Review Article - Interventional Cardiology (2010) Volume 2, Issue 2
Decision making in TAVI: ensuring the best possible clinical outcomes based on the selection of patients and techniques
- Corresponding Author:
- Antonio Colombo
Columbus Hospital, Via Buonarroti 48, 20145 Milan, Italy
Tel: +39 024 871 2422
Fax: +39 024 819 3433
E-mail: info@emocolumbus.it
Abstract
Keywords
delivery method selection, patient selection, transcatheter aortic valve implantation, valve selection
Surgical aortic valve replacement (AVR) is the most conventional treatment for severe aortic stenosis (AS). However, a high operative mortality of 7–10% is well recognized in selected high-risk groups [1,2]. As a result of these risks, 30–40% of elderly patients do not have surgery due to one or more of the following reasons: first, the patient is not referred for AVR by the cardiology team; second, the patient is not accepted for an operation by the cardiothoracic team; and finally, the patient declines aortic valve surgery [3–5]. Surgery is most likely to be denied in patients who are elderly, with left ventricular dysfunction and multiple comorbidities [5]. However, conservative management of patients with severe AS is known to have a poor prognosis [6]. The technique of transcatheter aortic valve implantation (TAVI) was developed to address this unmet need, and has been recommended as an alternative treatment strategy for patients in high-risk surgical groups [7,8]. TAVI is now widely practiced worldwide with many centers regularly implanting devices in patients in whom an AVR is deemed to be inappropriate.
Currently, there are two CE-marked devices with some similar fundamental design features that are used for TAVI, the CoreValve ReValving™ system (Medtronic Inc, MN, USA), and the Edwards SAPIEN™ prosthesis (Edward Lifesciences Inc, CA, USA). The CoreValve is available in two inf low diameter sizes, 26 and 29 mm; while the Edwards SAPIEN valve is available in 23 and 26 mm diameters. Three delivery routes have been developed: transfemoral, transapical and transaxillary/subclavian. Both the transfemoral and transaxillary/subclavian routes involve a retrograde approach while the transapical route involves an anterograde approach. The transfemoral route is the first choice for valve delivery for both prostheses in the majority of patients; however, in patients in whom the transfemoral route is contraindicated, the transapical route can be considered for Edwards SAPIEN implantation, and the transaxillary/subclavian route is an alternative for implantation of the CoreValve.
Since the first human TAVI by Cribier in 2002 [9], there have been multiple trials assessing the efficacy of TAVI in terms of procedural success, early mortality and short-term clinical outcomes [10–12]; however, long-term survival data remain limited. Selection criteria have a crucial influence on clinical outcomes following TAVI, and are focused on the selection of the most appropriate patient group, prosthesis type and size, and route of delivery [8]. As the accepted techniques, experience and skill of the operators have improved, the indications and criteria for patient selection have become broader. In this article, we aim to deliver an overview of the current recommended selection criteria and offer a critical appraisal of these guidelines. The objective of this paper is to offer a more comprehensive and up-to-date outline of the most appropriate investigative pathway pre-TAVI, thereby ensuring that ‘the right valve is implanted into the right patient by the right delivery method’.
Patient selection
In ideal practice a TAVI team should be a multidisciplinary team that encompasses the expertise of interventional cardiologists, imaging cardiologists, cardiac surgeons and cardiac anesthetists.
As TAVI remains a relatively new procedure, it is currently only recommended for those patients who are functionally limited as a result of severe AS, and are considered at high-risk for surgery or in whom surgery is contraindicated [7,8]. Current recommended patient-related selection criteria include one or more of the following:
▪ Logistic EuroSCORE greater than 20% [13];
▪ Society of Thoracic Surgeons (STS) score greater than 10% [14];
▪ Age 65 years or older with one or more of the following:
- Liver cirrhosis (Child class A or B);
- Pulmonary insufficiency defined as forced expiratory volume in 1 s (FEV1) less than 1 l;
- Pulmonary hypertension (pulmonary artery systolic pressure greater than 60 mmHg);
- Previous cardiac surgery (coronary artery bypass graft surgery or valvular surgery);
- Porcelain aorta;
- Recurrent pulmonary emboli;
- Right ventricular insufficiency;
- Contraindication to open chest surgery (e.g., previous radiation or severe chest burns);
- Cachexia defined as a body mass index less than or equal to 18 kg/m2.
These recommendations were stated in a position statement by a European committee of the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC) [7,8], they are still open to debate and should not be considered binding. It should also be remembered that risk scoring schemes and the estimation of risk have recognized limitations; clinical judgment is still paramount in patient selection for any intervention. For example, in the case of a patient aged 60 years with clear contraindications to open chest surgery but a low EuroSCORE, a TAVI may still be appropriate following thorough preassessment by an experienced team, despite the presence of a low surgical risk score.
Following on from this clinical assessment, patients will then go on to have a series of investigations to assess a number of anatomical and functional characteristics that are important in evaluating their suitability for TAVI.
Assessment of severity of aortic stenosis
Prior to consideration for TAVI, all patients must have investigations to confirm severe AS requiring intervention. Transthoracic echocardiography (TTE) is mandatory for all patients.
In the presence of clear echocardiographic data, further investigation may not be necessary. However, there are cases in which at least two investigative modalities are required to confirm severe AS. In these cases, either of the following investigations may also be performed: transesophageal echocardiography (TEE) or cardiac catheterization to assess aortography and the transaortic valvular gradient.
In many institutions the diagnosis of severe AS is confirmed in the majority of patients by measurement of the transvalvular gradient, and sometimes by assessment of the hemodynamic valve area, prior to consideration of TAVI. In our practice we often skip this evaluation if we have clear crisp data from the echocardiogram. Following these investigations the following criteria for severe AS should be satisfied: aortic valve area less than 1 cm2 and aortic valve index less than 0.6 cm2/m2.
In addition, in the presence of a left ventricular ejection fraction of more than 50%, the following criteria must also be satisfied: aortic peak jet velocity greater than 4.0 m/s and aortic mean pressure gradient greater than 40 mmHg.
In the presence of low gradient aortic stenosis, dobutamine stress echo may be required to allow further assessment of severity of the aortic valve disease and left ventricular contractile reserve.
Assessment of concomitant coronary artery disease
Coexistent significant coronary artery disease (CAD) may increase the risks of TAVI, and in addition it is likely to affect patient clinical outcomes postprocedure if left untreated. Therefore, it requires investigation as part of the routine screening of potential TAVI patients. Those patients with associated significant CAD may require percutaneous coronary intervention prior to undergoing TAVI; however, these decisions are generally made on an individual patient basis by the multidisciplinary team. Importantly, in those patients with severe CAD that is not amenable to intervention and that may affect prognosis, it is generally accepted that TAVI is not appropriate. The assessment of CAD is conducted using:
▪ Coronary angiography: this is the gold standard investigative modality and is performed in the majority of patients being considered for TAVI.
▪ Multislice coronary computed tomography (MSCT): this can be considered in those patients where there is a need to lower the risk of the TAVI preassessment process.
Furthermore, all patients must be clinically stable prior to a TAVI procedure and it is generally recommended that, in those patients with decompensated heart failure, medical therapy must be optimized and followed by balloon valvuloplasty to ensure improvement of their clinical status and left ventricular function prior to TAVI.
Valve type & size selection
The Edwards SAPIEN valve consists of a stainless steel balloon-expandable stent with three integrated valve leaflets composed of bovine pericardium. It is currently available in two sizes:
▪ 23 mm valve: suitable for aortic annulus size 18–21 mm. This is delivered via a 22F transfemoral introducer sheath and a 26F transapical sheath;
▪ 26 mm valve: suitable for aortic annulus size 21–25 mm. This is delivered via a 24F transfemoral introducer sheath and a 26F transapical sheath.
The minimal iliofemoral diameter required for delivery of the Edwards SAPIEN valve via the transfemoral route is 7–8 mm.
The CoreValve consists of a self-expanding nitinol frame with three integrated porcine pericardial leaflets. It is also currently available in two sizes:
▪ 26 mm valve: suitable for aortic annulus size 20–23 mm. This is delivered via an 18F transfemoral introducer sheath and an 18F transaxillary/ subclavian sheath.
▪ 29 mm valve: suitable for aortic annulus size 23–27 mm. This is delivered via an 18F transfemoral introducer sheath and an 18F transaxillary/subclavian sheath.
The minimal iliofemoral and subclavian artery diameter required for delivery of the CoreValve via the transfemoral route and transaxillary/subclavian routes respectively is 6 mm.
It is important to remember that the external size of the sheath is 3F larger than the internal size, which is the most commonly quoted measurement.
In order to assess the most suitable valve type and size for all patients being considered for TAVI, an evaluation of the aortic annulus size in conjunction with an assessment of the peripheral arteries is required to allow selection of the correct valve for each patient.
Assessment of the aortic annulus
Measurement of the size of the aortic annulus requires precise assessment in order to ensure appropriate valve selection, and minimize the risks of paravalvular leak and device migration. The size of the transcatheter heart valve chosen must be slightly larger than the aortic annulus size, thereby reducing the risk of paravalvular aortic insufficiency. There are a number of investigations used for this measurement:
▪ Transthoracic echocardiography: this is normally the primary investigative modality but is generally not used in isolation as it can lead to imprecise measurements [15];
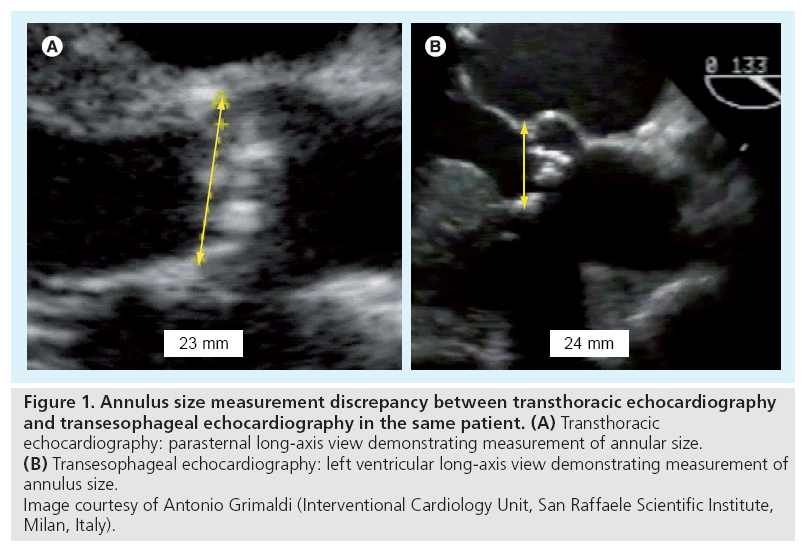
▪ Transesophageal echocardiography: this is particularly helpful in borderline cases where clear imaging is required to allow accurate measurements and in which the TTE quality is inadequate. When utilizing TTE and TEE for annulus size measurement it is important to measure the annulus in the preferred views (Figure 1);
Figure 1: Annulus size measurement discrepancy between transthoracic echocardiography and transesophageal echocardiography in the same patient. (A) Transthoracic echocardiography: parasternal long-axis view demonstrating measurement of annular size. (B) Transesophageal echocardiography: left ventricular long-axis view demonstrating measurement of annulus size. Image courtesy of Antonio Grimaldi (Interventional Cardiology Unit, San Raffaele Scientific Institute, Milan, Italy).
▪ Multislice CT: this can be used to combine the measurement of the size of the aortic annulus while also performing an assessment of the peripheral arteries [16,17];
▪ Aortography with graded pigtail catheter: performed in the right anterior oblique and left anterior oblique 15° projections.
In addition, the following investigations are not routinely used but can be useful adjunctive tests to add to knowledge of the valve anatomy:
▪ Rotational angiography: can be used as an additional tool for annular size assessment;
▪ 3D TEE: can provide a more detailed assessment of the anatomy of the aortic valve.
It may be pragmatic to evaluate the annular size first in the preassessment sequence as a size over 27 mm or below 18 mm will make the patient unsuitable for any TAVI. In addition, a size between 23 and 26 mm would require either a CoreValve if the common femoral and iliac arteries are smaller than 7–8 mm or an alternative delivery approach, such as the transaxillary/ subclavian or transapical route.
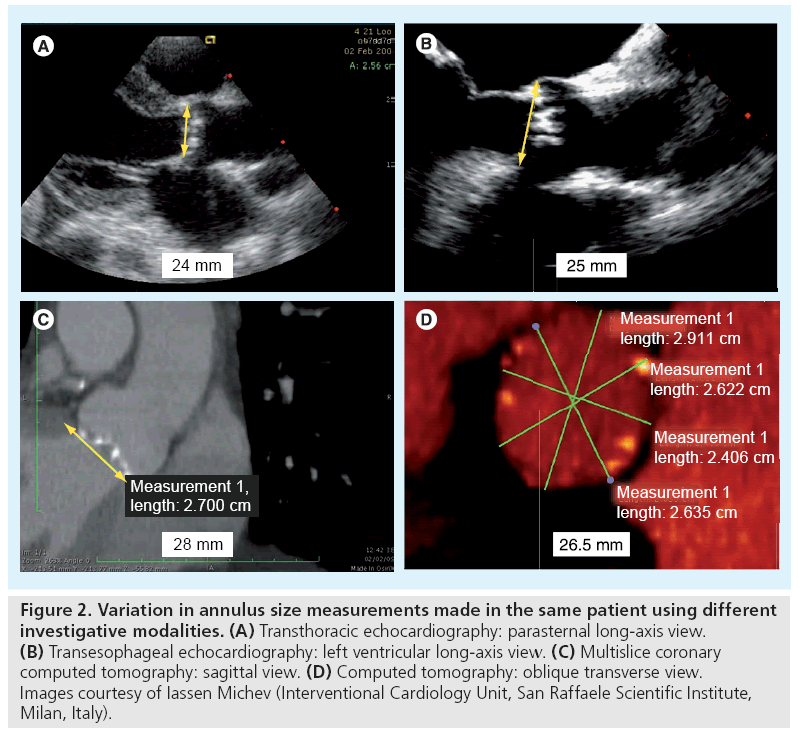
In addition, an integrated approach with the use of multiple modalities for annular assessment is usually recommended as there are cases in which there may be significant discrepancies in the measurements made using any one of the standard imaging techniques (Figure 2) [20].
Figure 2: Variation in annulus size measurements made in the same patient using different investigative modalities. (A) Transthoracic echocardiography: parasternal long-axis view. (B) Transesophageal echocardiography: left ventricular long-axis view. (C) Multislice coronary computed tomography: sagittal view. (D) Computed tomography: oblique transverse view. Images courtesy of Iassen Michev (Interventional Cardiology Unit, San Raffaele Scientific Institute, Milan, Italy).
Assessment of cardiac anatomy
Although it is crucially important to evaluate the aortic annular size, there are a number of other structural factors in the cardiac anatomy of the patient that affect procedural success or complication rates. These factors need to be taken into account and considered as part of the overall evaluation of the risk–benefit analysis prior to TAVI. They can be identified using a combination of TTE, TEE, MSCT, angiography and cardiac MRI and consist of:
▪ Left ventricular outf low tract (LVOT) diameter;
▪ LVOT diameter 18–21 mm requires a 23 mm Edwards SAPIEN valve;
▪ LVOT diameter greater than 21 mm requires a 26 mm Edwards SAPIEN valve or CoreValve. This is particularly important in the presence of left ventricular hypertrophy and a sigmoid septum. It may influence the choice of delivery method, such as in cases of a pronounced sigmoid septum in which the transapical approach may be preferred in order to allow adequate positioning and anchorage of the prosthesis [7];
▪ Bicuspid aortic valve: in these cases the valvular orifice is elliptical, which may cause significant paravalvular insufficiency following implantation of a cylindrical prosthesis [21]. Nevertheless, in our opinion, a bicuspid aortic valve represents a relative rather than absolute contraindication to TAVI. In such situations, a TAVI can still be considered in some cases; however, the operator must be more vigilant when assessing for paravalvular leaks and must also take into account the greater risk of ascending aorta dissection, particularly in cases of a dilated ascending aorta [22];
▪ Degree of angulation between the aorta and the heart: this condition can make accurate positioning more demanding, particularly in instances of a horizontal aortic root with a vertical aortic annulus;
▪ Calcified aortic wall (porcelain aorta): if this occurs in conjunction with a horizontal aortic root, delivery via the transfemoral or transaxillary/subclavian routes may increase the risk of aortic dissection or distal embolization, therefore making the transapical route the best option.
▪ Ventricular thrombus;
▪ Subaortic stenosis;
▪ Height of coronary ostia from the base of the aortic valve leaflets: these ideally need to be greater than 10 mm to ensure that the coronary arteries are not occluded upon implantation of the prosthesis [23–25]. The mean position of the right coronary ostium is 15–17 mm from the base of the annulus, while the left coronary ostium is positioned 13–17 mm from the base of the annulus [24,25]. In some situations this can be compensated for by controlled deep deployment of the CoreValve, thereby implanting a valve 1 or 2 mm lower than the recommended position. This results in positioning of the waist, the narrowest portion of the valve, adjacent to the position of the coronary ostia;
▪ Calcific valvular nodules: a degree of annulus calcification is present in all patients with degenerative AS [25]; however, the presence of large calcific nodules can be a contraindication to TAVI as they can predispose a patient to a risk of coronary occlusion or paravalvular leak after valve deployment;
▪ Mitral prosthesis: this can potentially interfere with the positioning of the aortic prosthesis. It is therefore imperative not to position the valve too low thereby affecting the function of the mitral prosthesis [26].
Assessment of the peripheral arterial system
The type of device chosen and the ideal delivery method is based on an assessment of the peripheral arteries as the transfemoral route is generally the preferred route by most operators worldwide. The transfemoral route allows minimally invasive vascular access via a percutaneous approach or surgical exposure of the femoral artery. This route shortens the length of hospital stay and has higher 1‑year survival rates when compared with the transapical route [27]. It is important to make an accurate assessment of the minimal luminal diameter of the aorta, iliac and common femoral arteries when considering this delivery route. In addition, the operator needs to know the degree of calcification and tortuosity of these arteries as the transfemoral route is contraindicated in those patients with severely calcified and tortuous peripheral arteries. To achieve an accurate assessment, at least two of the following investigative modalities are generally used:
▪ Peripheral angiography: this is a simple technique that allows assessment of vessel size and tortuosity but gives minimal indication as to the degree of calcification of vessels;
▪ Contrast-enhanced MSCT: this provides excellent noninvasive evaluation with good vessel resolution allowing assessment of vessel size, calcification and tortuosity. Furthermore, it allows cross-sectional evaluation of vessels (including the subclavian arteries) and 3D reconstruction to enhance the procedural planning process;
▪ Noncontrast-enhanced MSCT: this can be used as an alternative in patients in whom contrast load should be minimized, for example, patients with chronic kidney disease or contrast allergy, although it does not offer the same degree of definition between the vessel wall and blood interface as does contrast enhanced CT;
▪ MRI: is most informative when performed with gadolinium enhancement, therefore it should be used with caution in those patients with chronic kidney disease, as the nephrotoxicity of gadolinium is similar to that of regular contrast agents [28].
While in general, those patients with small, heavily calcified, tortuous arteries may require TAVI to be conducted via the transapical or transaxillary/subclavian routes, in the hands of experienced operators there are some cases in which the transfemoral route can still be considered. In arteries with circumferential vessel wall calcification, transfemoral access can still be attempted; however, the operator should only select those cases with a minimal vessel diameter that is greater than the reference ranges of 6 and 7–8 mm for the Edwards SAPIEN and CoreValve prostheses, respectively. In cases in which there are only focal areas of smaller diameter vessels, the transfemoral approach is still possible as long as the diameter mismatch is small. Vessel tortuosity can also be addressed in some cases by the careful use of a stiff guidewire to straighten out the arteries (Table 1). In all cases, particular care must be given when steering guidewires or introducer sheaths through those areas that are most commonly involved in vessel damage, namely, at the bifurcation of the iliac arteries and at the area of origin of the iliac arteries.
While a stiff guide wire is frequently preferred in tortuous anatomies, we should not forget that occasionally a softer wire may perform better as it minimizes friction against of the arterial walls once they have been straightened.
Delivery method selection
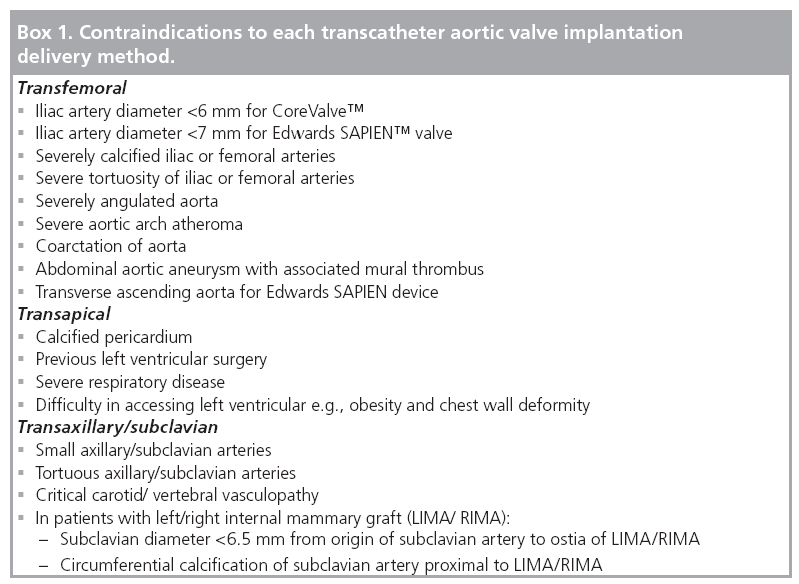
This is generally based on local expertise and a tailored approach to procedural planning by the multidisciplinary team in all TAVI patients as there are no comparative studies comparing the three approaches. The transaxillary/subclavian approach was introduced relatively recently as an alternative delivery method in patients with difficult transfemoral access for CoreValve implantation. Initial studies have reported excellent procedural success rates leading to the suggestion that this technique may provide a safer alternative to the transapical route in patients in whom the transfemoral route is contraindicated [29–30]. When selecting the best delivery method, the most commonly used pathway starts with an assessment of the transfemoral route in the first instance. If there are contraindications to the transfemoral route, the alternative delivery methods are then considered. There are guidelines as to the specific contraindications to each technique that can be used when making a selection of the most appropriate delivery method (Box 1).
Indications for pacing
Temporary pacing wire implantation is a necessity for all TAVI procedures as the atrioventricular (AV) node and its left bundle branch lie adjacent to the noncoronary cusp of the aortic valve, leading to a potential risk of AV conduction block postintervention [31]. Following conventional surgical AVR in octogenerians, 8.5% of patients require pacemaker implantation [32]. This is compared with a reported 5.4% pacing risk with implantation of the Edwards SAPIEN valve [12], and the need for a permanent pacemaker in 9.3–33% of patients following CoreValve implantation [11,33]. It is therefore widely accepted that the requirement for permanent pacing is greatest with the CoreValve prosthesis, and that this risk is even greater than with conventional surgical AVR. However, it should be noted that the reported frequency of permanent pacemaker requirement varies between studies due to differences in the clinical threshold and timing of pacing at TAVI centers. It also appears to be the case that the incidence of new left bundle branch block is approximately 40% following CoreValve implantation [34], compared with an incidence of 15.6% following surgical AVR [35]; however, the significance of this finding remains unknown. There is some evidence that AV conduction may improve over time post-TAVI, but studies of this phenomenon have been limited. The data on the physiological and anatomic factors that may predict pacing requirement are preliminary, but there does appear to be an increased risk of pacing in patients undergoing CoreValve implantation with evidence of conduction system disease at baseline, the presence of severe septal hypertrophy, increased thickness of the noncoronary cusp, and the presence of rate-limiting medication preprocedure [33]. As more studies are conducted in this field, we may find that pacing predictive parameters become a further factor influencing the choice of the most appropriate transcatheter valve prosthesis.
Overall contraindications to TAVI
Industry recommendations and guidelines suggest that there are cases in which a TAVI cannot be considered, and either surgical or conservative therapy is indicated instead [7]. Many of these contraindications to TAVI are based on the exclusion criteria for the initial TAVI trials and the ongoing randomized trial comparing surgical versus TAVI versus medical treatment in the Placement of Aortic Transcatheter Valve (PARTNER) trial. Contraindications include:
▪ Bicuspid aortic valve. This should not be viewed as an absolute contraindication. The operator should be aware of the higher risk of residual aortic insufficiency due to an asymmetric valve, and of the risk of aortic wall dissection due to aortic wall disease and dilatation;
▪ For the Edwards SAPIEN valve: aortic annular size less than 18 or more than 25 mm;
▪ For the CoreValve: aortic annular size less than 20 mm or more than 27 mm;
▪ Apical left ventricular thrombus;
▪ Subaortic stenosis;
▪ Grade 3–4 mitral regurgitation: it is felt that untreated severe mitral regurgitation may lead to ‘incomplete valvular intervention’ with poor long-term clinical outcomes. However, in our experience this is only a relative contraindication and should be considered on an individual patient basis;
▪ Calcif ic valvular nodules that may be dislocated towards the coronary ostia;
▪ Height of take-off of coronary ostia less than 10 mm;
▪ For the CoreValve: aortic root dimension more than 45 mm at the aorto-tubular junction;
▪ Life expectancy less than 1 year.
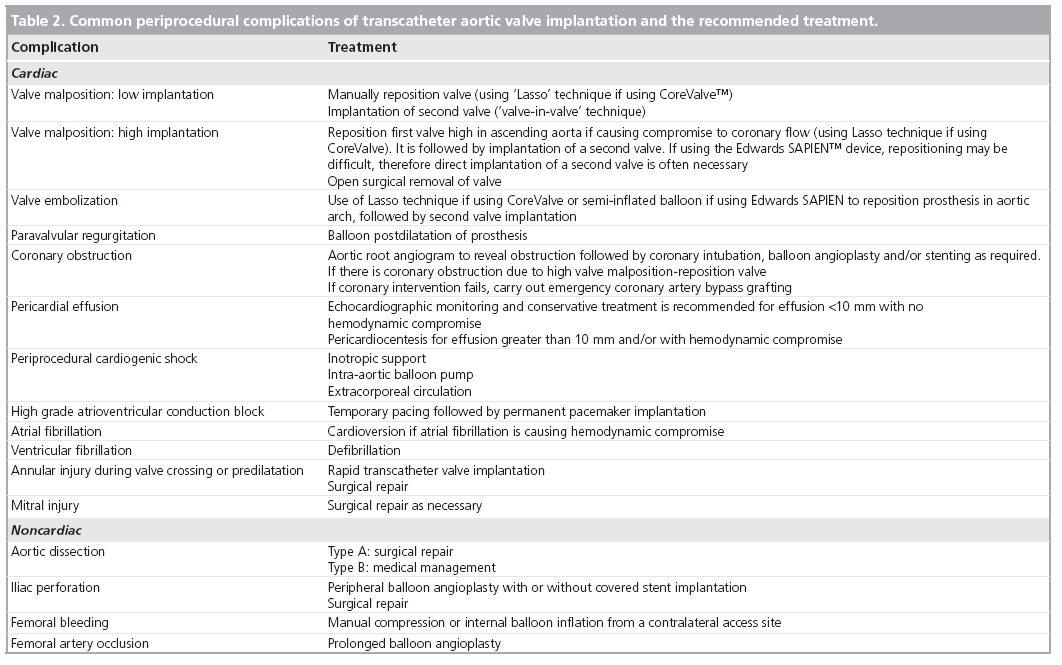
It should be noted that this list is not considered by most experienced operators to represent a series of absolute contraindications to TAVI. Some of these factors only represent relative contraindications that should be studied carefully before consideration of TAVI implantation; they may make TAVI implantation more challenging but can often be overcome in the hands of experienced physicians. These contraindications may also lead to a greater incidence of complications and, therefore, the operator must be cautious before considering a TAVI in these patients. The most frequent complications of TAVI are listed in Table 2.
Off-label use
In this article we have discussed the recommended ‘on‑label’ indications for TAVI; however, it is well recognized that centers and individual operators worldwide have become more proficient in this procedure. A greater level of experience in treating patients with a wide range of comorbidities, and anatomical and functional variables, has resulted in more frequent use of TAVI for ‘off‑label’ indications. TAVI has also been performed to treat bioprosthetic aortic valve failure resulting in severe aortic stenosis or regurgitation [36]. These ‘valve-in-valve’ procedures have been performed with good results, and while they represent an ‘off‑label’ indication for TAVI, they provide a growing application of the technique to this high-risk surgical subgroup. It appears that centers are now performing percutaneous valve implantation in patients who have a lower surgical risk than those in the initial study cohorts. This is to be expected as confidence in the therapy grows, and as the use of TAVI is expanded to address a wider proportion of the population. However, we must not overlook the fact that we currently only have up to 3-year follow-up from the initial TAVI patient cohorts, and we will need to have studied the much longer-term clinical outcomes of these patients before we can begin to offer TAVI as a viable alternative to conventional AVR in individuals with moderate or low surgical risk.
Piazza et al. recently assessed the frequency and outcomes with TAVI for ‘off‑label’ indications [37]. Of a group of 200 patients referred for TAVI between November 2005 and November 2008, 69 patients went on to have CoreValve implantation, of which 63 patients were treated with the third generation 18F system. The ‘on‑label’ indications for TAVI were defined as the widely reported standard criteria, as described within this review. In the same group, 42 patients (67%) had at least one ‘off-label’ criterion. A significant number of patients had at least one anatomical feature that is normally stated as a contraindication to TAVI, with the most common of these being the presence of grade 3–4 mitral regurgitation in 31% of patients, and an annulus diameter outside of the 20 to 27 mm range was found in 40% of patients. Furthermore, despite the presence of an iliofemoral diameter of less than 6 mm, the CoreValve was implanted via the transfemoral route in eight patients. The investigators went on to compare the overall technical success and procedural success between the ‘on-label’ group (n = 21) and the ‘off-label’ group (n = 42), with mean logistic EuroSCOREs of 19 and 14%, respectively. They found, perhaps surprisingly, that the overall technical and procedural success was highest in the ‘off‑label’ group, although this difference was not significant and may be a reflection of a small overall sample size. Given that a large proportion of patients had an aortic annular size outside the ‘on-label’ limits, it is also interesting to note that the frequency of moderate–severe aortic regurgitation postvalve dilatation or implantation of a second valve was not significantly different between the two groups. There was also no significant difference in the 30‑day rate of death, stroke, myocardial infarction or bleeding between the two groups, and furthermore the survival rate was similar between the two groups at 1‑year follow-up with an overall cumulative survival rate of 68.7%. While this study was small, and was the first to evaluate the ‘off‑label’ use of TAVI, it does lead to some important questions with respect to whether the current guidelines for TAVI are too strict, and may need revision now that our range of experience in the field has grown. A conservative approach was clearly important early on in the learning curve of TAVI but perhaps it is now time to widen the net to include more patients as our procedural success rates and clinical outcomes continue to improve.
Conclusion
Current guidelines for patient selection for TAVI implantation are based on early trials and experience with initial device implantation. Since these trials, the field of TAVI has continued to grow and develop, with operators becoming progressively more skilled in the various techniques. As experience has increased, the previously published absolute contraindications to TAVI have become more relative with operators choosing to implant devices in a growing number of highrisk cases. Furthermore, in the last 3 years the number of implantations has increased progressively, with an increasing length of follow-up and no reported cases of valve failure. This evidence can be used in making the argument that the indications for TAVI should be extended to lower-risk subgroups. While the guidelines were appropriate to address the initial learning curve and a growing level of experience in TAVI, it may be that we now need to readdress the patient selection criteria for TAVI in the light of a wider level of expertise.
Future perspective
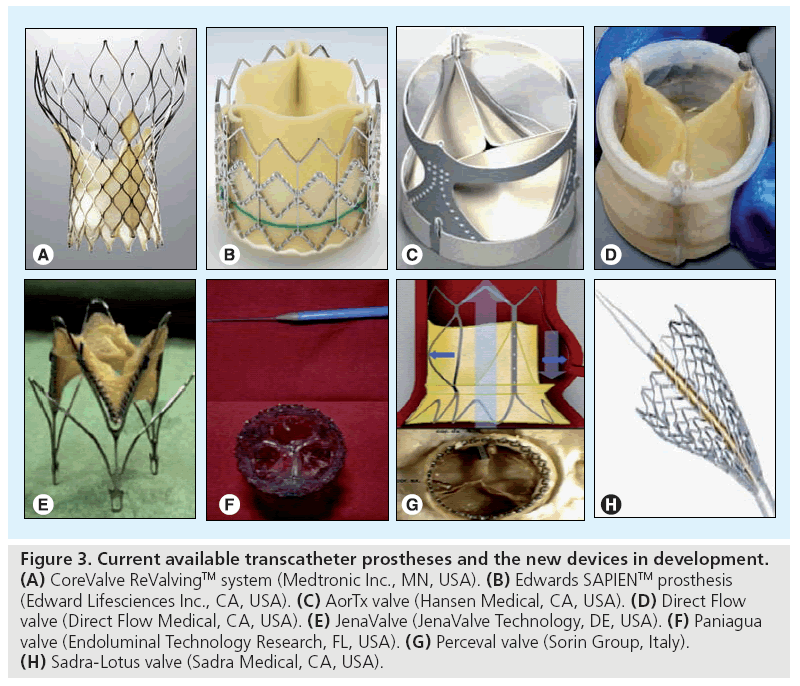
Given the success and widespread use of TAVI worldwide, new generations of the Edwards SAPIEN and the CoreValve are being developed. We await the availability of valves that are compatible with smaller sheath sizes, and are applicable to a wider range of annular dimensions. In addition, further devices are currently being developed that may add to the available therapies in this field. The development of these devices is aimed at producing design innovations that may allow treatment of patients with smaller arteries, the freedom to reposition the prosthesis and techniques to decrease the degree of perivalvular insufficiency. These devices are all in the preliminary stages of evaluation, and as yet, none of them are CE marked. A comprehensive review of all new TAVI prostheses is beyond the scope of this paper; however, the following are examples of a selection of new devices in the development phase (Figure 3).
Figure 3: Current available transcatheter prostheses and the new devices in development. (A) CoreValve ReValvingTM system (Medtronic Inc., MN, USA). (B) Edwards SAPIENTM prosthesis (Edward Lifesciences Inc., CA, USA). (C) AorTx valve (Hansen Medical, CA, USA). (D) Direct Flow valve (Direct Flow Medical, CA, USA). (E) JenaValve (JenaValve Technology, DE, USA). (F) Paniagua valve (Endoluminal Technology Research, FL, USA). (G) Perceval valve (Sorin Group, Italy). (H) Sadra-Lotus valve (Sadra Medical, CA, USA).
▪ AorTx valve
The AorTx valve (Hansen Medical, CA, USA) consists of a 24F-compatible device that can be repositioned, recaptured and redeployed. Proofof- concept for this device has been presented following implantation in eight patients prior to surgical AVR.
▪ Direct Flow valve
The Direct Flow valve (Direct Flow Medical, CA, USA) is a 22F-compatible stent, which consists of two hydrophilic coated rings with a bovine pericardial tissue valve sutured between the two rings. It can be repositioned and is retrievable before final release. In order to fix the valve in position, polymer is injected into the inflatable rings. It has been tested temporarily and permanently in humans [38].
▪ JenaValve™ The JenaValve™ (JenaValve Technology, DE, USA) is a low profile, self-expandable valve that has been implanted temporarily in patients prior to surgical AVR for proof-of-concept testing.
▪ Paniagua valve The Paniagua valve (Endoluminal Technology Research, FL, USA) consists of two models, a 16F-compatible balloon-expandable stent and a 12F-compatible self-expandable stent. One human implant has been reported and further trials are ongoing [39].
▪ Perceval valve
The Perceval valve (Sorin Group, Italy) is a self-expandable device that attempts to approximate the shape of the aortic root and sinuses of Valsalva, and has a double pericardial sheet that improves sealing against the native valve, thereby potentially reducing the incidence of paravalvular leak. First-man-studies have proven safety and feasibility and further studies are awaited.
▪ Sadra-Lotus™ valve
The Sadra-Lotus™ valve (Sadra Medical, CA, USA) is a repositionable, self-expandable device that has a gasket-like structure around the valve, which minimizes the risk of aortic regurgitation by filling any mismatch between the annulus and the valve.
There are a number of other new TAVI devices that are being developed, and these are currently in the preclinical stages of assessment, but they have yet to be tested in first-in-man trials. The advent of new technologies may mean improved procedural success rates with better long-term outcomes, but as yet it is too early to ascertain the exact details of the next generation of widely available transcatheter valve devices.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
▪ Severe aortic stenosis is conventionally treated with surgical aortic valve replacement. A high operative mortality of 7–10% is well recognized in selected high-risk groups; a resulting 30–40% of elderly patients do not have surgery.
▪ Transcatheter aortic valve implantation (TAVI) was developed to address this unmet need and is now widely practiced worldwide.
▪ Two transcatheter prostheses are currently available: the CoreValve ReValving™ system (Medtronic Inc, MN, USA) and the Edwards SAPIEN™ prosthesis (Edward Lifesciences Inc, Irvine, CA, USA).
Patient selection
▪ Patient selection should be performed by a dedicated multidisciplinary team.
▪ TAVI is recommended for patients with symptomatic severe aortic stenosis deemed to have high surgical risk for aortic valve surgery.
▪ Patients require preprocedural assessment of severity of aortic stenosis and concomitant coronary artery disease.
Valve type & size selection
▪ Edwards SAPIEN prosthesis is available in two sizes: 23 and 26 mm available for aortic annulus size 18–21 mm and 21–25 mm, respectively.
▪ CoreValve prosthesis is available in two sizes: 26 and 29 mm available for aortic annulus size 20–23 mm and 23–27 mm, respectively.
▪ In order to select the most suitable valve type and size an assessment of aortic annulus size, cardiac anatomy and peripheral arterial system is required.
Delivery method selection
▪ The Edwards SAPIEN prosthesis can be delivered via the transfemoral or transapical route.
▪ The CoreValve prosthesis can be delivered via the transfemoral or transaxillary/subclavian route.
▪ Selection of the most appropriate delivery method relies on an assessment of the peripheral arterial system and investigation of any contraindications to the use of a specific delivery route.
Indications for pacing
▪ There is a reported 5.4% pacing risk with implantation of the Edwards SAPIEN valve and 9.3–33% following CoreValve implantation.
Overall contraindications to TAVI
▪ A number of factors are considered to be contraindications to TAVI including aortic annulus size less than 18 mm or greater than 27 mm, subaortic stenosis, left ventricular thrombus, severe mitral regurgitation, low set coronary ostia, large calcific aortic nodules and most cases of bicuspid aortic valve.
▪ Residual aortic insufficiency and vascular complications represent the most important current limitations of TAVI.
Off-label use
▪ As TAVI becomes more widespread, it is being considered for patients with ‘off‑label’ indications.
▪ The ‘off‑label’ use of TAVI is appropriate in selected patients and may represent an indication that the current guidelines for TAVI are too strict and should be expanded to extend to a larger patient group.
Summary
▪ Selection of the most appropriate patients, valve prosthesis and delivery method is vital to the success and continued development of TAVI.
▪ Initial guidelines were appropriately strict given the relatively recent introduction of this technique. However, as experience in the field expands, TAVI may be applicable to a wider patient group.
Future perspective
▪ New developments in current TAVI technology and new devices are in continuous development and may offer an opportunity to expand the technique with improving efficacy and a reduction in the incidence of associated complications.
References
Papers of special note have been highlighted as:
▪▪ of considerable interest
- Asimakopoulos G, Edwards MB, Taylor KM: Aortic valve replacement in patients 80 years of age and older: survival and cause of death based on 1100 cases: collective results from the UK heart valve registry. Circulation 96(10), 3403–3408 (1997).
- Edwards MB, Taylor KM: Outcomes in nonagenarians after heart valve replacement operation. Ann. Thorac. Surg. 75(3), 830–834 (2003).
- Kojodjojo P, Gohil N, Barker D et al.: Outcomes of elderly patients aged 80 and over with symptomatic, severe aortic stenosis: impact of patient’s choice of refusing aortic valve replacement on survival. QJM 101(7), 567–573 (2008).
- Iung B, Baron G, Butchart EG et al.: A prospective survey of patients with valvular heart disease in europe: the Euro Heart Survey on valvular heart disease. Eur. Heart J. 24(13), 1231–1243 (2003).
- Iung B, Cachier A, Baron G et al.: Decisionmaking in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur. Heart J. 26(24), 2714–2720 (2005).
- Varadarajan P, Kapoor N, Bansal RC, Pai RG: Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann. Thorac. Surg. 82(6), 2111–2115 (2006).
- Vahanian A, Alfieri O, Al-Attar N et al.: Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 29(11), 1463–1470 (2008).
- Vahanian A, Alfieri OR, Al-Attar N et al.: Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-thoracic Surgery (EACT) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. J. Cardiothorac. Surg. 34(1), 1–8 (2008).
- Cribier A, Eltchaninoff H, Bash A et al.: Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106(24), 3006–3008 (2002).
- Grube E, Schuler G, Buellesfeld L et al.: Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current thirdgeneration self-expanding corevalve prosthesis: device success and 30-day clinical outcome. J. Am. Coll. Cardiol. 50(1), 69–76 (2007).
- Piazza N, Grube E, Gerckens U et al.: Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntervention 4(2), 242–249 (2008).
- Webb JG, Altwegg L, Boone RH et al.: Transcatheter aortic valve implantation: Impact on clinical and valve-related outcomes. Circulation 119(23), 3009–3016 (2009).
- Roques F, Nashef SA, Michel P: Risk factors for early mortality after valve surgery in europe in the 1990s: lessons from the euroscore pilot program. J. Heart Valve Dis. 10(5), 572–577; discussion 577–578 (2001).
- Ferguson TB Jr, Dziuban SW Jr, Edwards FH et al.: The sts national database: current changes and challenges for the new millennium. Committee to establish a national database in cardiothoracic surgery, the society of thoracic surgeons. Ann. Thorac. Surg. 69(3), 680–691 (2000).
- Moss RR, Ivens E, Pasupati S et al.: Role of echocardiography in percutaneous aortic valve implantation. JACC Cardiovasc. Imaging 1(1), 15–24 (2008).
- Tops LF, Wood DA, Delgado V et al.: Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc. Imaging 1(3), 321–330 (2008).
- Doddamani S, Grushko MJ, Makaryus AN et al.: Demonstration of left ventricular outflow tract eccentricity by 64-slice multi-detector CT. Int. J. Cardiovasc. Imaging 25(2), 175–181 (2009).
- Burgstahler C, Kunze M, Loffler C et al.: Assessment of left ventricular outflow tract geometry in non-stenotic and stenotic aortic valves by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 8(6), 825–829 (2006).
- Caruthers SD, Lin SJ, Brown P et al.: Practical value of cardiac magnetic resonance imaging for clinical quantification of aortic valve stenosis: comparison with echocardiography. Circulation 108(18), 2236–2243 (2003).
- Wood DA, Tops LF, Mayo JR et al.: Role of multislice computed tomography in transcatheter aortic valve replacement. Am. J. Cardiol. 103(9), 1295–1301 (2009).
- Zegdi R, Khabbaz Z, Ciobotaru V et al.: Calcific bicuspid aortic stenosis: a questionable indication for endovascular valve implantation? Ann. Thorac. Surg. 85(1), 342 (2008).
- Delgado V, Tops LF, Schuijf JD et al.: Successful deployment of a transcatheter aortic valve in bicuspid aortic stenosis: role of imaging with multislice computed tomography. Circ. Cardiovasc. Imaging 2(2), E12–E13 (2009).
- Tops LF, Krishnan SC, Schuijf JD, Schalij MJ, Bax JJ: Noncoronary applications of cardiac multidetector row computed tomography. JACC Cardiovasc. Imaging 1(1), 94–106 (2008).
- Knight J, Kurtcuoglu V, Muffly K et al.: Ex vivo and in vivo coronary ostial locations in humans. Surg. Radiol. Anat. 31(8), 597–604 (2009).
- Rivard AL, Bartel T, Bianco RW et al.: Evaluation of aortic root and valve calcifications by multi-detector computed tomography. J. Heart Valve Dis. 18(6), 662–670 (2009).
- Rodes-Cabau J, Dumont E, Miro S et al.: Apical aortic valve implantation in a patient with a mechanical valve prosthesis in mitral position. Circ. Cardiovasc. Interv. 1(3), 233 (2008).
- Himbert D, Descoutures F, Al-Attar N et al.: Results of transfemoral or transapical aortic valve implantation following a uniform assessment in high-risk patients with aortic stenosis. J. Am. Coll. Cardiol. 54(4), 303–311 (2009).
- Briguori C, Colombo A, Airoldi F et al.: Gadolinium-based contrast agents and nephrotoxicity in patients undergoing coronary artery procedures. Catheter Cardiovasc. Interv. 67(2), 175–180 (2006).
- Fraccaro C, Napodano M, Tarantini G et al.: Expanding the eligibility for transcatheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation corevalve revalving system. JACC Cardiovasc. Interv. 2(9), 828–833 (2009).
- Laborde J: Axillary/subclavian access with transcatheter aortic valve implantation: a new and promising approach. Presented at: EuroPCR, Barcelona, Spain, 19–22 May, 2009.
- Piazza N, de Jaegere P, Schultz C et al.: Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ. Cardiovasc. Intervent. 1, 74–81 (2009).
- Dawkins S, Hobson AR, Kalra PR et al.: Permanent pacemaker implantation after isolated aortic valve replacement: incidence, indications, and predictors. Ann. Thorac. Surg. 85(1), 108–112 (2008).
- Jilaihawi H, Chin D, Vasa-Nicotera M et al.: Predictors for permanent pacemaker requirement after transcatheter aortic valve implantation with the CoreValve bioprosthesis. Am. Heart J. 157(5), 860–866 (2009).
- Piazza N, Onuma Y, Jesserun E et al.: Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after percutaneous replacement of the aortic valve. JACC Cardiovasc. Interv. 1(3), 310–316 (2008).
- El-Khally Z, Thibault B, Staniloae C et al.: Prognostic significance of newly acquired bundle branch block after aortic valve replacement. Am. J. Cardiol. 94(8), 1008–1011 (2004).
- Olsen LK, Engstrom T, Sondergaard L: Transcatheter valve-in-valve implantation due to severe aortic regurgitation in a degenerated aortic homograft. J. Invasive Cardiol. 21(10), E197–E200 (2009).
- Piazza N, Otten A, Schultz C et al.: Adherence to patient selection criteria in patients undergoing transcatheter aortic valve implantation with the 18f CoreValve revalving™ system – results from a single-center study. Heart 96(1), 19–26 (2010).
- Schofer S, Treede H et al.: Retrograde transarterial implantation of a nonmetallic aortic valve prosthesis in high-surgical-risk patients with severe aortic stenosis. A first-in-man feasibility and safety study. Circ. Cardiovasc. Intervent. 1, 126–133 (2009).
- Paniagua D, Condado JA, Besso J et al.: First human case of retrograde transcatheter implantation of an aortic valve prosthesis. Tex. Heart Inst. J. 32(3), 393–398 (2005).
▪▪ Current European guidelines for transcatheter aortic valve implantation.
▪▪ Current European guidelines for transcatheter aortic valve implantation.
▪▪ Important article on outcomes following CoreValve™ implantation.
▪▪ Important article on outcomes following Edwards SAPIEN™ valve implantation.
▪▪ Interesting article describing off-label use of transcatheter aortic valve implantation.