by Doppler technique. Ann. Rheum. Dis. 60(7), 690–693 (2001). & Describes the color fraction.
- Ellegaard K, Torp-Pedersen S, Terslev L, Danneskiold-Samsøe B, Henriksen M, Bliddal H: Ultrasound color Doppler measurements in a single joint as measure of disease activity in patients with rheumatoid arthritis – assessment of concurrent validity. Rheumatology 48, 254–257 (2009).
- Terslev L, Torp-Pedersen S, Qvistgaard E, Bliddal H: Spectral Doppler and resistive index. A promising tool in ultrasonographic evaluation of inflammation in rheumatoid arthritis. Acta Radiol. 44(6), 645–652 (2003).