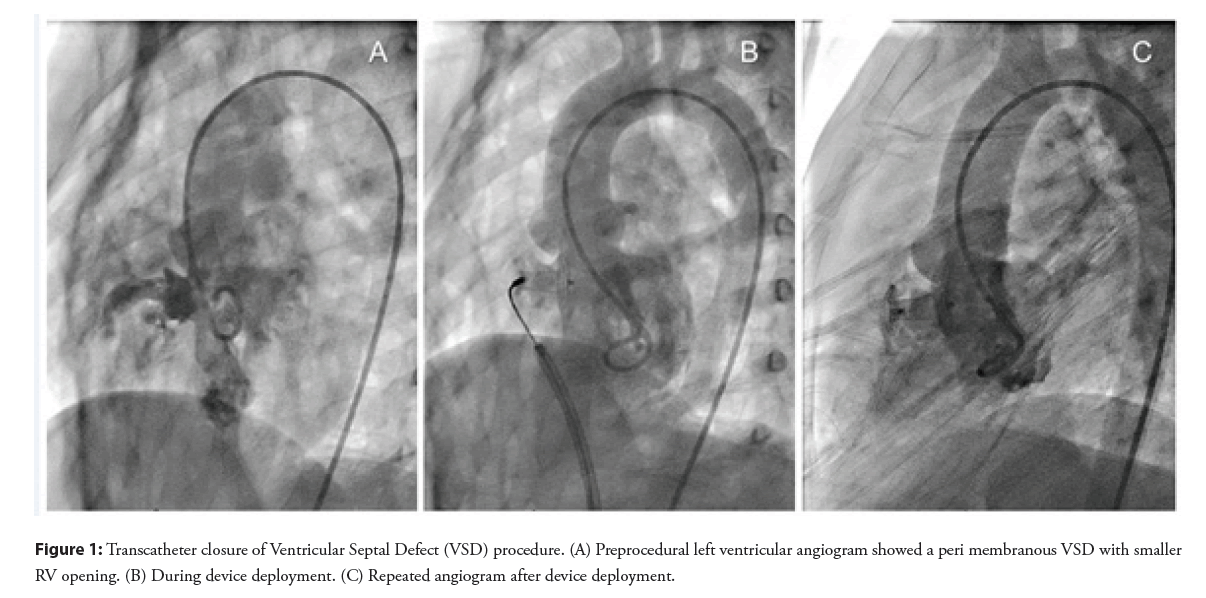
Case Report - Interventional Cardiology (2022) Volume 14, Issue 6
Delayed onset of atrioventricular block after transcatheter closure of peri membranous ventricular septal defect recovered after surgical axplantation: A case report and review of literature
- Corresponding Author:
- Pornthep Lertsapcharoen
Division of Pediatric Cardiology,
Department of Pediatrics,
Chulalongkorn University and Cardiac Center,
King Chulalongkorn Memorial Hospital,
The Thai Red Cross Society,
Bangkok,
Thailand,
E-mail: lpornthep@yahoo.com
Received date: 11-Nov-2022, Manuscript No. FMIC-22-79537Editor assigned: 14-Nov-2022, PreQC No. FMIC-22-79537 (PQ)Reviewed date: 28-Nov-2022, QC No. FMIC-22-79537Revised date: 05-Dec-2022, Manuscript No. FMIC-22-79537 (R)Published date: 15-Dec-2022, DOI: 10.37532/1755-5310.2022.14(6). 586
Abstract
Background: Atrioventricular block (AV block) is still a major concern following transcatheter closure of Ventricular Septal Defect (VSD) despite an improvement in post-procedural outcome in the current era. Late onset of AV block has rarely been reported thus no consensus on definite treatment options exists.
Case presentation: We report a case of delayed onset of AV block which occurred 3 years after transcatheter VSD closure in a 4-year-old boy with peri membranous VSD. The VSD size was 8 mm at the Left Ventricular (LV) opening and 4 mm at the Right Ventricular (RV) opening and aneurysmal-type Cocoon ventricular septal occluder 8-6/10 mm (the device has a central waist diameter at the distal site of 8 mm and at the proximal site of 6 mm with its length of 10 mm) was deployed without immediate post-procedural complication. The ECG remained in normal sinus rhythm throughout the regular follow-up period. He developed exercise intolerance 3 years later and ECG showed a new onset of complete AV block with a junctional rate of 60 beats/minute. Surgical removal of the device with VSD closure was successfully performed and resulted in a restoration of sinus rhythm within 3 months after the operation. The patient had regained normal functional capacity and a good exercise tolerance without the need for permanent pacemaker implantation.
Conclusion: Progressive perinodal inflammation can damage the conduction system and result in the late onset of AV block. Surgical removal of the device can be a possible treatment option although permanent pacemaker implantation is frequently required. Comprehensive long-term follow-up focusing on conduction system disturbance is crucial after transcatheter closure of VSD.
Keywords
Atrioventricular block • Device closure • Transcatheter closure •Ventricular septal defect • Surgical explantation
Introduction
Ventricular Septal Defect (VSD) is one of the most common congenital cardiac defects in the pediatric population. Surgical closure of the defect under cardiopulmonary bypass has been a gold-standard treatment until percutaneous transcatheter VSD closure was introduced in 1988 [1]. The development of the device and delivery system together with an improvement of interventional technique has resulted in better safety and feasibility of this procedure. Over 90% success rate following transcatheter VSD closure with various types of devices has been reported [2-5].
However, disturbance of Atrioventricular conduction is still a major concern following the transcatheter device closure. The incidence of complete Atrioventricular (AV) block from recent studies varies from 1% to 3% [2,5-8]. Conduction disturbance mostly occurs immediately after device deployment. Late onset of AV block up to months or years has rarely been described [9-11]. We report a case of delayed onset of AV block which occurred three years after transcatheter VSD closure and recovered after surgical removal of the VSD device.
Case Presentation
A 4-year-old boy diagnosed with a moderate-size Peri membranous Ventricular Septal Defect (PmVSD) was scheduled for an elective transcatheter closure. Transthoracic echocardiography revealed an 8 mm PmVSD partially covered with membrane-formed aneurysm resulting in an effective orifice of 3-4 mm with left to right flow across the defect. Pre-procedural Electrocardiography (ECG) was in sinus rhythm and showed left ventricular hypertrophy.
During cardiac catheterization, hemodynamic data revealed the ratio of pulmonary to systemic flow (Qp: Qs) of 1.4 to 1. Left ventricular angiography confirmed an aneurysmal PmVSD measured 8 mm at the left ventricular orifice and 4 mm at the right ventricular orifice. An aneurysmal-type Cocoon ventricular septal occluder, size 8-6/10 mm (the device has a central waist diameter at the distal site of 8 mm and at the proximal site of 6 mm with its length of 10 mm), was successfully deployed by transvenous approach without complication. Post-procedural angiography and transthoracic echocardiography showed VSD device was in a proper position with minimal leakage and no regurgitation of the tricuspid and the aortic valves was observed (Figure 1).
Oral administration of aspirin 5 mg/kg/day was initiated. After continuous monitoring of ECG and hemodynamic parameters ensured no complication, the patient was discharged home the following day in stable condition.
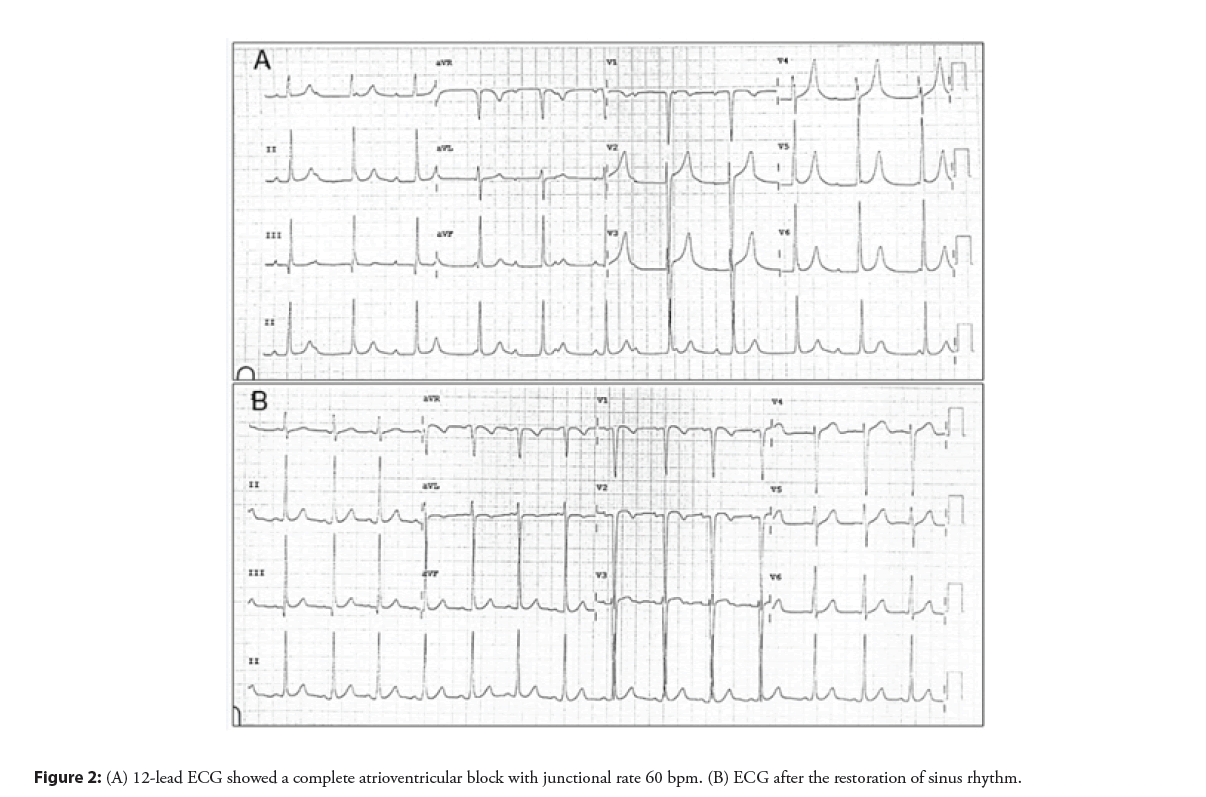
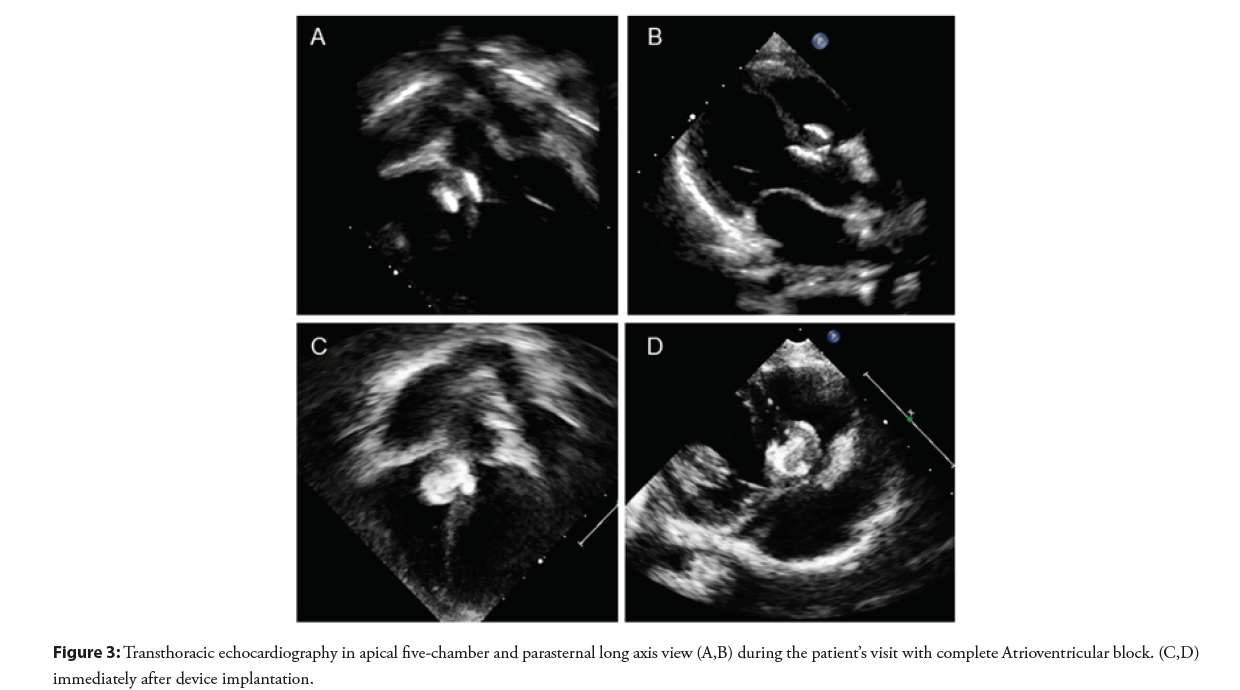
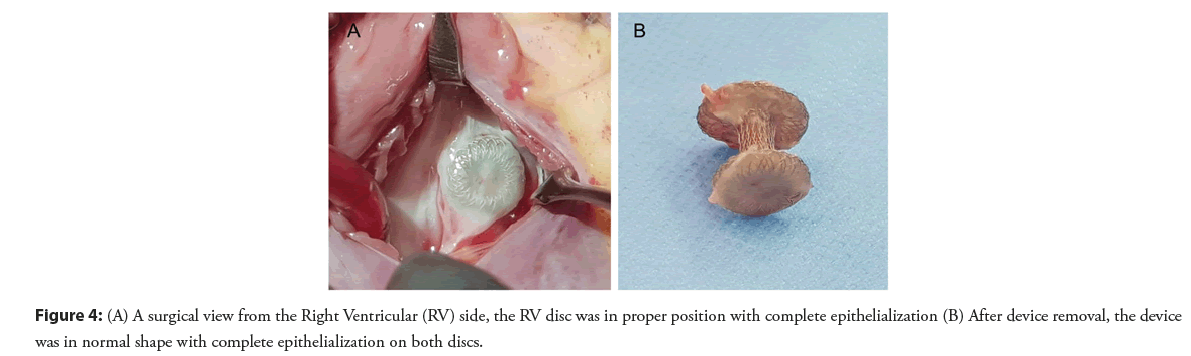
The patient was doing well without evidence of electrical disturbance during the regular follow-up visits for three years. He then gradually developed easily fatigue and exercise intolerance without syncopal episodes. Physical examination was unremarkable except for bradycardia. ECG showed a complete AV block with a junctional escape rate of 60 bpm (Figure 2A). Echocardiography confirmed a stable device position and good ventricular function. However, there was a slight flattening of the VSD device compared with when it was immediately implanted (Figure 3). The exercise stress test failed to increase the patient’s heart rate and early ended due to chronotropic incompetence. No other explanation for the cause of AV block except for the consequence of the transcatheter VSD closure procedure. After a discussion with the parents, a surgical operation was performed to remove the VSD device and close the defect. Intraoperative findings demonstrated that the VSD device was in a proper position with complete epithelialization on both right and left ventricular discs (Figure 4). The rhythm remained in complete AV block post-operatively with good junctional escape and the patient was in a stable hemodynamic status. We agreed to delay permanent pacemaker implantation and planned to closely follow up. Three months after the operation, ECG had turned back to normal sinus rhythm without evidence of conduction abnormalities (Figure 2B). The patient had regained a normal functional capacity and a good exercise tolerance.
Figure 3: Transthoracic echocardiography in apical five-chamber and parasternal long axis view (A,B) during the patient’s visit with complete Atrioventricular block. (C,D) immediately after device implantation.
Results and Discussion
There was a higher incidence of conduction abnormalities following either surgical or device closure of peri membranous VSD, as compared with other types of VSD, due to the proximity of the AV node and conduction system to the defect margin. Various types of double disc devices have been developed for a better outcome of transcatheter closure and avoidance of potential complications. The Cocoon VSD Occluder (Vascular Innovations, Co., Thailand) is a self-expandable double disc device manufactured from braided Nitinol wires coated with platinum. The two discs are linked together by a connecting waist corresponding to the defect size. Cocoon VSD occluders have three different designs to match VSD morphology: membranous, muscular, and aneurysmal type. Transcatheter closure with this device has been reported in different centers with favorable outcomes and low incidence of conduction system abnormalities [5,12] .
Many mechanisms have been proposed to be the cause of AV block following transcatheter device closure, yet no definite consensus on the treatment options exists. After device deployment, the device can cause direct injury or compression to the AV node and conduction system [9,10,13]. This damage can be from excessive clamping force as frequently seen in double disc devices. Moreover, the excessive radial force from oversize devices stretching the conduction system has also been postulated. AV block from these mechanisms mostly occurs immediately or early within 24-48 hours after device deployment [9]. In case an AV block occurs immediately during the procedure, it is recommended to abandon the procedure and proceed to surgery. Steroids may show a slight response and the patients eventually need surgical removal of the device and closure of the defect [10,13].
Additionally, the inflammation around the AV node and conduction system following device closure can cause progressive perinodal fibrosis that leads to a delayed onset of AV block (months to years) [9,14]. This can occur despite no prior evidence of conduction disturbance during the immediate post-procedural period. Permanent pacemaker implantation is usually required, and surgical removal of the device often shows no improvement since there is potentially irreversible damage to the conduction system [9,11].
The device shape may change over time and the flattening of the previously oversized device can cause ongoing damage to the conduction system resulting in a late AV block [9,13]. Shao et al. [11], reported the possibly latest onset of AV block occurred 10 years after Amplatzer muscular VSD device implantation in a 5-year-old patient. Device flattening was documented from echocardiography and angiography; thus this mechanism was proposed to be the cause. The patient did not respond to steroid injection and a permanent pacemaker was eventually implanted.
Surgical explantation of the VSD device has been proven to restore the conduction system in the immediate AV block [7,10,15]. However, there was no sufficient data regarding the effectiveness of device removal for the late onset of AV block. Since the conduction system in such cases can be permanently damaged. Furthermore, surgical removal of the device in late AV block may not be possible due to a complete epithelialization of the device. Kumar et al. [16], reported a successful surgical explantation of a VSD device in a patient who developed Left Bundle Branch Block (LBBB) and LV dysfunction several months after device deployment. The LBBB recovered one month after the surgery and LV function was gradually restored.
We hypothesized that ongoing fibrosis around the AV nodal area which eventually alters the AV conduction may explain the late onset of AV block in our patient. Since the device seemed not to be oversized and there was only a slight change of the device shape, in our point of view, the device flattening may not solely contribute to the development of AV block in this patient. In correlation with the recent onset of the patient’s symptoms just within a few months, we thought that surgical removal would be a feasible treatment option to modify possible causative factors and regain the AV function. The complete recovery of AV conduction after device removal signified no direct permanent damage to the AV node and conduction system. However, due to complete epithelialization on both sides of the device, surgical removal should be performed with great caution to avoid further injury to the conduction system. Despite a complete recovery of AV conduction, this patient is in need of a long-term (possibly life-long) follow-up for a potential deterioration of the conduction system.
Conclusion
Conduction system disturbance following transcatheter closure of VSD mostly occurs during the immediate post-procedural period, but the late onset of AV block up to years has been reported. A comprehensive long-term follow-up focusing on conduction system disturbance is crucial in this group of patients. Device flattening or localized inflammation causing damage to the conduction system has been proposed to be the cause of the late onset of AV block. Despite the need for permanent pacemaker implantation in the majority of the cases, we have reported a surgical explantation as a possible treatment option to successfully restore sinus rhythm in a particular case.
Data Availability Statement
The data that support this case report are available from the corresponding author upon reasonable request. The data are not publicly available due to the patient confidentiality.
Funding statement: The author received no specific grant from any funding agency, commercial or non-profit organization.
Conflicts of Interest
No disclosure
Ethics Approval Statement
Ethics approval was exempted by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (COE No. 040/2022, IRB No. 0542/65).
Patient Consent Statement
The patient and parents provided consent for this article to be published.
Permission to Reproduce Material from other Sources
Not applicable
Clinical Trial Registration
Not applicable
References
- Lock JE, Block PC, McKay RG, et al. Transcatheter closure of ventricular septal defects. Circulation. 78(2): 361-368 (1988).
- Santhanama H, Yang L, Chen Z, et al. A meta-analysis of transcatheter device closure of peri membranous ventricular septal defect. Int J Cardiol. 254: 75-83 (2018).
[Cross ref] [Google Scholar] [PubMed]
- Walavalkar V, Mauya S, Pujar S, et al. Percutaneous device closure of congenital isolated ventricular septal defects: A single‑center retrospective database study amongst 412 cases. Pediatr Cardiol. 41:591-598 (2020).
[Cross ref] [Google Scholar] [PubMed]
- Jiang D, Han B, Zhao L, et al. Transcatheter device closure of perimembranous and intracristal ventricular septal defects in children: Medium-and long-term results. J Am Heart Assoc. 10(11): e020417 (2021).
[Cross ref] [Google Scholar] [PubMed]
- Arora R, Trehan V, Kumar A, et al. Transcatheter closure of congenital ventricular septal defect: Experiences with various devices. J Interven Cardiol. 16(1): 83-91 (2003).
[Cross ref] [Google Scholar] [PubMed]
- Bai Y, Xu XD, Li CY, et al. Complete atrioventricular block after percutaneous device closure of peri membranous ventricular septal defect: A single-center experience on 1046 cases. Heart Rhythm. 12: 2132-2140 (2015).
[Cross ref] [Google Scholar] [PubMed]
- Chen Q, Cao H, Zhang GC, et al. Atrioventricular block of intraoperative device closure peri membranous ventricular septal defects: A serious complication. BMC Cardiovasc. 12: 1-21 (2012).
[Cross ref] [Google Scholar] [PubMed]
- Shah JH, Thakkar BM. Cardiac rhythm abnormalities after transcatheter device closure of peri membranous ventricular septal defects in pediatric patients at intermediate term follow up. Int J Pediatr Res. 3(10): 718-23 (2016).
- Ghosh S, Mukherji A, Pathak NL, et al. Delayed onset atrioventricular block following transcatheter closure of peri-membranous VSD using Amplatzer duct occluder-II device: Discussion with review of literature. Prog Pediatr Cardiol.
- Kabbani M, Munshi F, Alhabshan F, et al. Unusual delayed presentation of life-threatening complete heart block after Ventricular Septal Defect (VSD) closure with Amplatzer Device. Eur Heart J Suppl. 16(B): B72-B74 (2014).
- Shao S, Luo C, Zhou K, et al. Very late-onset complete atrioventricular block following deployment of Amplatzer membranous ventricular septal defect occluder. Medicine. 98(51): e18412 (2019).
[Cross ref] [Google Scholar] [PubMed]
- Park H, Song J, Kim ES, et al. Early experiences using cocoon occluders for closure of a ventricular septal defect. J Cardiovasc Imaging. 26(3): 165-74 (2018).
[Cross ref] [Google Scholar] [PubMed]
- Walsh MA, Bialkowski J, Szkutnik M, et al. Atrioventricular block after transcatheter closure of perimembranous ventricular septal defects. Heart. 92(9): 1295-1297 (2006).
[Cross ref] [Google Scholar] [PubMed]
- Predescu D, Chaturvedi RR, Friedberg MK, et al. Complete heart block associated with device closure of perimembranous ventricular septal defects. J Thorac Cardiovasc Surg. 136(5): 1223-1228 (2008).
[Cross ref] [Google Scholar] [PubMed]
- Ovaert C, Dragulescu A, Sluysmans T, et al. Early surgical removal of membranous ventricular septal device might allow recovery of atrioventricular block. Pediatr Cardiol. 29(5): 971-975 (2008).
[Cross ref] [Google Scholar] [PubMed]
- Kumar HVJ, Mathew A, Subramanian A, et al. Role of late surgical explantation of device from perimembranous ventricular septal defect for left bundle branch block and left ventricular dysfunction. Heart. Case Rep. 6(4): 178-82 (2020).
[Cross ref] [Google Scholar] [PubMed]