Review Article - Interventional Cardiology (2012) Volume 4, Issue 4
Deciphering the role of cardiac computed tomography in interventional cardiology: 2012 and beyond
- Corresponding Author:
- Sujith K Seneviratne
Monash Cardiovascular Research Centre, Monash Heart
Department of Medicine, Monash Medical Centre (MMC)
Southern Health & Monash University, 246 Clayton Road, Clayton
3168 VIC, Melbourne, Australia
Tel: +1 613 9594 6666
E-mail: sujith.seneviratne@southernhealth.org.au
Abstract
Keywords
aortic valve, bypass graft, chronic total occlusion, computed tomography, coronary artery disease, coronary intervention, coronary stent, mitral valve, myocardial ischemia
Transcatheter-based therapies have revolutionized the practice of cardiology and have provided significant improvements in the symptoms and survival of patients with coronary artery and structural heart disease [1–3]. Invasive and noninvasive imaging play a crucial role in choosing patients who are suitable for these therapies, guiding the procedure and identifying post-procedural complications. While invasive angiography (ICA) is the mainstay imaging modality by providing real-time iterative feedback and guidance, its main limitation predominantly stems from its 2D projection format and the lack of information it provides regarding surrounding structures. Computed tomography (CT) offers 3D volumetric assessment of the heart and its surrounding structures. Using this dataset, elaborate multiplanar reconstructions can be performed that enable cardiologists to better understand cardiac anatomy across multiple planes. In this article, we highlight how CT is being increasingly utilized in assisting both coronary and structural interventions, and its future role in interventional cardiology.
Advances in multidetector CT
The technological advances in CT have undoubtedly played an important role in its integration into the practice of interventional cardiology. Over the past decade, cardiac CT has evolved rapidly from the introduction of 4-detector row systems to those with 64 detectors, dual-source CT and, more recently, 320-detector systems. This has been accompanied by improvements in spatial and temporal resolution, extended longitudinal coverage and decreased scan times and radiation exposure (Table 1).
Current generation multidetector CT (MDCT) scanners have a spatial resolution that ranges from 0.3 to 0.5 mm, which is slightly lower than the 0.1 and 0.2 mm afforded by intravascular ultrasound (IVUS) and ICA, respectively. This permits accurate and consistent assessment of native coronary arteries. Prior studies evaluating the diagnostic performance of MDCT have shown high accuracy for the detection and exclusion of obstructive coronary artery disease (CAD) with sensitivity and negative predictive values both in excess of 95% [4,5]. The temporal resolution of modern scanners ranges from 75 to 175 ms which has been vastly reduced from 300 ms using 4-detector CT. As a result, image quality is improved and coronary assessment can be performed, even in patients with higher heart rates [6,7]. Furthermore, wide-detector CT offers an extended longitudinal coverage of 16 cm, This allows the entire volume of the heart to be imaged in a single heart beat, compared with the 4–8 heart beats required using narrowdetector CT. Accordingly, image acquisition is less vulnerable to breathing artefacts in patients who are not fully cooperative and the occurrence of arrhythmias or ectopic beats. CT acquisition has evolved from traditional low-pitch helical scanning using retrospective ECG gating to prospective ECG-gated image acquisition, which has vastly reduced the radiation exposure required in coronary assessment from 16–20 mSv [8] down to 1–4 mSv [9,10]. Lastly, new techniques in CT image reconstruction have been introduced using adaptive statistic iterative reconstruction (ASIR). When compared with the traditional filtered back projection techniques, ASIR results in significant reductions in image noise and improved image quality while maintaining image spatial resolution [11]. This may allow for further reductions in tube current and, hence, up to 44% of the required radiation dose [11]. These reductions in radiation exposure will ultimately broaden the applicability of cardiac CT in interventional cardiology.
Role of cardiac CT in percutaneous coronary interventions
▪ Cardiac CT in selection of suitable patients for percutaneous coronary intervention
While ICA is considered the gold standard for the detection of coronary artery stenosis, the decisions involved in selecting patients suitable for revascularization are complex and take into account symptoms, results of functional studies and severity of stenosis on invasive angiography. Cardiac CT in its current form, similar to ICA alone, plays a limited role in the routine selection of patients who may benefit from coronary revascularization.
Computed tomography coronary angiography (CTA) offers coronary assessment that most closely resembles invasive angiography among all other noninvasive imaging modalities. This has been demonstrated in large multicenter trials, particularly in patients with a low–intermediate risk of CAD in whom 64-detector CT identified significant coronary artery stenosis with a sensitivity of 95–99%, specificity of 64–83% and a negative predictive value of 97–99% [4,12]. As a result of the high sensitivity and negative predictive value, current guidelines recommend the use of cardiac CT to exclude significant CAD in this population [13].
However, the specificity of CTA is comparatively lower, especially in calcified stenoses. Calcific densities enhance the Hounsfield units of adjacent contrast by the partial volume effect of the denser calcium (blooming effect), which may result in an overestimation of the degree of luminal stenosis [14]. For this reason, patients who are identified to have significant stenosis on CT often require further assessment using invasive angiography or functional testing.
Nevertheless, upfront use of CTA in patients with low–intermediate risk of CAD may reduce unnecessary downstream ICA referrals and lower overall costs when compared with upfront use of single photon emission computed tomography- myocardial perfusion imaging (SPECTMPI) [15] with no excess adverse events [16]. The downstream utilization of this approach is currently being investigated in the Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter (CONFIRM) registry [17]. Apart from stenosis assessment, CTA permits atherosclerotic plaque characterization and quantification. The latter has been demonstrated to provide incremental prognostic information beyond the analysis of conventional cardiovascular risk factors [18–21].
▪ Cardiac CT in the preprocedural planning of coronary interventions
Information derived from CTA may provide the interventionist with important knowledge above and beyond that of invasive angiography to guide percutaneous coronary intervention (PCI). Lesion visualization on invasive angiography can be hampered by adjacent vessel overlap, but this problem is not encountered in CT as each vessel is tracked independently. In addition, lesion assessment on invasive angiography can be difficult when it has not been possible to selectively cannulate a vessel or bypass graft. As such, CT can be particularly useful, such as in the assessment of anomalous coronary arteries, right internal mammary grafts and vein grafts originating from unanticipated aortic locations [22]. Even after successful selective invasive angiography, unresolved questions may remain, and CTA can be invaluable in determining whether PCI is required. This includes differentiating ostial disease from coronary spasm unrelieved by intracoronary nitroglycerin, determining the potentially malignant anterior versus the benign posterior course of anomalous coronaries, and deciphering path and length of chronic total occlusions. CTA may also provide information regarding the size and angulation of the aortic root as well as the plaque burden and morphology in the ostium of the coronary arteries, which may allow better selection of guide catheters and may shorten procedural duration [22,23].
Invasive angiography is limited in its ability to inform the interventionist about the vessel wall, hence, the provision of reliable reference vessel and luminal area measurements can be challenging. This information is particularly important when PCI is considered in coronary segments with positive or negative remodeling. This can be obtained from preprocedural CT, which may aid the interventional cardiologist in the selection of the optimal size and length of stents, therefore resulting in better lesion coverage and larger post-procedural in-stent and reference diameters [22,23]. While IVUS has been traditionally used for this purpose in the assessment of proximal coronary lesions, it has been demonstrated that CT detects the presence of calcified and noncalcified plaque with comparable accuracy [24]. Furthermore, CT-derived plaque and luminal area measurements have been demonstrated to correlate with IVUS-derived measurements, although they are consistently lower than the invasive reference [24,25], which may result in the underestimation of luminal stenosis.
Chronic total occlusion interventions
The role of preprocedural CTA in guiding interventions has been extensively studied in chronic total occlusion (CTO) interventions. While successful recanalization of a CTO is associated with substantial improvement in symptoms, quality of life and survival [1,26], the procedure remains a challenge for interventional cardiologists due to lack of ability to visualize the occluded segment. As such, these procedures are often lengthy and complex, with elevated radiation exposure, increased contrast load and a higher risk of complications [27]. Success rates in most experienced centers range from 55 to 80% [28].
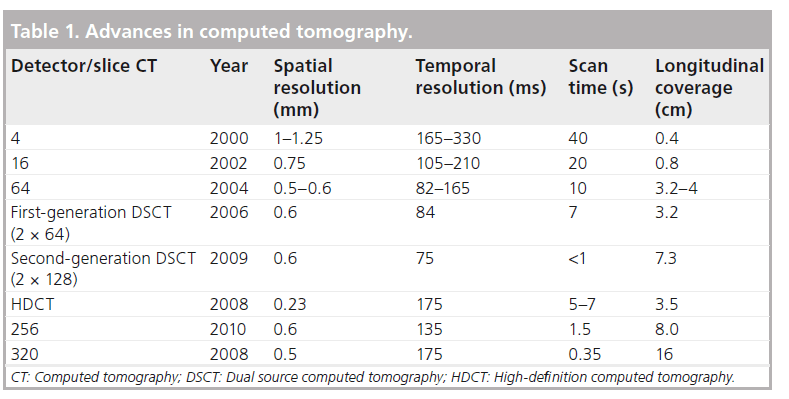
Angiographic features including the length, trajectory, tortuosity of the occluded segment, and the severity and distribution of calcification within the CTO, are considered to be the most important angiographic predictors for procedural failure, even in expert luminary centers with high procedural success rates [29]. All these features can be identified reliably on CTA and, by contrast, can be difficult to identify with ICA, which is largely limited by its 2D projection format (Figure 1) [30]. While real-time 3D reconstructions of the coronary arteries can be approximated from images acquired during rotational invasive angiography with encouraging results for CTO procedural success [31,32], these are limited by the need for a dedicated system to perform additional data processing, operator expertise to interpret the 3D reconstructions and prolonged injection into the coronary arteries to acquire the images, which poses an arrhythmic concern [31].
Figure 1: Computed tomography assessment of a chronic total occlusion. A 56-year-old was investigated for chest pain. (A) Invasive coronary angiography demonstrated a chronic total occlusion in the mid-right coronary artery. (B) There were collaterals from a right ventricular branch, which filled the right posterior descending artery distal to the occluded segment. (C) Computed tomography coronary angiography demonstrated a long, straight segment of chronic total occlusion (44 mm), which consisted of predominantly noncalcified plaque with minor calcification within the occluded segment. (D) Given the latter, percutaneous coronary intervention was attempted and successfully performed.
Multiple studies have demonstrated that the presence of CTO length >15 mm [33,34] and severe calcification, defined by the presence of calcium which occupies >50% of the luminal cross section area, are negative CT predictors of success [33–36]. Notably, the presence of severe calcification on CT has been demonstrated in multiple prospective registries to be the most significant independent predictor for procedural failure beyond conventional characteristics on ICA [34–37].
While CT may identify important features that may predict procedure failure, there are limited data on whether its use may improve procedural success. Ueno et al. demonstrated, in a retrospective cohort of 100 patients, that the use of cardiac CT was not associated with an increased prevalence of procedural success (80 vs 77.5%; p = 0.76) [38] and did not reduce irradiation time or contrast usage during PCI, but was associated with a significantly lower prevalence of complications (23.3 vs 7.5%; p = 0.039). This was driven by a lower occurrence of coronary perforation in patients who had had preprocedural CT assessment, yet demonstrated a similar occurrence of other major adverse cardiac events. These data suggest that CT is most useful in the cases with undesirable demographic and lesion characteristics to reduce complications during CTO interventions. In our experience, preprocedural cardiac CT should be applied to assess CTO lesions that are difficult to visualize on ICA or possess angiographic features on ICA that are negative predictors for procedural success, and in lesions that have had previous failed attempts at recanalization.
CT has a number of limitations in its assessment of CTOs. It remains difficult to differentiate between calcified subtotal and total occlusions. This may be overcome by dual-energy CT, which uses two different tube voltages to generate a pair of imaging datasets and may improve tissue characterization and the ability to discriminate between calcification and iodine contrast. This is currently under investigation. The lack of soft tissue contrast attenuation decreases the ability to accurately delineate the luminal and vessel borders in the occluded segment. Owing to its limited spatial resolution, the visualization and characterization of the distal segment beyond the occluded segment and its collaterals can be challenging. CT is highly accurate in identifying the presence or absence of collaterals (sensitivity: 91%; specificity: 100%) [39] but is moderately accurate for detection of individual collaterals across each CTO [39,40]. Specifically, it is not possible on CT to identify bridging collaterals or septal collaterals owing to their intramyocardial location [30]. The use of helical CT increases the overall radiation exposure of CTO interventions by 8.5 to 12 mSv using 64-detector CT scanners, [35,41]. Notably, significant dose reduction in CT can be achieved using prospective ECG-gated acquisition, which allows no compromise in image quality at a minimum radiation expense (1–3 mSv) [10]. Further dose reduction to <1 msV has been reported with the use of high-pitch spiral acquisition in secondgeneration dual-source CT scanners [42]. Lastly, given the need for iodinated contrast, the use of CT requires caution in patients with renal impairment. Accordingly, CT is ideally performed 1 week prior to scheduled intervention.
There have been reports of the use of hybrid CTA/ICA in guiding CTO interventions. This aims to provide real-time co-registration of data obtained from the two modalities, which can be presented as a fused image to guide interventionalists during PCI [43]. This technology is currently under investigation but has the potential to reduce contrast usage and procedural time, and to improve the success rates of CTO recanalization in the future.
Left main coronary artery interventions
Since the introduction of drug-eluting stents, percutaneous intervention in the left main coronary artery (LMCA) using drug-eluting stents is increasingly performed with low procedural complication rates and encouraging long-term outcomes [44]. While ICA is considered the gold standard to assess the LMCA, it is often difficult and can be unreliable [45–47]. Hence, IVUS, which confers the ability to accurately assess luminal dimensions, plaque composition and vessel remodeling, is the current preferred method to assess angiographically borderline LMCA lesions [48,49].
Given the large dimensions of the left main coronary artery, there has been interest in the incremental value of preprocedural CT. CT may better identify the exact location, length and composition of disease in the LMCA [50]. It also provides important information regarding the extent of plaque extension into the left anterior descending and left circumflex arteries, and the bifurcation angle. Dragu et al. compared the accuracy of CT assessment with IVUS in 20 patients with intermediate left main stenosis on invasive angiography. The study demonstrated a good correlation between CT and IVUS measurements in minimal luminal diameter (r = 0.77; p < 0.01), minimal luminal area (r = 0.93; p < 0.01), luminal area stenosis (r = 0.83; p < 0.01) and plaque burden (r = 0.94; p < 0.01) [51]. CT detected noncalcified plaque on IVUS with 100% sensitivity and calcified plaque with 75% sensitivity. While this may suggest that CT has a potential role in patient selection and procedure planning prior to LMCA intervention [52], further studies are required to further define the role of CT in LMCA interventions.
▪ Accuracy of CT to evaluate stent patency after coronary intervention
In-stent restenosis and resultant target lesion revascularization remain prevalent after stent implantation. Binary angiographic restenosis can occur in upto 30% of patients after baremetal stenting [53] and in 3–20% of patients after drug eluting stent implantation [54].
The sensitivity of CTA in the detection of in-stent restenosis is lower than reported for significant native artery stenoses [55–58]. The assessment of coronary stent patency and restenosis using cardiac CT is hampered by both underlying coronary calcification beneath the stent and artificial enlargement of the metallic stent struts, resulting in blooming artefacts, which can preclude appropriate assessment of the in-stent lumen. The impact of this is inversely related to stent diameter [59].
In a large meta-analysis including 854 patients from 15 studies using 4-, 16- and 64-detector CT, CTA detected coronary in-stent restenosis (>50%) with 85% pooled sensitivity and 97% specificity when compared with invasive angiography [58]. 64-detector CT had superior visualization of stent lumen when compared with 16-detector CT, which was reflected by its superior sensitivity (87 vs 81%).
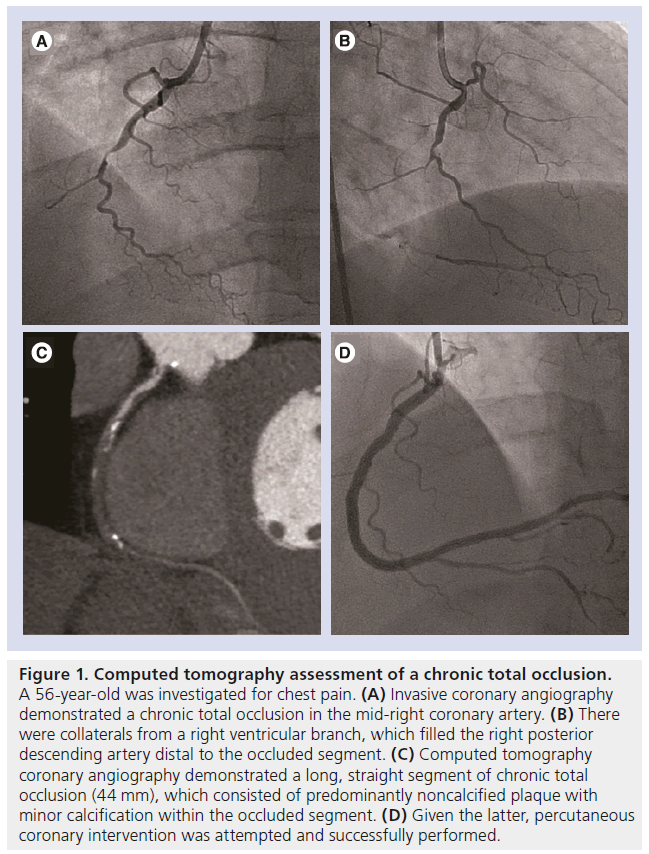
Andreini et al. demonstrated, in a retrospective cohort of 100 patients, that the accuracy of 64-detector CT is highly dependent on the diameter of the stent [57]. The ratio of evaluable segments and total number of segments is significantly higher in stents with a diameter of ≥3 mm than in stents with a <3-mm diameter (99 vs 74%; p < 0.05) [57]. The corresponding accuracy of CT was 98 versus 75%, which was a result of a higher specificity (100 vs 78%; p < 0.05) and a higher positive predictive value (100 vs 57%; p < 0.05). Similar findings were reported using 320-detector CT [56] and dual-source CT [55]. Pugliese et al. reported on the use of dual-source CT in an evaluation of 133 patients including 178 stents, and demonstrated 100% accuracy in stents with a diameter of ≥3.5 mm (n = 78) [55]. For this reason, CTA is recommended in asymptomatic patients to assess for stent patency and in-stent restenosis in stents which are ≥3 mm in diameter, but is not recommended in <3-mm stents according to the American Heart Association/American College of Cardiology appropriate use criteria (Figure 2A & B) [13].
Figure 2: Stent evaluation using multidetector computed tomography. (A) Computed tomography (CT) luminal assessment of a 2.0-mm diameter stent (B) compared with a 3.5-mm diameter stent. (C) Stent visualization using standard-definition 64-detector CT with 0.63-mm spatial resolution (D) compared with high-definition 64-detector CT with 0.23-mm spatial resolution. CT evaluation of a bioresorbable stent. The metallic stent struts have been resorbed and are no longer seen. (E) Only the platinum markers at the proximal and distal ends of the stent remain and are visible on CT. (C & D) Reproduced from [61] with permission from Elsevier.
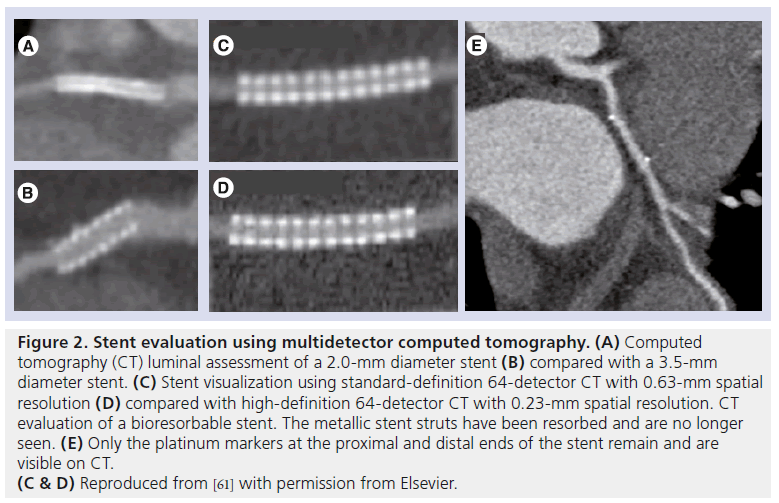
Given high accuracy in larger stents, the question begs as to whether it can accurately diagnose in-stent restenosis in the LMCA. Van Mieghem et al. demonstrated in 74 patients that 16-detector (27 patients) and 64-detector CT (43 patients) detected LMCA in-stent restenosis with a sensitivity, specificity and overall accuracy of 100, 91 and 93%, respectively, compared with invasive angiography [60]. Sub-analysis demonstrated accuracy to be higher in patients who received isolated stenting of the LMCA with or without extension into a single major side branch than in patients who had had both branches of the LMCA bifurcation stented (98 vs 83%). CT assessment of stent diameter and area was shown to have a good correlation with that of IVUS (r = 0.78 and 0.73, respectively). Accordingly, it is appropriate for CT to be used to assess LMCA stent patency in accordance with American Heart Association/American College of Cardiology appropriate use criteria (Figure 3) [13].
Figure 3: Multidetector computed tomography assessment of stent patency in the left main coronary artery. Chronic total occlusion was performed after recurrence of angina in a 52-year-old woman, 4 months after bifurcational distal left main coronary artery stenting. This was performed using the culotte technique, and two 3.5 × 18 mm zotarolimus-eluting stents were inserted into the left anterior descending and left circumflex arteries. (A) Chronic total occlusion demonstrated a black hypodense area at the left main coronary artery bifurcation, consistent with significant in-stent restenosis (arrow). (B) This was confirmed on coronary angiography, which demonstrated severe in-stent restenosis of the distal left main artery, involving the ostial and proximal portions of the left anterior descending and left circumflex artery stents. Reprinted from [61] with permission from Elsevier.
Recently, a high-definition CT (HDCT) scanner has been introduced, which has improved inplane spatial resolution of 0.23 mm and possesses the ability to reconstruct images using the novel ASIR algorithm. Min et al. reported that HDCT yielded substantially larger luminal area visualization when compared with present-generation 64-detector CT in the ex-vivo assessment of stents (Figure 2C & D) [61]. Stent diameter was higher and image noise was lower with the use of HDCT and ASIR image-reconstruction techniques. Further in-vivo studies are needed to determine its future role and relevance in the evaluation of coronary stents. Looking ahead, the future uptake in the use of stents with a fully bioresorbable platform may remove the difficulty encountered in imaging metallic stents on CT (Figure 2E).
Lastly, in-stent restenosis can occasionally be caused by the presence of stent fracture. In vitro studies demonstrated that the presence of stent fracture can be diagnosed on CT with higher accuracy than with IVUS or invasive angiography [62,63]. This finding is supported in vivo by a number of case reports [64,65].
▪ Accuracy of CT to evaluate graft patency after coronary artery bypass graft surgery
While invasive angiography remains the standard of reference for detection of native coronary artery and graft stenoses after surgical revascularization, this can be cumbersome and challenging at times. As such, the engagement and visualization of venous and arterial bypass grafts can be associated with prolonged procedure time, larger contrast usage and increased radiation exposure [52]. Recently, coronary CT has emerged as an appropriate alternative investigation technique to assess for graft patency and stenosis in symptomatic patients [13]. These recommendations have been based on 93–100% and 96–100% accuracy in detection of significant graft stenosis and graft patency, respectively, using contemporary scanners [52,66,67]. Notably, the accuracy in the detection of severe stenoses in the distal runoff arteries beyond the graft anastomoses and in the ungrafted native coronary arteries is lower at 89–99% and 80–93%, respectively [52,66,67]. This lower accuracy is attributable to the smaller calibre of native arteries and the significant calcific burden in patients who have had previous surgical revascularization.
Role of coronary CT in structural interventions
▪ Transcatheter aortic valvular replacement
Transcatheter aortic valvular replacement (TAVR) is an alternative treatment for patients with severe symptomatic aortic stenosis who are at a too-high risk for conventional aortic valve replacement [3,68] and is reported to have noninferior outcomes when compared with surgery in high-risk surgical candidates [2]. Noninvasive imaging, including CT and echocardiography, plays a vital role in the preoperative setting in assessing the aortic valve complex and access-site anatomy. The main role of CT is to evaluate aortic annular dimensions, root anatomy, implantation angle, distance between the annulus and coronary arteries, and iliofemoral anatomy.
Sizing of annulus
Accurate evaluation of the native aortic valve annular dimensions is critical to optimize selection of the correct bioprosthetic valve size. Incorrect valve sizing may lead to paravalvular aortic regurgitation, which is a predictor of worse long-term outcome [69], valve embolization, patient prosthesis mismatch or catastrophic annular rupture [70].
Current recommendations for prosthesis sizing are largely based on annulus measurements performed using 2D transthoracic echocardiogram or transesophageal echocardiography (TEE). However, annular assessment by 2D transthoracic echocardiogram is limited by single annular plane measurements. Hence, it is difficult to fully appreciate the elliptical nature of the annulus and its complex 3D structure. Surgical correlation demonstrated that TEE-derived diameters can underestimate the true diameter by a mean of 1.2 mm and not infrequently by more than 2 mm [71]. While the current practice of TEEbased annular determination and routine oversizing of the valve by 1–2 mm has provided good outcomes, there remains a percentage of patients with paravalvular regurgitation after the procedure, resulting in many investigators pursuing the use of MDCT annular measurements when screening patients for TAVR [72,73].
Computed tomography assessment may better account for the 3D elliptical shape of the annulus. Measurements are typically taken as the average of the short and long dimensions of the basal ring or derived from the basal ring area (Figure 4). In a recent cohort of 50 patients, Gurvitch et al. demonstrated these measurements to be reproducible and typically 1–1.5 mm larger than TEE measurements of the annulus [72]. Based on this information, a CT-based sizing scale has been proposed for TAVR and for current valve sizing thresholds, which are based on TEE measurements, to increase by 1–1.5 mm when CT measurements are used. Future large prospective multicenter studies will be required to gain more understanding of the optimal method for annulus sizing using CT.
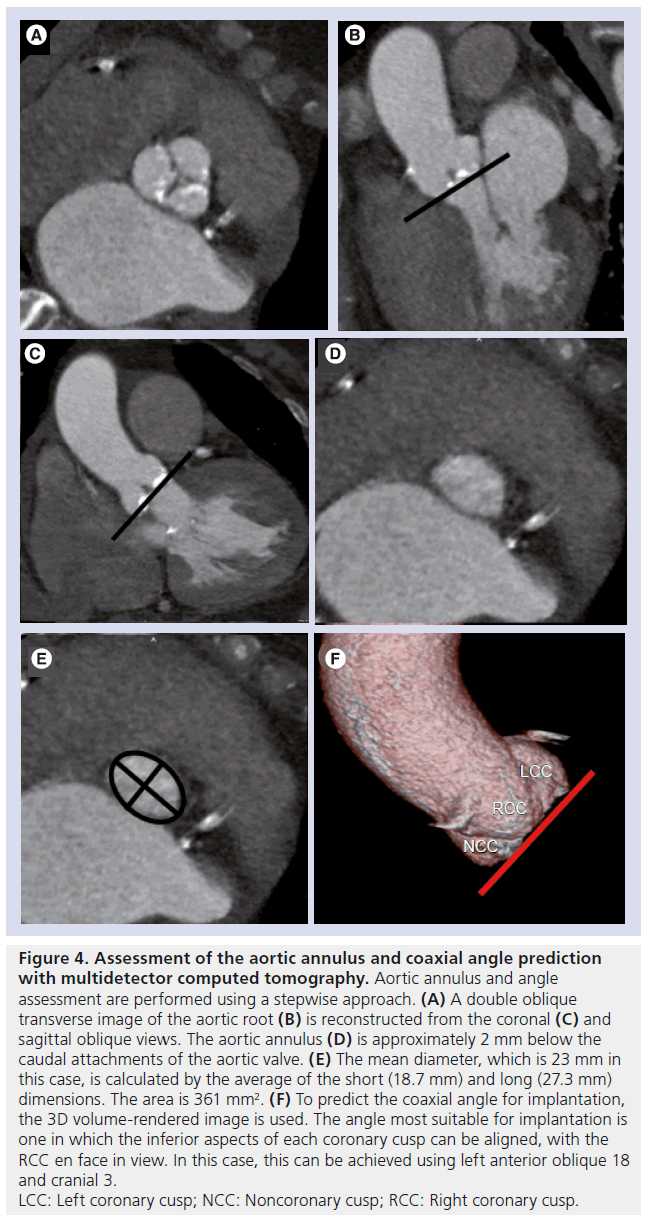
Figure 4: Assessment of the aortic annulus and coaxial angle prediction
with multidetector computed tomography. Aortic annulus and angle
assessment are performed using a stepwise approach. (A) A double oblique
transverse image of the aortic root (B) is reconstructed from the coronal (C) and
sagittal oblique views. The aortic annulus (D) is approximately 2 mm below the
caudal attachments of the aortic valve. (E) The mean diameter, which is 23 mm in
this case, is calculated by the average of the short (18.7 mm) and long (27.3 mm)
dimensions. The area is 361 mm2. (F) To predict the coaxial angle for implantation,
the 3D volume-rendered image is used. The angle most suitable for implantation is
one in which the inferior aspects of each coronary cusp can be aligned, with the
RCC en face in view. In this case, this can be achieved using left anterior oblique 18
and cranial 3.
LCC: Left coronary cusp; NCC: Noncoronary cusp; RCC: Right coronary cusp.
Coaxial angle of deployment prediction
Preprocedural determination of the optimal valve implantation angle is a crucial step for interventional cardiologists to increase the likelihood that the valve is deployed perpendicular to the aortic root [74]. Given the orientation of the aortic valve, the optimal coaxial angle for implantation is often a slight caudal angulation when in the right anterior oblique projection and a slight cranial angulation when in the left anterior oblique projection. Traditionally, this has been determined by using repeated catheter aortograms in one or two orthogonal planes before commencing the procedure.
Preprocedural CT examinations have the potential advantage of reducing the number of aortograms required during the procedure, and hence, shortening both procedural time and contrast usage. Furthermore, they provide a dataset that can be used to determine the orientation of the aortic root and therefore in the prediction of optimal angles for valve implantation. Gurvitch et al. demonstrated, using data from CT, that there is a wide range of right anterior oblique or left anterior oblique projections, as long as appropriate caudal or cranial angulation is added, in each individual for which the aortic valve would be perpendicular to the aortic root [74]. This additional information is particularly useful in patients with atypical angles, which are most commonly observed in patients with musculoskeletal abnormalities and tortuous unfolded aortas.
Iliofemoral artery access assessment
Vascular complications in the iliofemoral arteries may occur as a result of the passage of the large delivery catheters required during TAVR. These constitute a major cause of mortality and morbidity. CT allows a thorough and complete 3D assessment of the iliofemoral system, including an evaluation of plaque burden and vessel tortuosity, which provides important data for procedural planning, given the common association of peripheral vascular disease and severe aortic stenosis [75]. MDCT provides more accurate luminal assessment than single-plane angiography owing to the additional data afforded by its multiplanar capabilities stemming from the isotropic voxels that are acquired. This potential allows for elaborate 3D reconstructions and accurate assessments of the minimal luminal diameter, integrating data from the coronal, sagittal and transverse axial planes.
Accurate luminal assessment on CT can be facilitated by measurements taken in a plane orthogonal to the vessel rather than in the transverse axial plane by adopting a center line approach which typically elongates the curved and tortuous iliofemoral arteries [76]. Importantly, the use of external sheaths with a larger diameter than the CT-derived minimal luminal diameter is a predictor of access complications (23 vs 5%; p = 0.01) [77].
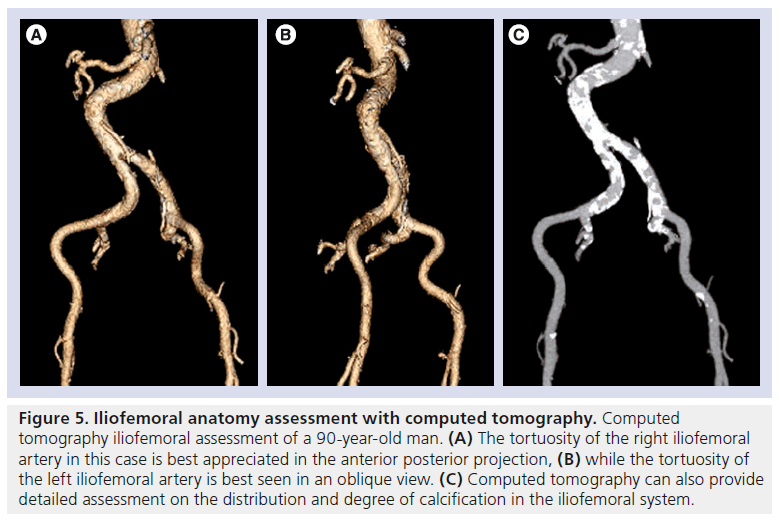
CT can assess vessel tortuosity and the burden and pattern of calcification (Figure 5). Toggweiler et al. demonstrated, in a cohort of 82 subjects, that the presence of moderate or severe calcification on CT was a significant predictor of vascular complications (23 vs 9%; p = 0.03) [77]. This additional information also allows identification of circumferential or horseshoe calcification within small calibre vessels or stenotic segments, which may not be appreciated on screening 2D angiography [78]. The presence of circumferential calcification may preclude the safe passage of large-profile delivery catheters and, hence, be considered as a contraindication for vascular access.
Figure 5: Iliofemoral anatomy assessment with computed tomography. Computed tomography iliofemoral assessment of a 90-year-old man. (A) The tortuosity of the right iliofemoral artery in this case is best appreciated in the anterior posterior projection, (B) while the tortuosity of the left iliofemoral artery is best seen in an oblique view. (C) Computed tomography can also provide detailed assessment on the distribution and degree of calcification in the iliofemoral system.
▪ Percutaneous mitral valve repair
Two techniques for transcatheter mitral valve repair have been studied to date, including: attempting to create a double-orifice mitral valve by using a percutaneous edge to edge technique with a clip device or stitch [79]; and remodeling of the annulus of the mitral valve with suturebased techniques, application of radiofrequency energy and device implantation [80].
There is currently no described role for the use of cardiac CT in preoperative assessment of patients prior to mitral clip device insertion, possibly as a result of the inferior temporal resolution of MDCT in comparison with echocardiography in the assessment of the dynamic nature of mitral valve dysfunction. In the case of coronary sinus annuloplasty, cardiac CT may play a role in delineating the anatomy of the mitral annulus, coronary sinus and great cardiac vein, and their relationship with the left circumflex artery [81]. This is important as earlier imaging studies have demonstrated that branches of the circumflex artery travel deep to the great cardiac vein in >50% of patients [82].
▪ Other structural interventions
In adult patients with a patent ductus arteriosus, cardiac CT can be a useful adjunct to echocardiography in demonstrating the 3D ductal anatomy, ductal dimensions and complicating features such as calcification or aneurysm formation, which may guide interventional strategy [83].
Furthermore, in patients awaiting left atrial appendage (LAA) closure, preprocedural CT may exclude the presence of thrombus in the LAA, which is a contraindication for closure and provides detailed and 3D morphological assessment of the LAA, which may guide device selection for closure [84]. During followup, failure to achieve complete closure can be demonstrated on CT by the presence of persistent communication between the left atrium and LAA, and incomplete device endothelialization [85].
Future applications under investigation
▪ Assessment for vulnerable plaque
Currently, the role of CTA in the identification of vulnerable plaques remains investigational. CTA offers the ability to identify important plaque characteristics that may be associated with acute coronary syndrome (ACS). Motoyama et al. demonstrated, in a retrospective cohort of 71 patients including 38 patients with ACS and 33 patients with stable angina, that the combined presence of low-attenuation plaque, positive remodeling and spotty calcification on CT had a high positive predictive value for culprit ACS plaques [86].
The question begs whether CT plaque features may be used to identify rupture-prone plaque and predict future ACS. Kashiwagi et al. reported that the presence of plaque that is surrounded by a ring of high contrast attenuation (<130 HU) on CT is highly specific (96%) for thin-cap fibroatheroma on OCT, which is believed to represent rupture-prone plaques [87]. This ring enhancement is speculated to represent the presence of highly active neovascularizations in vasa vasorum within a vulnerable plaque [88]. Furthermore, a prospective study including 1059 patients demonstrated that patients with positively remodeled coronary segments and low attenuation plaques on CT were at a higher risk of ACS developing over time when compared with patients without these characteristics (22 vs 0.5%; p < 0.001) [89]. Whilst vulnerable plaque features on CT, like other imaging modalities, are associated with a higher incidence of adverse outcomes, currently, these features cannot be used to predict ACS or influence treatment.
▪ Combined assessment of coronary stenoses & their hemodynamic significance
The combined evaluation of coronary stenoses and their hemodynamic significance is the holy grail of noninvasive diagnostic imaging. Cardiac CT in its current form is limited in predicting the hemodynamic significance of coronary stenoses [90]. New methods are being investigated to provide this information, including the use of CT fractional flow reserve (FFR) and CT stress myocardial perfusion imaging.
CT FFR can be derived by using computational fluid dynamics with CTA vessel data. Indeed, computational fluid dynamics enables derivation of other important hemodynamic data including flow velocity, wall shear stress and wall shear stress gradients, which may be play a role in atherogenesis [91,92]. Its major advantage is that these parameters can be derived without additional image reconstruction, acquisition or administration of medications. In a retrospective cohort of 159 vessels in 103 patients, Koo et al. demonstrated that CT FFR, using a threshold of ≤0.8, detected FFR-significant (≤0.8) stenoses with a sensitivity of 84% and specificity of 82% [93]. Overall there was a good correlation between CT FFR and FFR (r = 0.72; p < 0.001) and area under the receiver operator curve was 0.90. Further studies evaluating its diagnostic performance are underway, including the Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography (DeFACTO) study, which will be the first prospective multicenter study [94]. An alternative approach currently under investigation is to measure the gradient of intraluminal attenuation along the course of a coronary artery in an attempt to extract flow-related information within the coronary vessel [95,96].
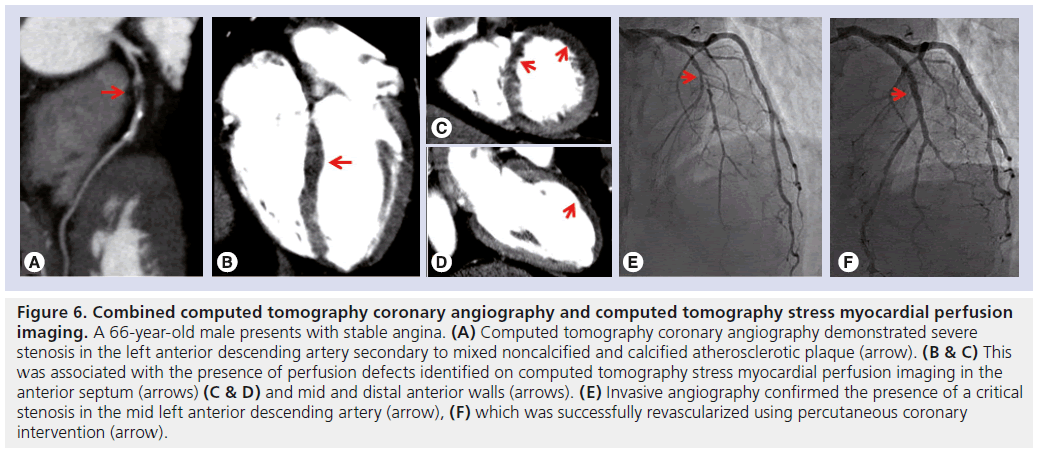
CT stress myocardial perfusion imaging (CTP) is an emerging technique to detect myocardial ischemia (Figure 6). Images are typically acquired during first pass of iodinated contrast and vasodilator stress (adenosine), using prospective ECG gating or dynamic imaging. Myocardial perfusion analysis can be performed qualitatively by visual assessment or quantified using various techniques. In patients with known CAD, the added use of CTP may improve the accuracy of CTA in the detection of myocardial ischemia on SPECT-MPI [97] and magnetic resonance perfusion imaging [98], and, importantly, of occlusive disease on ICA [99] and hemodynamically significant stenoses on FFR [100,101]. A recent study demonstrated, in 40 patients (120 vessels) with suspected CAD, combined CTA and CTP identified FFR significant stenosis with 87% sensitivity and 95% specificity, and can be achieved with a short on-CT–table duration (43 mins) and an acceptable radiation dose (9.2 mSv) [102]. The Core 320 is a multicenter international study currently recruiting participants with suspected and known CAD, and is designed to determine the diagnostic accuracy of 320-detector CT in the combined assessment of coronary arteries and perfusion when compared against invasive angiography and SPECT-MPI [103].
Figure 6: Combined computed tomography coronary angiography and computed tomography stress myocardial perfusion imaging. A 66-year-old male presents with stable angina. (A) Computed tomography coronary angiography demonstrated severe stenosis in the left anterior descending artery secondary to mixed noncalcified and calcified atherosclerotic plaque (arrow). (B & C) This was associated with the presence of perfusion defects identified on computed tomography stress myocardial perfusion imaging in the anterior septum (arrows) (C & D) and mid and distal anterior walls (arrows). (E) Invasive angiography confirmed the presence of a critical stenosis in the mid left anterior descending artery (arrow), (F) which was successfully revascularized using percutaneous coronary intervention (arrow).
Future perspective
In the near future, technological advances in MDCT systems will undoubtedly allow prospective scanners to combine longitudinal coverage, multiple x-ray tubes, faster gantry speed to further enhance diagnostic performance and image quality, and decrease scan time and radiation exposure. For this reason, as the scope of transcatheter interventions in cardiology broadens, there is little doubt that cardiac CT will be increasingly utilized in the selection of patients suitable for intervention and as an adjunctive imaging tool in guiding interventions. Prospective studies exploring the clinical benefits in the use of CT will continue to be much needed to clarify its role in coronary and structural interventions.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Advances in multidetector computed tomography
▪ The improved spatial and temporal resolution inherent in current-generation computed tomography (CT) scanners permits accurate and convenient assessment of coronary arteries.
▪ The use of prospective ECG gating has vastly reduced the radiation dose required for coronary assessment.
Cardiac CT in percutaneous coronary interventions
▪ CT coronary angiography in its current form plays a minimal role in selecting patients who may benefit from coronary revascularization.
▪ In chronic total occlusion interventions, CT coronary angiography can identify features that may predict procedural failure. Its use may reduce complication rates but it has not been demonstrated to enhance procedural success.
▪ CT can reliably assess stent patency in stents that have a ≥3 mm diameter, including left main stents.
Cardiac CT in percutaneous structural interventions
▪ In transcatheter aortic valvular replacement, CT plays an important role in the assessment of iliofemoral anatomy, the prediction of optimal angle for valve implantation and annular sizing. The latter is traditionally guided using a transesophageal echocardiogram, hence, sizing thresholds based on CT measurements will need to be developed before wider adaptation.
▪ In percutaneous mitral interventions, CT is utilized to delineate the anatomy of the coronary sinus and its relationship with the left circumflex artery.
Future applications under investigation
▪ While the presence of certain CT plaque features is associated with a higher prevalence of acute coronary syndromes, CT may not be used to predict acute coronary syndromes or to influence management.
▪ CT fractional flow reserve and CT stress myocardial perfusion imaging represent promising techniques to allow the combined assessment of coronary stenoses and their hemodynamic significance.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Jones DA, Weerackody R, Rathod K et al. Successful recanalization of chronic total occlusions is associated with improved long-term survival. JACC Cardiovasc. Interv. 5(4), 380–388 (2012).
- Smith CR, Leon MB, Mack MJ et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 364(23), 2187–2198 (2011).
- Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363(17), 1597–1607 (2010).
- Budoff MJ, Dowe D, Jollis JG et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 52(21), 1724–1732 (2008).
- Hamon M, Biondi-Zoccai GG, Malagutti P, Agostoni P, Morello R, Valgimigli M. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J. Am. Coll. Cardiol. 48(9), 1896–1910 (2006).
- Achenbach S, Ropers U, Kuettner A et al. Randomized comparison of 64-slice single- and dual-source computed tomography coronary angiography for the detection of coronary artery disease. JACC Cardiovasc. Imaging 1(2), 177–186 (2008).
- Ropers U, Ropers D, Pflederer T et al. Influence of heart rate on the diagnostic accuracy of dual-source computed tomography coronary angiography. J. Am. Coll. Cardiol. 50(25), 2393–2398 (2007).
- Miller JM, Rochitte CE, Dewey M et al. Diagnostic performance of coronary angiography by 64-row CT. N. Engl. J. Med. 359(22), 2324–2336 (2008).
- Dewey M, Zimmermann E, Deissenrieder F et al. Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation 120(10), 867–875 (2009).
- Husmann L, Valenta I, Gaemperli O et al. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur. Heart J. 29(2), 191–197 (2008).
- Leipsic J, Labounty TM, Heilbron B et al. Estimated radiation dose reduction using adaptive statistical iterative reconstruction in coronary CT angiography: the ERASIR study. AJR Am. J. Roentgenol. 195(3), 655–660 (2010).
- Meijboom WB, Meijs MF, Schuijf JD et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J. Am. Coll. Cardiol. 52(25), 2135–2144 (2008).
- Taylor AJ, Cerqueira M, Hodgson JM et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/ NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J. Cardiovasc. Comput. Tomogr. 4(6), 407 E401–E433 (2010).
- Hecht HS, Bhatti T. How much calcium is too much calcium for coronary computerized tomographic angiography? J. Cardiovasc. Comput. Tomogr. 2(3), 183–187 (2008).
- Cheezum MK, Hulten EA, Taylor AJ et al. Cardiac CT angiography compared with myocardial perfusion stress testing on downstream resource utilization. J. Cardiovasc. Comput. Tomogr. 5(2), 101–109 (2011).
- Menon M, Lesser JR, Hara H et al. Multidetector CT coronary angiography for patient triage to invasive coronary angiography: performance and cost in ambulatory patients with equivocal or suspected inaccurate noninvasive stress tests. Catheter Cardiovasc. Interv. 73(4), 497–502 (2009).
- Min JK, Dunning A, Lin FY et al. Rationale and design of the CONFIRM (Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter) Registry. J. Cardiovasc. Comput. Tomogr. 5(2), 84–92 (2011).
- Pundziute G, Schuijf JD, Jukema JW et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J. Am. Coll. Cardiol. 49(1), 62–70 (2007).
- Van Werkhoven JM, Schuijf JD, Gaemperli O et al. Incremental prognostic value of multi-slice computed tomography coronary angiography over coronary artery calcium scoring in patients with suspected coronary artery disease. Eur. Heart J. 30(21), 2622–2629 (2009).
- Hadamitzky M, Freissmuth B, Meyer T et al. Prognostic value of coronary computed tomographic angiography for prediction of cardiac events in patients with suspected coronary artery disease. JACC Cardiovasc. Imaging 2(4), 404–411 (2009).
- Ostrom MP, Gopal A, Ahmadi N et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J. Am. Coll. Cardiol. 52(16), 1335–1343 (2008).
- Hecht HS. Applications of multislice coronary computed tomographic angiography to percutaneous coronary intervention: how did we ever do without it? Catheter Cardiovasc. Interv. 71(4), 490–503 (2008).
- Rodriguez-Granillo GA, Rosales MA, Llaurado C, Ivanc TB, Rodriguez AE. Guidance of percutaneous coronary interventions by multidetector row computed tomography coronary angiography. EuroIntervention 6(6), 773–778 (2011).
- Achenbach S, Moselewski F, Ropers D et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrastenhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation 109(1), 14–17 (2004).
- Leber AW, Knez A, Von Ziegler F et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J. Am. Coll. Cardiol. 46(1), 147–154 (2005).
- Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: results from the FlowCardia’s Approach to Chronic Total Occlusion Recanalization (FACTOR) Trial. Circ. Cardiovasc. Qual. Outcomes 3(3), 284–290 (2010).
- Suzuki S, Furui S, Kohtake H et al. Radiation exposure to patient’s skin during percutaneous coronary intervention for various lesions, including chronic total occlusion. Circ. J. 70(1), 44–48 (2006).
- Stone GW, Reifart NJ, Moussa I et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part II. Circulation 112(16), 2530–2537 (2005).
- Tsuchikane E, Katoh O, Suzuki T. Chronic total occlusion. In: Practical Handbook of advanced interventional cardiology tips and tricks (3rd Edition). Blackwell Publishing, MA, USA, 173–204 (2008).
- Hoe J. CT coronary angiography of chronic total occlusions of the coronary arteries: how to recognize and evaluate and usefulness for planning percutaneous coronary interventions. Int. J. Cardiovasc. Imaging 25(Suppl. 1), S43–S54 (2009).
- Dvir D, Kornowski R. Real-time 3D imaging in the cardiac catheterization laboratory. Future Cardiol. 6(4), 463–471 (2010).
- Dvir D, Assali A, Kornowski R. Percutaneous coronary intervention for chronic total occlusion: novel 3-dimensional imaging and quantitative analysis. Catheter Cardiovasc. Interv. 71(6), 784–789 (2008).
- Mollet NR, Hoye A, Lemos PA et al. Value of preprocedure multislice computed tomographic coronary angiography to predict the outcome of percutaneous recanalization of chronic total occlusions. Am. J. Cardiol. 95(2), 240–243 (2005).
- Garcia-Garcia HM, Van Mieghem CA, Gonzalo N et al. Computed tomography in total coronary occlusions (CTTO registry): radiation exposure and predictors of successful percutaneous intervention. EuroIntervention 4(5), 607–616 (2009).
- Cho JR, Kim YJ, Ahn CM et al. Quantification of regional calcium burden in chronic total occlusion by 64-slice multidetector computed tomography and procedural outcomes of percutaneous coronary intervention. Int. J. Cardiol. 145(1), 9–14 (2010).
- Soon KH, Cox N, Wong A et al. CT coronary angiography predicts the outcome of percutaneous coronary intervention of chronic total occlusion. J. Interv. Cardiol. 20(5), 359–366 (2007).
- Choi JH, Song YB, Hahn JY et al. Threedimensional quantitative volumetry of chronic total occlusion plaque using coronary multidetector computed tomography. Circ. J. 75(2), 366–375 (2011).
- Ueno K, Kawamura A, Onizuka T et al. Effect of preoperative evaluation by multidetector computed tomography in percutaneous coronary interventions of chronic total occlusions. Int. J. Cardiol. 156(1), 76–79 (2012).
- Rieber J, Sheth TN, Mooyaart EA et al. Assessment of the presence and extent of coronary collateralization by coronary computed tomographic angiography in patients with total occlusions. Int. J. Cardiovasc. Imaging 25(3), 331–337 (2009).
- Weinstock M, Rutkin B, Makaryus AN. CT angiography versus invasive angiography for the diagnosis of total occlusion. Int. J. Cardiovasc. Imaging 26(2), 121–122 (2010).
- Kaneda H, Saito S, Shiono T, Miyashita Y, Takahashi S, Domae H. Sixty-four-slice computed tomography-facilitated percutaneous coronary intervention for chronic total occlusion. Int. J. Cardiol. 115(1), 130–132 (2007).
- Achenbach S, Marwan M, Ropers D et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur. Heart J. 31(3), 340–346 (2010).
- Roguin A, Abadi S, Engel A, Beyar R. Novel method for real-time hybrid cardiac CT and coronary angiography image registration: visualising beyond luminology, proof-ofconcept. EuroIntervention 4(5), 648–653 (2009).
- Biondi-Zoccai GG, Lotrionte M, Moretti C et al. A collaborative systematic review and meta-analysis on 1278 patients undergoing percutaneous drug-eluting stenting for unprotected left main coronary artery disease. Am. Heart J. 155(2), 274–283 (2008).
- Isner JM, Kishel J, Kent KM, Ronan JA Jr, Ross AM, Roberts WC. Accuracy of angiographic determination of left main coronary arterial narrowing. Angiographic– histologic correlative analysis in 28 patients. Circulation 63(5), 1056–1064 (1981).
- Cameron A, Kemp HG Jr, Fisher LD et al. Left main coronary artery stenosis: angiographic determination. Circulation 68(3), 484–489 (1983).
- Bergelson BA, Tommaso CL. Left main coronary artery disease: assessment, diagnosis, and therapy. Am. Heart J. 129(2), 350–359 (1995).
- Abizaid AS, Mintz GS, Abizaid A et al. One-year follow-up after intravascular ultrasound assessment of moderate left main coronary artery disease in patients with ambiguous angiograms. J. Am. Coll. Cardiol. 34(3), 707–715 (1999).
- Park SJ, Hong MK, Lee CW et al. Elective stenting of unprotected left main coronary artery stenosis: effect of debulking before stenting and intravascular ultrasound guidance. J. Am. Coll. Cardiol. 38(4), 1054–1060 (2001).
- Weustink AC, Schinkel AF, Van Der Ent M, De Feyter PJ. Pre-procedural dual source 64-slice computed tomography in unprotected left main intervention. JACC Cardiovasc. Interv. 2(5), 470–471 (2009).
- Dragu R, Kerner A, Gruberg L et al. Angiographically uncertain left main coronary artery narrowings: correlation with multidetector computed tomography and intravascular ultrasound. Int. J. Cardiovasc. Imaging 24(5), 557–563 (2008).
- Weustink AC, Nieman K, Pugliese F et al. Diagnostic accuracy of computed tomography angiography in patients after bypass grafting: comparison with invasive coronary angiography. JACC Cardiovasc. Imaging 2(7), 816–824 (2009).
- Cutlip DE, Chauhan MS, Baim DS et al. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J. Am. Coll. Cardiol. 40(12), 2082–2089 (2002).
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J. Am. Coll. Cardiol. 56(23), 1897–1907 (2010).
- Pugliese F, Weustink AC, Van Mieghem C et al. Dual source coronary computed tomography angiography for detecting in-stent restenosis. Heart 94(7), 848–854 (2008).
- De Graaf FR, Schuijf JD, Van Velzen JE et al. Diagnostic accuracy of 320-row multidetector computed tomography coronary angiography to noninvasively assess in-stent restenosis. Invest. Radiol. 45(6), 331–340 (2010).
- Andreini D, Pontone G, Bartorelli AL et al. Comparison of feasibility and diagnostic accuracy of 64-slice multidetector computed tomographic coronary angiography versus invasive coronary angiography versus intravascular ultrasound for evaluation of in-stent restenosis. Am. J. Cardiol. 103(10), 1349–1358 (2009).
- Sun Z, Davidson R, Lin CH. Multi-detector row CT angiography in the assessment of coronary in-stent restenosis: a systematic review. Eur. J. Radiol. 69(3), 489–495 (2009).
- Schuijf JD, Bax JJ, Jukema JW et al. Feasibility of assessment of coronary stent patency using 16-slice computed tomography. Am. J. Cardiol. 94(4), 427–430 (2004).
- Van Mieghem CA, Cademartiri F, Mollet NR et al. Multislice spiral computed tomography for the evaluation of stent patency after left main coronary artery stenting: a comparison with conventional coronary angiography and intravascular ultrasound. Circulation 114(7), 645–653 (2006).
- Min JK, Swaminathan RV, Vass M, Gallagher S, Weinsaft JW. High-definition multidetector computed tomography for evaluation of coronary artery stents: comparison to standard-definition 64-detector row computed tomography. J. Cardiovasc. Comput. Tomogr. 3(4), 246–251 (2009).
- Lim HB, Hur G, Kim SY et al. Coronary stent fracture: detection with 64-section multidetector CT angiography in patients and in vitro. Radiology 249(3), 810–819 (2008).
- Pang JH, Kim D, Beohar N, Meyers SN, Lloyd-Jones D, Yaghmai V. Detection of stent fractures: a comparison of 64-slice CT, conventional cine-angiography, and intravascular ultrasonography. Acad. Radiol. 16(4), 412–417 (2009).
- Ito T, Ehara M, Suzuki T. Multiple stent fractures detected by multislice computed tomography after full metal jacket stents. Eur. Heart J. Cardiovasc. Imaging 13(7), 631 (2012).
- Sozzi FB, Civaia F, Rossi P, Rusek S, Dor V. Coronary stent fracture and in-stent restenosis at coronary computed tomography. J. Am. Coll. Cardiol. 54(23), 2199 (2009).
- Ropers D, Pohle FK, Kuettner A et al. Diagnostic accuracy of noninvasive coronary angiography in patients after bypass surgery using 64-slice spiral computed tomography with 330-ms gantry rotation. Circulation 114(22), 2334–2341, quiz 2334 (2006).
- De Graaf FR, Van Velzen JE, Witkowska AJ et al. Diagnostic performance of 320-slice multidetector computed tomography coronary angiography in patients after coronary artery bypass grafting. Eur. Radiol. 21(11), 2285–2296 (2011).
- Grube E, Schuler G, Buellesfeld L et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current thirdgeneration self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J. Am. Coll. Cardiol. 50(1), 69–76 (2007).
- Tamburino C, Capodanno D, Ramondo A et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 123(3), 299–308 (2011).
- Webb JG, Pasupati S, Humphries K et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 116(7), 755–763 (2007).
- Kurra V, Kapadia SR, Tuzcu EM et al. Pre-procedural imaging of aortic root orientation and dimensions: comparison between x-ray angiographic planar imaging and 3-dimensional multidetector row computed tomography. JACC Cardiovasc. Interv. 3(1), 105–113 (2010).
- Gurvitch R, Webb JG, Yuan R et al. Aortic annulus diameter determination by multidetector computed tomography: reproducibility, applicability, and implications for transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 4(11), 1235–1245 (2011).
- Schultz C, Moelker A, Tzikas A et al. The use of MSCT for the evaluation of the aortic root before transcutaneous aortic valve implantation: the Rotterdam approach. EuroIntervention 6(4), 505–511 (2010).
- Gurvitch R, Wood DA, Leipsic J et al. Multislice computed tomography for prediction of optimal angiographic deployment projections during transcatheter aortic valve implantation. JACC Cardiovasc. Interv. 3(11), 1157–1165 (2010).
- Kurra V, Schoenhagen P, Roselli EE et al. Prevalence of significant peripheral artery disease in patients evaluated for percutaneous aortic valve insertion: preprocedural assessment with multidetector computed tomography. J. Thorac. Cardiovasc. Surg. 137(5), 1258–1264 (2009).
- Leipsic J, Hague CJ, Gurvitch R, Ajlan AM, Labounty TM, Min JK. MDCT to guide transcatheter aortic valve replacement and mitral valve repair. Cardiol. Clin. 30(1), 147–160 (2012).
- Toggweiler S, Gurvitch R, Leipsic J et al. Percutaneous aortic valve replacement: vascular outcomes with a fully percutaneous procedure. J. Am. Coll. Cardiol. 59(2), 113–118 (2012).
- Descoutures F, Himbert D, Lepage L et al. Contemporary surgical or percutaneous management of severe aortic stenosis in the elderly. Eur. Heart J. 29(11), 1410–1417 (2008).
- Feldman T, Wasserman HS, Herrmann HC et al. Percutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST Phase I clinical trial. J. Am. Coll. Cardiol. 46(11), 2134–2140 (2005).
- Webb JG, Harnek J, Munt BI et al. Percutaneous transvenous mitral annuloplasty: initial human experience with device implantation in the coronary sinus. Circulation 113(6), 851–855 (2006).
- Masson JB, Webb JG. Percutaneous treatment of mitral regurgitation. Circ. Cardiovasc. Interv. 2(2), 140–146 (2009).
- Tops LF, Van De Veire NR, Schuijf JD et al. Noninvasive evaluation of coronary sinus anatomy and its relation to the mitral valve annulus: implications for percutaneous mitral annuloplasty. Circulation 115(11), 1426–1432 (2007).
- Thai WE, Harper RW, Seneviratne S. Dynamic volume 320-slice CT in the assessment of patent ductus arteriosus for percutaneous closure. Heart 96(4), 321 (2010).
- Wang Y, Di Biase L, Horton RP, Nguyen T, Morhanty P, Natale A. Left atrial appendage studied by computed tomography to help planning for appendage closure device placement. J. Cardiovasc. Electrophysiol. 21(9), 973–982 (2010).
- Lockwood SM, Alison JF, Obeyesekere MN, Mottram PM. Imaging the left atrial appendage prior to, during, and after occlusion. JACC Cardiovasc. Imaging 4(3), 303–306 (2011).
- Motoyama S, Kondo T, Sarai M et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J. Am. Coll. Cardiol. 50(4), 319–326 (2007).
- Kashiwagi M, Tanaka A, Kitabata H et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc. Imaging 2(12), 1412–1419 (2009).
- Tanaka A, Shimada K, Yoshida K et al. Non-invasive assessment of plaque rupture by 64-slice multidetector computed tomography – comparison with intravascular ultrasound. Circ. J. 72(8), 1276–1281 (2008).
- Motoyama S, Sarai M, Harigaya H et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J. Am. Coll. Cardiol. 54(1), 49–57 (2009).
- Meijboom WB, Van Mieghem CA, Van Pelt N et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J. Am. Coll. Cardiol. 52(8), 636–643 (2008).
- Chaichana T, Sun Z, Jewkes J. Computation of hemodynamics in the left coronary artery with variable angulations. J. Biomech. 44(10), 1869–1878 (2011).
- Farmakis TM, Soulis JV, Giannoglou GD, Zioupos GJ, Louridas GE. Wall shear stress gradient topography in the normal left coronary arterial tree: possible implications for atherogenesis. Curr. Med. Res. Opin. 20(5), 587–596 (2004).
- Koo BK, Erglis A, Doh JH et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J. Am. Coll. Cardiol. 58(19), 1989–1997 (2011).
- Min JK, Berman DS, Budoff MJ et al. Rationale and design of the DeFACTO (Determination of Fractional Flow Reserve by Anatomic Computed Tomographic AngiOgraphy) study. J. Cardiovasc. Comput. Tomogr. 5(5), 301–309 (2011).
- Choi JH, Min JK, Labounty TM et al. Intracoronary transluminal attenuation gradient in coronary CT angiography for determining coronary artery stenosis. JACC Cardiovasc. Imaging 4(11), 1149–1157 (2011).
- Steigner ML, Mitsouras D, Whitmore AG et al. Iodinated contrast opacification gradients in normal coronary arteries imaged with prospectively ECG-gated single heart beat 320-detector row computed tomography. Circ. Cardiovasc. Imaging 3(2), 179–186 (2010).
- Blankstein R, Shturman LD, Rogers IS et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J. Am. Coll. Cardiol. 54(12), 1072–1084 (2009).
- Feuchtner G, Goetti R, Plass A et al. Adenosine stress high-pitch 128-slice dual-source myocardial computed tomography perfusion for imaging of reversible myocardial ischemia: comparison with magnetic resonance imaging. Circ. Cardiovasc. Imaging 4(5), 540–549 (2011).
- Rocha-Filho JA, Blankstein R, Shturman LD et al. Incremental value of adenosineinduced stress myocardial perfusion imaging with dual-source CT at cardiac CT angiography. Radiology 254(2), 410–419 (2010).
- Ko BS, Cameron JD, Meredith IT et al. Computed tomography stress myocardial perfusion imaging in patients considered for revascularization: a comparison with fractional flow reserve. Eur. Heart J. 33(1), 67–77 (2012).
- Bamberg F, Becker A, Schwarz F et al. Detection of hemodynamically significant coronary artery stenosis: incremental diagnostic value of dynamic CT-based myocardial perfusion imaging. Radiology 260(3), 689–698 (2011).
- Ko BS, Cameron JD, Meredith I et al. Incremental value of quantitative and qualitative CT myocardial perufsion assessment in combination with CT coroanry angiography for determination of functionally significant coronary artery diseasecomparison with fractional flow reserve. Circulation 124, A15108 (2011).
- Vavere AL, Simon GG, George RT et al. Diagnostic performance of combined noninvasive coronary angiography and myocardial perfusion imaging using 320 row detector computed tomography: design and implementation of the CORE320 multicenter, multinational diagnostic study. J. Cardiovasc. Comput. Tomogr. 5(6), 370–381 (2011).
▪▪ Multicenter study that demonstrated that computed tomography coronary angiography (CTA) is highly sensitive in the detection of coronary artery disease and has a high negative predictive value to exclude significant coronary artery disease.
▪▪ Outlines indications for computed tomography use that are considered appropriate, uncertain or inappropriate.
▪ Highlighted the difference in the accuracy of 64-detector computed tomography in assessment of in-stent restenosis in stents with a diameter of ≥3 mm compared with stents with a diameter of <3 mm.
▪ Outlines the reproducibility of aortic annular measurements made on cardiac computed tomography and their relationship to measurements obtained using echocardiography.
▪ First report on the use of CTA to identify plaque characteristics that are associated with culprit lesions in acute coronary syndromes.
▪ First report on the diagnostic accuracy of the use of noninvasive fractional flow reserve-derived from CTA when compared with invasive fractional flow reserve.