Short Article - Interventional Cardiology (2011) Volume 3, Issue 3
Deciding on the best course of action for grade III coronary perforations
- Corresponding Author:
- Eric Eeckhout
Centre Hospitalier Universitaire Vaudois, Division of Cardiology
1011 Lausanne, Switzerland
Tel: +41 795 568 862
Abstract
Keywords
complications, coronary angioplasty, coronary perforations
Coronary perforation remains one of the most serious complications in the catheterization laboratory, with multiple studies demonstrating very poor early and late outcomes. Although preventable to a great extent, perforation is inevitable in any high volume center. With conventional balloon angioplasty, the incidence is estimated to be 0.1–0.2% [1–7], whereas substantially higher rates of up to 3% have been reported with the use of atherectomy devices (i.e., directional atherectomy, rotablation, excimer laser angioplasty or extractional atherectomy) [8–10].
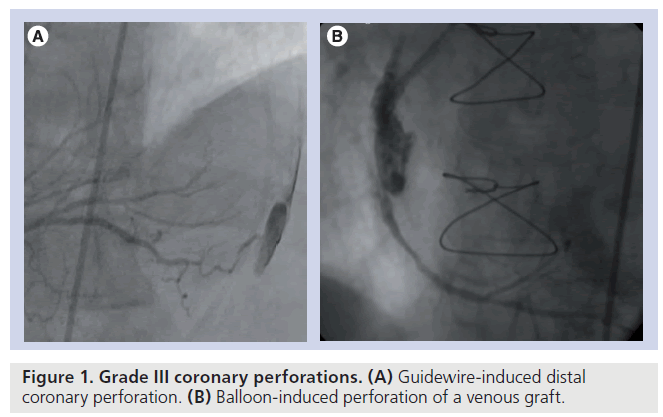
Ellis et al. proposed a classification for coronary perforation, based on angiographic appearance [1]. In this classification, a type I perforation is an extraluminal crater limited to the media or adventitia without contrast extravasations, a type II perforation is a myocardial or pericardial blush without contrast jet extravasations, while a type III perforation is an extravasation through a 1 mm or larger perforation. If the jet is directed toward an anatomic cavity (coronary sinus and ventricle), the perforation is classified as cavity spilling type or type IV or alternatively type IIIB, as opposed to the IIIA where the jet is directed toward the pericardium (Figure 1). Muller et al. recently proposed a modification of the Ellis classification, adding a type V perforation describing the distal coronary perforation by the guidewire [11].
Mechanisms, incidence & outcome
Several mechanisms can be implicated in perforation appearance:
▪ Excessive ablation of atheroma tissue by an atherectomy device [7–10];
▪ Perforation of the arterial wall by a sharp object, such as the end of a guidewire [12–14];
▪ Perforation caused by the excessive stretching of the vessel wall by an oversized balloon or stent [15].
Grade III coronary perforation or coronary rupture is an extremely rare, albeit life threatening, complication of percutaneous coronary intervention. It is associated with an increased rate of in-hospital and long-term adverse events, which include emergency surgery, myocardial infarction and death. These outcomes are mostly the consequence of hemodynamic compromise, resulting from pericardial effusion and tamponade, myocardial ischemia due to failure to treat the target lesion or intended vessel occlusion.
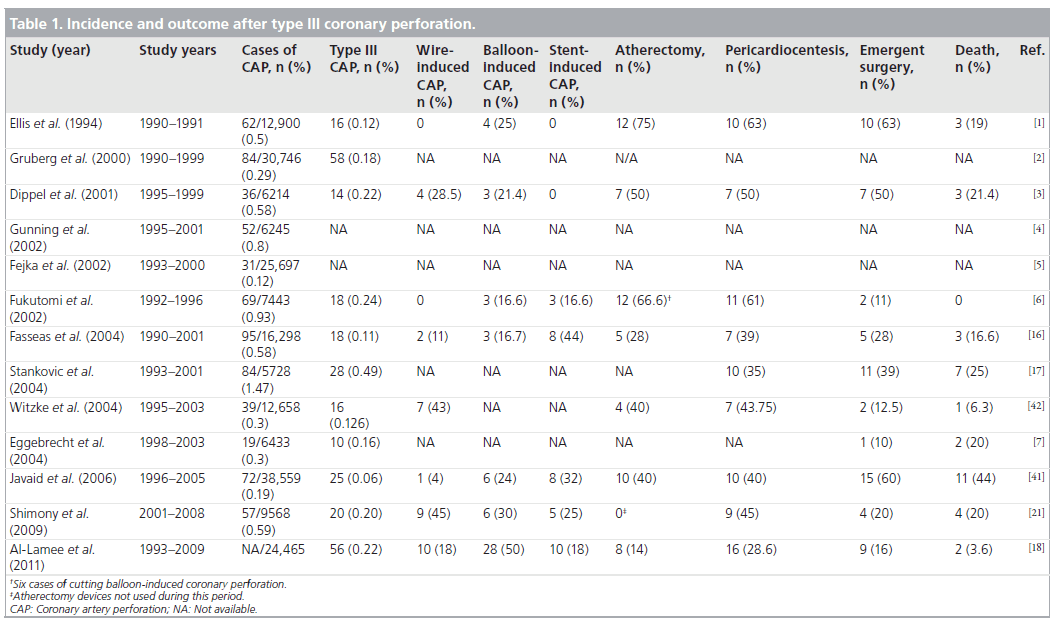
The reported incidence of grade III coronary perforations ranges from 0.06 to 0.49%. According to the literature (Table 1), tamponade may complicate 21–55% of cases [16,17], with pericardiocentesis needed in 28–63% of patients with grade III coronary perforations [1,18]. Emergency surgery was reported in 28–63% in earlier series [1,16]. More recent reports demonstrate a lower incidence of emergency surgery (2–20%) [19–21]. This reduction is likely related to a decreased use of atheroablative devices, a higher proportion of guidewire-induced distal coronary perforations that can be managed often conservatively or percutaneously, and the availability of covered stents. Grade III coronary perforations are clearly associated with far worse outcome than types I and II. The mortality in type III perforation is 19% [1]. When pericardial tamponade occurs, mortality is 42% [5].
Management
Treatment of coronary perforation depends on the size of the perforation, the extent of contrast medium extravasations and the hemodynamic status of the patient. The management strategy is dictated by the need to stop coronary extravasations and to relieve hemodynamic compromise. Generally, this can be achieved by means of prolonged balloon inflation and, if necessary, a perfusion balloon catheter to reduce myocardial ischemia. A pericardiocentesis set should be available in the case of cardiac tamponade and the blood pressure should be maintained by all means (intravenous fluids, inotropes and balloon pump). Discontinuation of glycoprotein (GP) IIb/IIIa antagonist therapy is needed; reversal of the anticoagulation with protamine, if heparin was used, should be discussed, since there are no universal guidelines for reversing the heparin effect (described later) and the decision should be individualized according to the hemodynamic status of the patient, concomitant GP IIb/IIIa, activated clotting time and the duration of prolonged balloon inflation. If the patient becomes hemodynamically unstable or pericardiocentesis must be performed, a second experienced physician can be asked, if available. The echocardiogram is certainly important in determining the hemodynamic effect of perforation and whether or not it has been solved. Covered stents have revolutionized the treatment of the coronary perforations and various lengths and sizes should be available in each catheterization laboratory. Foam or microcoil embolization may also be helpful in selected cases. If the aforementioned methods fail to restore perforation, emergency surgery should be considered.
▪ Management algorithm
Few algorithms for treating coronary perforations have been proposed recently [3,11,18]. Type III perforations require immediate actions with prolonged balloon inflation, hemodynamic supportive therapy, discontinuation and reversal of anticoagulant and antithrombotic therapy (if possible), echocardiographic assessment of pericardial effusion and pericardiocentesis, and percutaneous or surgical treatment of the perforation.
▪ First step: block the perforation
As soon as a grade III perforation has been diagnosed the most important thing to do, even before starting cardiopulmonary resuscitation, is to corrctly advance a balloon angioplasty in order to close the perforation. The fastest way to get a balloon in place is to re-advance the last balloon used. For this reason, it is good practice to use post-balloon inflation or post-stenting, an injection while the balloon is still in the guiding catheter. Inflations for 10 min long at low pressure (2–5 atmospheres) are often needed. Detection of residual extravasations should be evaluated by repeated contrast injections at several time intervals [22].
▪ Second step: hemodynamic stabilization
It is very important to assure, by any means, hemodynamic stability. Following major perforation, blood pressure can fall very quickly. This is due to a combination of blood loss, pericardial tamponade and a vagal-mediated reflex from sudden distension of the pericardium. Blood pressure should be maintained with intravenous fluids, vasopressors and inotropic therapy. Placement of an intraaortic balloon pump may also be required in the presence of depressed left ventricular function or large ongoing ischemia. Cardiopulmonary resuscitation may sometimes become rapidly necessary following coronary perforation.
In the setting of hemodynamically significant cardiac tamponade, immediate pericardiocentesis and deployment of a pericardial drainage catheter is a life-saving maneuver [23]. Pericardial effusion is usually visible by angiography. Nonetheless, echocardiographical evaluation should be performed immediately. Even if pericardial hemorrhage is evident, pericardiocentesis should only be performed in the presence of hemodynamic or echographic cardiac compromise. The pericardiocentesis needle should be exchanged for a multiple side holes catheter, allowing continuous aspiration and monitoring of pericardial blood. The catheter should be maintained in place for 24 h, and in the absence of recurrence can be removed after a control echocardiography.
▪ Step three: discontinuation and/or reversal of anticoagulant/ antithrombotic therapy
Reversal of unfractionated heparin anticoagulation is most easily and rapidly achieved through the intravenous administration of protamine sulfate. However, controversy still persists regarding heparin reversal and this is related to the fact that on one hand, it may assist in sealing a perforation, but on the other hand, prolonged balloon inflation and simultaneous heparin reversal may lead to proximal vessel thrombosis. It is our belief that no universal guidelines can be given and decision should be individualized based on the hemodynamic status of the patient, concomitant GP IIb/IIIa, activated clotting time and the duration of prolonged balloon inflation [24]. Adverse reactions to protamine are very rare [25]. Complete reversal of anticoagulation cannot be achieved if the patient is receiving low-molecular- weight heparin, fondaparinux or bivalirudin. Bivalirudin is a synthetic direct inhibitor that does not have a specific antidote, and has a short half-life (25 min) with a return of coagulation in 1–2 h after the discontinuation of the drug.
Platelet GP IIb/IIIa receptor antagonists should be discontinued once perforation occurs. The strategy of platelet transfusion applies specifically to abciximab-treated patients. Abciximab demonstrates a long half-life, high-affinity platelet receptor binding and rapid plasma clearing with low free plasma levels, and can be overcome with platelet transfusions. Conversely, small molecules such as tirofiban and eptifibatide maintain high plasma concentration, have a shorter half-life and their antiplatelet effects resolve within a few hours and platelet transfusion is of little value.
▪ Step four: perforation sealing
Coronary perforation with persistent contrast extravasations, despite reversal of anticoagulation and prolonged balloon inflation can be successfully treated with implantation of covered stents [26–29]. The JOSTENT Graftmaster coronary stent graft system (Abbott Vascular) is a balloon expandable, slotted tube stent manufactured by sandwiching a layer of polytetrafluoroethylene (PTFE) between two stents of reduced wall thickness. There are now several reports of the use of a covered stent to seal coronary perforation with good effect [30–32]. The problem with the stent grafts is that they are more difficult to use than ordinary stents. Owing to their bulk and stiffness, they may not negotiate very tortuous or diffusely diseased, calcified lesions. They cannot be used to treat small vessels or branches. Side branches represent a major problem for covered stents. If the stent covers the ostium of the side branch, the branch will be sacrificed. Since they are extremely stiff devices, it is imperative to deploy the stent grafts at high pressure. To avoid endoleak, the stent graft should be long enough to cover the perforation with 4 mm margins on each side. If a shorter margin is needed in order to avoid covering a side branch, the operator needs to weight the importance of losing the side branch versus the possibility of not sealing the perforation.
Brigouri et al. reported from their experience that PTFE-covered stents successfully sealed 91% of the coronary perforations when the other conservative approaches failed [29]. At angiographic follow-up, 29% restenosis was reported. Some cases of early/late stent thrombosis in stent restenosis have been reported in some PTFEimplanted patients. In a study by Al-Lamee et al. , three cases of documented stent thrombosis have been described, at 7 days, 3 months and 4 months, respectively, post coronary perforation and implantation of PTFE-covered stents, with all the patients receiving a dual antiplatelet therapy [18]. In the study by Eggebrecht et al. , four of the six patients undergoing covered stent implantation had an angiographic follow-up, with one patient developing subacute stent thrombosis, in-stent restenosis occurred in one patient, while in two patients angiography revealed occlusion of the stent graft [7]. Animal data have shown that bare metal stents develop a full endothelial covering faster than PTFE-covered stents.
In rare cases, additional noncovered stents have been used to seal a coronary perforation [33]. Such a procedure may not be desirable, since balloonexpandable stents could potentially increase the size of vessel rupture.
Other reported percutaneous strategies for treatment of distal coronary perforation include embolizations of coils [34,35], gelfoam [36], polyvinyl alcohol [37], thrombin [38], glue [39] or autologous blood clot [40]. These are solutions that have been successfully used, especially in distal guidewire-induced coronary perforations.
Surgical indication
In the case of persistent contrast extravasations despite the application of all the measures mentioned earlier, or in case of hemodynamic instability due to a large ischemic territory, surgical management should be considered. Emergency surgery should be performed to control hemorrhage, repair the perforation or ligature the vessel and bypass all the vessels containing significant stenosis. Before transferring the patient to the operating room, a balloon catheter should be kept inflated at low pressure at the perforation site.
Cavity spilling
Patients with cavity spilling may respond to prolonged balloon inf lation or the vascular communication may even spontaneously close in follow-up. In general, the outcome is favorable with conservative therapy.
Prevention
Determinants of coronary perforation include patient, vessel and procedural characteristics [20]. Patient characteristics include age, female gender and history of previous coronary artery bypass graft. Vessel determinants include lesion severity with American College of Cardiology/American Heart Association type B2 or C lesions with total occlusions, tortuous and calcified vessels, and bifurcated and angulated lesions. Procedural characteristics that have been associated with an increased risk of vessel perforation include use of an atheroablative device (rotational or directional atherectomy, excimer laser angioplasty), the use of particular guidewires (stiff or hydrophilic guidewires), device oversizing (balloon angioplasty or stenting) or balloon rupture (jet effect), and the use of intravascular ultrasound. Some of these determinants cannot be influenced (age, sex, antecedents of coronary artery bypass graft). Most of the procedural factors that have been indicated as predictors for a coronary perforation should be recognized and appropriate adjustment in treatment strategies can prevent problems that would otherwise occur.
Highly calcified lesions should be properly prepared before implanting a stent by means of rotational atherectomy or cutting balloon. The risk of perforation is increased when overinflation of a semi-compliant balloon is used to overcome a calcified lesion, or when using a balloon with a balloon:artery ratio of more than 1.2:1.3. Cases of perforations caused by stenting have been reported, especially after excessive overdilatation or after implantation of an oversized stent compared with the calibre of the artery. Javaid et al. reported in their study, a decrease of 25% in the incidence of coronary perforation since drug-eluted stents are used [41]. It is likely that the efficacy of active stents in preventing restenosis will reduce the need for stent overexpansion.
The use of atheroablative devices has also been associated with an increased incidence of degree III perforations. In fact, when they are used correctly they can prevent perforation by changing the compliance of the diseased arteries and can allow ballooning and stenting, avoiding inflation at high pressures. When used incorrectly, those devices can be a source of massive perforation. Perforations secondary to rotablator mostly occurs when used in treating bended lesions or larger burr sizes. Ablating devices must be used with caution when treating bifurcation lesions, especially in the direction of the side branches, because side branches generally arise at steep angles. A burr:artery ratio of 0.5:0.6 is recommended in high-risk lesions.
It is interesting to note that the percentage of perforations induced by guidewire (crossing lesion, distal wire perforation) is increasing and may be related to the introduction of hydrophilic coronary wires and special stiff wires designed to recanalize total chronic occlusions. Even if a guidewire-exit perforation causes small leaks, it is possible that in association with concomitant GPIIb/IIIa can determine a grade III coronary perforation. In the group of patients reported by Witzke, 51% of the perforations were guidewire induced [42]. Caution should be taken when advancing the wire and also in positioning the tip of the guidewire distally, especially when a hydrophilic wire is used. If a perforation occurs during a percutaneous coronary intervention for a chronic total occlusion and a covered stent is implanted to seal the perforation, a controlateral injection to check for persisting bleeding via collaterals can be performed.
Conclusion
Although perforation is a relatively uncommon complication, the consequences may be devastating and all measures must be taken to prevent it. The best way to prevent a perforation is to recognize situations of increased risk and take appropriate precautions. When a grade III perforation occurs, the window of opportunity for effective treatment can be very short, sometimes less than 1 min. Early diagnosis, adjusted risk evaluation and immediate implementation of the therapeutic resources are key to controling the coronary perforation.
Future perspective
Even if the use of covered stents is considered to have the highest potential to seal a large perforation and to considerably reduce the need for emergent surgery in type III coronary perforations, this method is far from being the ideal tool for treating coronary perforations. First, the double layer design confers to this stent a bad crossing profile. Second, the long-term results are still suboptimal owing to a high incidence of restenosis and/or late thrombotic occlusion. The type of long-term antiplatelet strategy is not well established. Prasugrel, owing to its lack of intrinsic resistance, might be considered as the thienopiridine of choice, although there are no available data to support this.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Type III coronary perforation is a severe complication of percutaneous coronary intervention, associated with significant morbidity and mortality, and all measures must be taken to prevent it.
▪ Early recognition and familiarity with all the treatment algorithms for coronary perforations are paramount in the era of complex percutaneous coronary intervention.
▪ Covered stents have the highest potential to seal a large perforation and to considerably reduce the need for emergent surgery in type III coronary perforations and should be available in every catheterization laboratory.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Ellis SG, Ajluni S, Arnold AZ et al.: Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation 90, 2725–2730 (1994).
- Gruberg L, Pinnow E, Flood R et al.: Incidence, management and outcome of coronary artery perforation during percutaneous coronary intervention. Am. J. Cardiol. 86(15), 680–682 (2000).
- Dippel EJ, Kereiakes DJ, Tramuta DA et al.: Coronary perforation during percutaneous coronary intervention in the era of abciximab platelet glycoprotein IIb/IIIa blockade: an algorithm for percutaneous management. Catheter Cardiovasc. Intervent. 52, 279–286 (2001).
- Gunning MG, Williams IL, Jewitt DE, Shah AM, Wainwright RJ, Thomas MR: Coronary artery perforation during percutaneous intervention: incidence and outcome. Heart 88, 495– 498 (2002).
- Fejka M, Dixon S, Safian R et al.: Diagnosis, management and clinical outcome of cardiac tamponade complicating percutaneous intervention. Am. J. Cardiol. 90, 1183–1186 (2002).
- Fukutomi T, Suzuki T, Popma JJ et al.: Early and late clinical outcomes following coronary perforation in patients undergoing percutaneous coronary intervention. Circ. J. 66, 349–356 (2002).
- Eggebrecht H, Ritzel A, von Birgelen C et al.: Acute and long-term outcome after coronary perforation during percutaneous coronary interventions. Z. Kardiol. 93, 791–798 (2004).
- Bittl JA, Ryan TJ Jr, Keaney JF Jr et al.: Coronary artery perforation during excimer laser coronary angioplasty. The Percutaneous Excimer Laser Coronary Angioplasty Registry. J. Am. Coll. Cardiol. 21, 1158–1165 (1993).
- Holmes DR Jr, Reeder GS, Ghazzal ZM et al.: Coronary perforation after excimer laser coronary angioplasty: The Excimer Laser Coronary Angioplasty Registry experience. J. Am. Coll. Cardiol. 23, 330–335 (1994).
- Litvack F, Eigler N, Margolis J et al.: Percutaneous excimer laser coronary angioplasty: results in the first consecutive 3000 patients. The ELCA Investigators. J. Am. Coll. Cardiol. 23, 323–329 (1994).
- Muller O, Windecker S, Cuisset T et al.: Management of two major complications in the cardiac catheterization laboratory: the no reflow phenolmenon and coronary perforations. EuroIntervention 4, 181–192 (2008).
- Melchior JP, Meier B, Urban P et al.: Percutaneous transluminal coronary angioplasty for chronic total coronary arterial occlusion. Am J Cardiol. 59, 535–538 (1987).
- Tamura M, Oda H, Miida T et al.: Coronary perforation to left ventricular cavity by a guide wire during coronary angioplasty. Jpn Heart J. 34, 633–637 (1993).
- Wong CM, Kwong Mak GY, Chung DT: Distal coronary artery perforation resulting from the use of hydrophilic coated guidewire in tortuous vessels. Catheter Cardiovasc. Diagn. 44, 93–96 (1998).
- Schiele F, Meneveau N, Gilard M et al.: Intravascular ultrasound-guided balloon angioplasty compared with stent: immediate and 6-month results of a multicenter, randomized Balloon Equivalent to Stent Study (BEST). Circulation 107, 545–551 (2003).
- Fasseas P, Orford JL, Panetta CJ et al.: Incidence, correlates, management, and clinical outcome of coronary perforation: analysis of 16,298 procedures. Am. Heart J. 147, 140–145 (2004).
- Stankovic G, Orlic D, Corvaja N et al.: Incidence, predictors, inhospital, and late outcomes of coronary artery perforations. Am. J. Cardiol. 93, 213–216 (2004).
- Al-Lamee R, Ielasi A, Colombo A et al.: Incidence, predictors, management, immediate and long-term outcomes following grade III coronary perforation. J. Am. Coll. Cardiol. Cardiovasc. Interv. 4, 87–95 (2011).
- Ramana RK, Arab D, Joyal D et al.: Coronary artery perforation during percutaneous coronary intervention: incidence and outcome in the new interventional era. J. Invasive Cardiol. 17, 603–605 (2005).
- Kini AS, Rafael OC, Sarkar K et al.: Changing outcomes and treatment strategies for wire induced coronary perforations in the era of bivalirudin use. Catheter Cardiovasc. Interv. 74, 700–707 (2009).
- Shimony A, Zahger D, Van Straten M et al.: Incidence, risk factors, management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am. J. Cardiol. 104, 1674–1677 (2009).
- Stack RS, Quigley PJ, Collins G, Phillips HR 3rd: Perfusion balloon catheter. Am. J. Cardiol. 61, 1196–1197 (1988).
- Altman F, Yazdanfar S, Wertheimer J, Ghosh S, Kotler M: Cardiac tamponade following perforation of the left anterior descending coronary system during percutaneous transluminal coronary angioplasty: successful treatment by pericardial dreanage. Am. Heart J. 111, 1196–1197 (1986).
- Eeckhout Eric, De Palma R: Coronary perforation, an inconvenient complication. JACC: Cardiovasc. Interv. 4(1), 96–97 (2011).
- Stoelting RK: Allergic reactions during anesthesia. Anesth. Analg. 62, 341–356 (1983).
- Caputo RP, Amin N, Marvasti M, Wagner S et al.: Successful treatment of saphenous vein graft perforation with an autologous vein-covered stent. Catheter Cardiovasc. Interv. 48, 382–386 (1999).
- Colombo A, Itoh A, Di Mario C et al.: Successful closure of a coronary vessel rupture with a vein graft stent: case report. Catheter Cardiovasc. Interv. 38, 172–174 (1996).
- Stefanadis C, Toutouzas K, Tsiamis E et al. Implantation of stents covered by autologous arterial grafts in human coronary arteries: a new technique. J. Invasive Cardiol. 12, 7–12 (2000).
- Brigouri C, Nishida T, Anzuini A, Di Mario C, Grube E, Colombo A: Emergency polytetrafluotoethylene-covered stent implantation to treat coronary ruptures. Circulation 102, 3028–3031(2000).
- Campbell PG, Hall JA, Harcombe AA et al.: The Jomed covered stent graft for coronary artery aneurysms and acute perforation: a successful device which needs careful deployment and may not reduce restenosis. J. Invasive Cardiol. 12, 272–276 (2000).
- Casella G, Werner F, Klauss VV et al.: Successful treatment of coronary artery perforation during angioplasty using a new membrane-coated stent. J. Invasive Cardiol. 11, 622–626 (1999).
- Nageh T, Thomas MR: Coronary artery rupture treated with a polytetrafluoroethylenecoated stent. N. Engl. J. Med. 342, 1922–1924 (2000).
- Hammoud T, Tanguay JF, Rios F, Bilodeau L: Repair of the left anterior descending coronary artery perforation by Magic Wallstent implantation. Catheter Cardiovasc. Intervent. 48, 304–307 (1999).
- Aslam MS, Messersmith RN, Gilbert J, Lakier JB: Successful management or coronary artery perforation with helical platinum microcoil embolization. Catheter Cardiovasc. Intervent. 51, 320–322 (2000).
- Assali AR, Moustapha A, Sdringola S, Rihner M, Smalling RW: Successful treatment of coronary perforation in an abciximab-treated patient by microcoil embolization. Catheter Cardiovasc. Intervent. 51, 487–489 (2000).
- Hadjimiltiades S, Paraskevaides S, Kazinakis G, Louridas G: Coronary vessel perforation during balloon angioplasty: a case report. Catheter Cardiovasc. Diagn. 45, 417–420 (1998).
- Yoo BS, Yoon J, Lee SH et al.: Guidewireinduced coronary artery perforation treated with transcatheter injection of polyvinyl alcohol form. Catheter Cardiovasc. Intervent. 52, 231–234 (2001).
- Fischell TA, Korban AH, Lauer MA: Successful treatment of distal coronary guidewire induced perforation with balloon catheter delivery of intracoronary thrombin. Catheter Cardiovasc. Intervent. 58, 370–374 (2003).
- Storger H, Ruef J: Closure of guide wire induced coronary artery perforation with a two-component fibrin glue. Catheter Cardiovasc. Intervent. 70, 237–240 (2007).
- Cordero H, Gupta N, Underwood PL, Gogte ST, Heuser RR: Intracoronary autologous blood to seal a coronary perforation. Herz. 26, 157–160 (2001).
- Javaid A, Buch AN, Satler LF et al.: Management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am. J. Cardiol. 98, 911–914 (2006).
- Witzke C, Martin-Herrero F, Clarke S, Pomerantzev E, Palacios I: The changing pattern of coronary perforation during percutaneous coronary intervention in the new device era. J. Invasive Cardiol. 16, 257–301 (2004).
▪▪ Proposed angiographic classification of coronary perforation which has since been adopted by the majority of other studies in this area. The authors observed a higher incidence of coronary perforations associated with the use of atheroablative devices.
▪ The largest consecutive series of coronary perforations from a single center with almost half related to atheroablative device use.
▪▪ In this study, an increased incidence and severity of coronary perforations was observed with the use of atheroablative technologies for percutaneous coronary intervention, but no association between abciximab use with either the incidence or the angiographic classification for coronary perforation was observed. An angiographic class-specific algorithm for coronary perforation is also presented.
▪ Represents a large series of patients with cardiac tamponade complicating percutaneous coronary intervention and highlights several important aspects related to diagnosis, management and clinical outcomes based on the mode of presentation.
▪▪ Algorithm proposed for the management of grade III coronary perforation. High incidence of in-stent restenosis and definite stent thrombosis in the polytetrafluoroethylene (PTFE)-covered stent implantation.
▪▪ Supports the utility of PTFE-covered stents for the nonsurgical treatment of vessel rupture. A high rate of in-stent restenosis in the PTFE-covered stent patients was observed.
▪▪ In this study, the use of PTFE-covered stents in treating coronary perforations did not significantly change the rates of adverse outcomes.
▪▪ Increased incidence of cardiac tamponade in patients receiving glycoprotein IIb/IIIa antagonists, without affecting the outcome.