Review Article - Interventional Cardiology (2021) Volume 13, Issue 5
Deoxyribonucleic acid repair in atherosclerotic coronary artery disease
- Corresponding Author:
- Mahmoudi M
Coronary Research Group,
University Hospital Southampton NHS Foundation Trust,
Southampton,
UK,
E-mail: m.mahmoudi@soton.ac.uk
Received date: June 28, 2021 Accepted date: July 12, 2021 Published date: July 19, 2021
Abstract
Despite recent advances in the therapeutic and interventional treatment of coronary artery disease the global prevalence of this disease is increasing and the associated mortality and morbidity remain high. Alongside well-established risk factors such as smoking, hypertension, hypercholesterolaemia and diabetes mellitus, deoxyribonucleic acid (DNA) damage, cascade protein signalling and the associated repair pathways are becoming increasingly recognised as major causative co-factors in the pathogenesis of atherosclerosis. A number of in vitro studies have shown defective DNA repair is instrumental in the pathogenesis and progression of atherosclerotic plaques with a positive correlation observed between the level of DNA damage and the severity of the atherosclerotic lesions. In knockdown mouse models of atherosclerosis, the DNA repair signalling cascade has been shown to be amenable to pharmacological manipulation and overexpression of specific repair proteins attenuate atherogenesis. However, to date there is little in-human data which supports these findings. This review will explore the current evidence and understanding of the role of DNA damage and repair in the pathogenesis of atherosclerosis and address possible therapeutic interventions for treatment.
Keywords
Cardiovascular disease • Coronary artery • DNA repair • Atherosclerosis
Introduction
Despite advances in the medical and invasive treatment of Coronary Artery Disease (CAD) the global incidence and mortality remain high [1,2]. Information generated from the latest Global Burden of Disease Study 2019 confirms historical data that cardiovascular disease (CVD) due to atherosclerosis remains the leading cause of overall mortality [2].
Whilst new coronary interventional procedural techniques such as the concordant use of fractional flow reserve assessment of ischaemia [3], intracoronary imaging (intravascular ultrasound (IVUS) and Optical Coherence Tomography (OCT)) have been associated with improvements in clinical outcomes in patients undergoing Percutaneous Coronary Intervention (PCI) [4], there have been very few advances in the medical therapy of CAD in recent years [5]. Progress still needs to be made in the primary prevention of cardiovascular events.
The rapid expansion and improvement in genomic research techniques in recent times has highlighted DNA damage within the atherosclerotic plaque and in circulating leukocytes as key factors in the pathogenesis of atherosclerosis [6-8].
Overview of Deoxyribonucleic Acid (DNA) Damage and the Response Pathways
DNA damage in human cells occurs via endogenous and exogenous pathways. Typically, endogenous damage is caused by Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) formed as a result of oxidative stress, metabolic processes and inflammatory responses to a variety of stimuli [9,10]. Exogenous DNA damage primarily occurs as a result of exposure to ionising radiation, ultraviolet radiation and mutagenic chemicals such as organophosphates but can also occur following exposure to toxic metals, mercury being a primary example [11]. The clinical consequences of both pathways include a wide variety of disease processes such as cancer and atherosclerosis.
Of the endogenous and exogenous pathways resulting in DNA damage, endogenous ROS, at both physiological and pathological levels, are the most common cause. Whilst producing adenosine triphosphate (ATP) the mitochondrial electron transport chain, as a double-edged sword, is the primary source for intracellular ROS [12]. Several ROS have been shown to increase the progression of atherosclerosis including superoxide anion, hydrogen peroxide, hydroxyl radicals and oxidised lipids such as oxidised-low density lipoprotein [13,14], acting primarily by disruption of mitochondrial antioxidant pathways [15]. Initially, superoxide and hydrogen peroxide are inert to DNA, but following conversion into hydroxyl radicals via the Fenton reaction they can subsequently cause extensive damage to mitochondrial DNA (mtDNA) and nuclear DNA which results in single stand breaks, double strand breaks, glycolytic damage and mispairing [16,17].
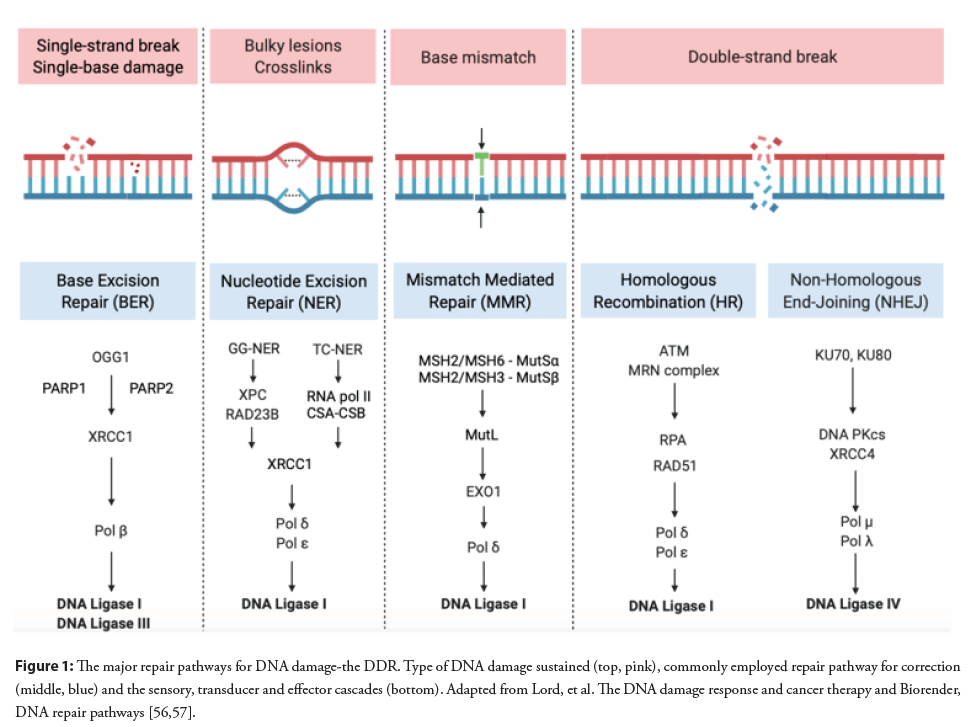
In order to protect against the deleterious effects of DNA damage, human cells have developed several pathways collectively known as the DNA Damage Response (DDR) [18]. A number of DDR pathways have been identified to date, which include Base Excision Repair (BER) [19], Double Strand Break Repair (DSBR) subdivided into homologous and non-homologous recombination alternatively known as non-homologous end-joining [20], Nucleotide Excision Repair (NER) [21,22], and mismatch repair (MMR) [23]. BER and DSBR are the most relevant repair mechanisms resulting from oxidative stress in atherosclerosis (Figure 1).
Figure 1: The major repair pathways for DNA damage-the DDR. Type of DNA damage sustained (top, pink), commonly employed repair pathway for correction (middle, blue) and the sensory, transducer and effector cascades (bottom). Adapted from Lord, et al. The DNA damage response and cancer therapy and Biorender, DNA repair pathways [56,57].
The repair mechanisms are broadly governed by a series of modulatory and recruiting proteins acting in three stages. Sensor proteins detect the initial damage to DNA [24,25], transducer/ adaptor proteins initiate a signalling cascade [26], which in turn results in effector proteins enacting the final stage of the pathway, cell cycle arrest, delay, DNA repair or apoptosis [27].
DNA repair pathways
Base Excision Repair (BER): When damage occurs as a result of oxidative stress, alkylation and abasic single base damage that does not result in significant distortion to the integrity of the DNA helix, base excision repair may correct the issue [28]. Chromatin remodelling of the damaged bases results in the recruitment of a variety of DNA glycosylases which in turn remove the distorted lesions forming an Abasic Site (AP) [29]. DNA glycosylases are either monofunctional, possessing only glycosylase activity, commonly uracil glycosylases, for example N-methylpurine DNA Glycosylase (MPG), or are bifunctional possessing additional β-lyase activity: Examples include Nth-like DNA glycosylase-1 (NTHL1) and Nei-like DNA glycosylase 1 (NEIL1) [30]. It is important to note that some are able to act as both monofunctional or bifunctional glycosylases, for example the ubiquitous 8-oxoguanine DNA glycosylase (OGG1) [31].
Monofunctional glycosylases initiate short-patch repair in which a 5’ phosphodiester incision is made by apurinic endonuclease-1 (APE-1) to the AP site. The single gap is filled by DNA polymerase-β (Pol-β) and then ligated by either DNA ligase I (LIG1) or the DNA ligase III (LIG3) X-Ray repair Cross-Complementing protein 1 (XRCC1) complex [32]. Long-patch repair is started as the result of bifunctional glycosylases, under the action of poly (ADPribose) polymerase 1 (PARP1) and poly (ADP-ribose) polymerase 2 (PARP2) [33]. Following the initial DNA glycosylase incision, the resultant AP site is customised by the 3’ phosphodiesterase activity of APE-1, after which DNA polymerase δ/ε (Pol δ/ε) begin synthesising in a strand displacement manner, creating a flap of typically 2-10 nucleotides, this is finally ligated by LIG1 [28,34].
Double Strand Break Repair (DSBR): Double strand DNA breaks have a significantly more detrimental effect on cellular longevity and DNA integrity. They commonly arise as a result of exposure to ROS, ionising radiation, V(D)J recombination and immunoglobulin class switching processes [35]. The repair of double strand breaks is primarily via two pathways, Homologous Recombination (HR) and Non-Homologous End-Joining (NEHJ) [28].
In NHEJ the repair process is governed by the tumour suppressor p53 binding protein 1 (53BP1). It is responsible for recruiting many of the other repair components and, importantly, facilitating the joining of the two break ends [36]. The Ku heterodimer (Ku70 and Ku80) moves rapidly to stabilise the break and prevent end resection and, in turn, recruits DNA-dependent protein kinasecatalytic subunit (DNA-PKcs) [37,38]. Ku subsequently moves inwards on the DNA whilst, simultaneously, X-ray Repair Cross- Complementing protein 4 (XRCC4) stabilises the whole complex by tethering the ends together [39]. The groups which are blocking the strand ends are resected and the resultant gaps are filled by DNA polymerase μ (Pol μ) and DNA polymerase λ (Pol λ). DNA ligase 4 (LIG4) completes the NHEJ procedure by joining the ends [40,41].
Nucleotide Excision Repair (NER): Nucleotide excision repair is often employed in helix distorting damage that is classically a result of ultraviolet radiation [46]. In a similar fashion to DSBR, NER employs two distinct pathways to repair the damage; global genome NER (GG-NER) and transcription-coupled NER (TC-NER) [47]. In TC-NER damaged lesions are excised from transcribed stands following exposure as a result RNA polymerase II reverse translocation which is stimulated by a complex of DNA excision repair protein-8 (ERCC8) and DNA excision repair protein-6 (ERCC6) also known as the CSA-CSB complex [48]. In GG-NER the damage sensor Xeroderma pigmentosum complementation group C (XPC)-UV excision repair protein RAD23 homolog B (RAD23B) complex scans the genome for transient single-stranded DNA (ssDNA) caused by disrupted base pairing due to the lesion, thus exposing the damage [49]. The excision of the damaged nucleotide and finishing process is shared by both the GG-NER and TC-NER pathways and culminates in a myriad of DNA polymerases, typically δ and , completing the gap filling and LIG1 or XRCC1-LIG3 implementing ligation [50]. Whilst there is currently limited evidence of increased NER activity in atherosclerosis the importance of NER was shown in two NER defective mouse models ERCC1d/- and XPRTTD. Specifically, increased vascular cell senescence, stiffness, hypertension and impaired vasomotor function were all noted [21].
Mismatch Repair (MMR): The MMR pathway is an evolutionarily conserved pathway which typically repairs base mismatches that occurred during replication and insertion-deletion loops that have resulted from strand slippage events [51]. It is further utilised in numerous other cellular processes such as DNA-damage signalling, apoptosis, meiotic and mitotic recombination and microsatellite instability [52]. Chromatin modification is the initial step in the MMR pathway and allows cascade signalling proteins access to the DNA lesion in order to initiate repair [53]. In humans base mismatches and short, meaning single or double insertion/deletion loops, are recognised by the DNA mismatch repair protein Msh2 (MSH2)/MSH6 heterodimer, otherwise known as MutSα, with longer loops recognised by the MutSβ heterodimer (MSH2/ MSH3) [54]. Several MutL complexes are recruited, with MutLα, in particular, having a central role since it controls the cessation of mismatched-provoked excision along with influencing the 3’ nickdirected digestion by Exonuclease 1 (EXO1) [55-57]. The final steps in the pathway involve various DNA polymerases and LIGI1 synthesising and sealing the gap.
Evidence for DNA Damage in Atherosclerosis
The pathogenesis of atherosclerosis is a multifaceted process and DNA damage has been shown to be a key component in the process at both micro and macros levels [58]. Several studies have shown that cells in the systemic circulation and those found within the mature atherosclerotic plaque contain increased levels of DNA damage compared to controls without significant atherosclerotic disease [59-62].
DNA base damage
As mentioned previously, DNA bases can be particularly vulnerable to damage by ROS, in particular guanine due to its low redox potential, which results in base modification to 7,8-dihydro-8- oxoguanine (8-oxoG) [63]. The consequence of this can be G:C and T:A transverse mutations [64]. Martinet, et al. found high levels of cytoplasmic and nuclear immunoreactivity for 8-oxoG in atherosclerotic Vascular Smooth Muscle Cells (VSMC), endothelial cells and, importantly, in monocytes. This was not replicated in surrounding normal media or arteries [60,61]. In a recent landmark study from Shah, et al. the key BER enzyme OGG1 was found to have significantly reduced activity resulting in defective oxidative DNA repair in human VSMCs [65]. 8-oxoG repair in VSMCs from human carotid plaques was compared to age and sex matched controls (normal aortic tissue), plaque VSMC had reduced nuclear 8-oxoG activity, as shown by fluorescently labelled 8-oxoG containing molecular beacon assay. Following isolated in vitro OGG1 knockdown of exon 1 and 7 in rat VSMCs (confirmed by normal expression of other BER enzymes NEIL1 and NTH), oxidative damage was induced by tert-butyl hydroperoxide (t-BHP). It was found that t-BHP increased intracellular 8-oxoG significantly in OGG1 knockdown VSMCs compared to controls, thus confirming OGG1 is a major BER enzyme repairing 8-oxoG. OGG1-/-mice had more extensive atherosclerosis compared to controls following a lipid rich diet, as expected, but importantly, correction of OGG1 levels resulted in improved BER and a subsequent reduction in atherosclerotic burden. This indicates that oxidative DNA damage directly promotes atherogenesis [65].
Telomere attrition
Shortened telomeres have been shown to be present in atherosclerotic VSMC and contribute to the pathogenesis of ischaemic heart disease [66]. Telomere shortening and reduced telomere lengths have been observed in many previous studies in a variety of cell lines known to be instrumental in the pathogenesis of atherosclerosis. Reduced telomere length was found in atherosclerotic endothelial cells [67] and cultured sub-endothelial progenitor cells from patients with coronary artery disease [68]. Brouilette, et al. showed that circulating leukocytes also show reduced telomere length in patients with ischaemic heart disease and previous myocardial infarction compared with controls [69], a finding further replicated by Spyridopoulos, et al. [70,71]. Reduced telomere length is inversely correlated to cardiovascular disease risk in patients with subclinical atherosclerotic disease, and furthermore reduced telomere length is also found to be more prevalent in men [72]. This has important clinical consequences. In the landmark study published [73], Brouilette, et al. reported that statin treatment in those deemed at higher risk of adverse cardiovascular events, based on their telomere length, yielded a reduction in clinical events compared to controls. This finding was deemed to be independent of blood lipid concentrations or inflammation as there was no difference in LDL, HDL, triglycerides, CRP or fibrinogen levels between the groups. They suggested another potential protective mechanism of statins is via increased expression of the telomere capping protein Telomeric Repeat-binding Factor 2 (TRF2) [73,74].
Reactive oxygen species and mitochondrial DNA (mtDNA) damage
In addition to nuclear DNA damage, mtDNA damage can occur by similar deleterious mechanisms. The mitochondrial deletion mtDNA 4977 has been shown to result in mitochondrial dysfunction [75], with circulating leukocytes in patients who have severe coronary artery disease showing a greater degree of this deletion compared to controls [76]. However, a causal link was not established.
More concrete evidence from Ballinger, et al. found higher levels of mtDNA damage in aortas of patients undergoing surgery for severe atherosclerotic vascular disease compared to normal aorta from transplant donors at the time of organ harvesting, high mtDNA damage was also found in aortas from apolipoprotein E knockdown (ApoE-/-) mice suggesting a possible causal link [15]. Increased mitochondrial reactive species generation which is as a direct result of a deficiency in the protective mitochondrial superoxide dismutase (SOD2) antioxidant enzyme increases mtDNA damage and accelerates further atherosclerosis development, perpetuating the destructive cycle [15,77].
Yu, et al. again looked at ApoE-/- mice that were also deficient for mitochondrial polymerase-γ (polG) proof reading activity. They found ApoE-/-/polG-/- mice (possessing high levels of mtDNA damage and oxidative phosphorylation) had increased atherosclerosis and impaired proliferation and apoptosis of VSMCs [78]. Furthermore, ApoE-/-/polG-/- monocytes had increased apoptosis and inflammatory cytokine release, known to perpetuate atherosclerosis. In the human population of the study the authors found increased mtDNA damage in atherosclerotic plaques compared with normal vessels. Furthermore, leucocyte mtDNA damage was associated with higher risk, vulnerable plaques as characterised on virtual histology-IVUS during coronary angiography [78]. It is important to note that high risk coronary atherosclerotic plaques such as thin capped fibroatheroma have been shown to be associated with a higher frequency of major adverse cardiovascular events, as elegantly described in the PROSPECT trial [79]. This suggests a possible causative link between leucocyte mtDNA damage and major adverse cardiovascular events that warrants further investigation.
Human studies
The most recent key piece of research addressing DNA damage in atherosclerosis is the DECODE I study from Shah, et al. [80]. A total of 89 patients, who were scheduled to undergo PCI for stable angina or Non-ST Elevation Myocardial Infarction (NSTEMI), were prospectively enrolled. DNA ligase I, III and IV activity were measured in PBMCs as a marker of total DNA repair activity. The presence of healed coronary plaque rupture (which has been shown in previous autopsy and ex vivo studies to be related to sudden death in an Acute Coronary Syndrome (ACS) population [81,82] was assessed using intracoronary OCT imaging during angiography. The presence of healed coronary plaque was greater in individuals with higher level of DNA ligase activity and had a positive and direct correlation. The authors postulated that in advanced and diffuse atherosclerosis the DNA damage and repair signalling cascade may contribute to the development and progression of atherosclerotic plaque formation directly.
Consequences of DNA Damage
DNA repair
The assembly and disassembly of the DDR factors facilitates DNA repair by the pathways described earlier. Disassembly of DDR factors is key to allow return of normal cellular function and replication. Several pathways share a common end process, for example DNA-dependent protein kinase catalytic subunit (DNAPKcs) autophosphorylation mediates its dissociation from double strand breaks and E3 ubiquitin-protein ligase RNF8 ubiquitination allows the removal of Ku from the damaged site [83-85].
The choice of DNA repair pathway has been shown not only to be a consequence of the type of damage but also the stage of the cell cycle at which the lesion occurs. HR repair requires substantially resected DSB DNA; Cyclin-Dependent Kinases (CDKs) control the C-terminal Interacting Protein (CtIP) nucleolytic function in a cell cycle dependent manner, and CtIP is phosphorylated in S and G2 phase of the cell cycle. This increases its activity and interaction with breast cancer type 1 susceptibility protein (BRCA1) [86,87]. Contrary to this, DSB resection may be stalled by ATM-dependent phosphorylation of 53BP1, preventing BRCA1 migration to the DSB during G1 [88]. This suggests a ‘choice’ exists between the fast, more error prone NHEJ, acting during G1, and the more accurate HR, acting during S and G2 with 53BP1 and BRCA1 antagonising each other, influencing the repair undertaken [89,90].
Growth arrest, cell senescence and inflammation
The normal cell cycle is delayed by DNA damage checkpoints, thereby allowing adequate time for lesion repair. However, if the employed repair process is unsuccessful then irreversible growth arrest results. Cellular growth inhibition typically occurs during G1 and is controlled by the p16/pRB and p53/p21 pathways [91,92]. Senescence is typically recognised by the expression series of markers including Senescence-associated beta Galactosidase (SaβG) [93]. More recently, studies have shown that there are between 40 and 80 secretory intracellular signaling factors released as a consequence of senescence and are colloquially known as the Senescence-Associated Secretory Phenotype (SASP) [94]. The DDR is crucial for the regulation of SASP and controlling the rate of cellular senescence. It has been observed that a reduction in key regulatory factors (ATM, NBS1 and checkpoint kinase 2 (Chk2)) responding to DDR also reduces many SASP factors including interleukin (IL)-6 and IL-8 [95].
Many of the SAPS factors released during senescence can themselves promote and perpetuate the cycle of atherosclerosis through inflammation and recruitment of modulatory immune cells [96]. Further to this, the autocrine and paracrine function SAPS results in further recruitment of inflammatory matrix metalloproteinases (MMPs) from non-senescent neighbouring cells, such as MMP-1, MMP-3 and MMP-10 [97,98]. These matrix metalloproteinases are known to contribute to extracellular matrix degradation and further damage to the vascular wall [99].
Apoptosis
Cellular apoptosis may occur if DNA damage cannot be fully repaired [100]. Whilst attempting to repair damage, the DDR simultaneously activates pro-apoptotic and growth arrest pathways, with the degree of damage present dictating the dominant pathway [16]. As previously described in homologous recombination of DSBR, ATM signalling results in Replication Protein A (RPA) and RAD51 initiating strand invasion [44,45]. However, simultaneously ATM, and to a lesser extent ataxia telangiectasia and Rad3-related protein (ATR), trigger transcriptional activation of tumour protein p53 via checkpoint kinases 1 (Chk1) and 2.
P53 in turn upregulates pro-apoptotic genes (Bax, Puma, Bid [101]) resulting in the release of cytochrome c which, along with activating actor 1 (APF-1) and caspase 9, result in cell death via caspase 3 activation [102]. Apoptotic bodies are formed within the intimal space following cellular morphological changes such as nuclear fragmentation and chromatin condensation and these are subsequently phagocytosed by nearby cells [103,104].
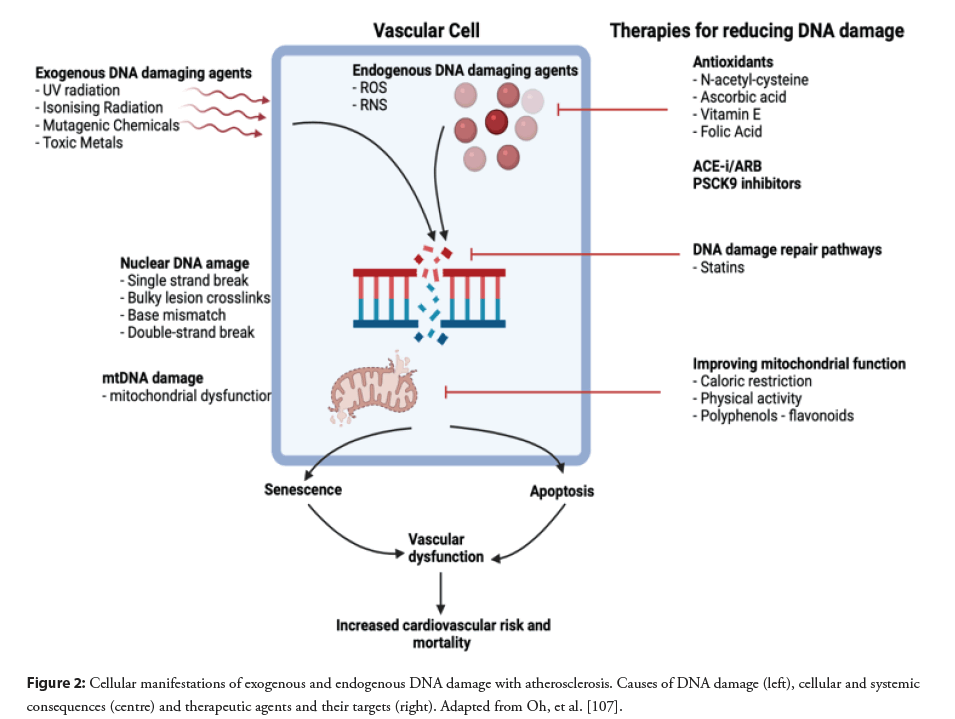
Clarke, et al. showed that chronic apoptosis of VSMCs accelerates atherogenesis and progression of atherosclerosis in established plaques [105]. The group had previously shown that smooth muscle protein 22α-human diphtheria toxin receptor (SM22α-hDTR) mice have VSMC specific apoptosis in both normal arteries and within plaque, conditionally induced by the transgenic expression of the hDTR from the minimal SM22α promoter [106] (Table 1). SM22α-hDTR/ApoE-/- mice atheroma was also noted to have increased high risk features characterised by a larger necrotic core, fibrous cap thinning as compared to ApoE-/- controls, which has also been noted in human studies [79] suggesting a possible causal link between the rate of VSMC apoptosis and atherosclerotic events which warrants further investigation (Figure 2).
Figure 2: Cellular manifestations of exogenous and endogenous DNA damage with atherosclerosis. Causes of DNA damage (left), cellular and systemic consequences (centre) and therapeutic agents and their targets (right). Adapted from Oh, et al. [107].
| Authors | Title | Journal | Study Population | Findings |
|---|---|---|---|---|
| Martinet, et al. [60] | Oxidative DNA damage and repair in experimental atherosclerosis are reversed by dietary lipid lowering | Circulation Research | Male New Zealand white rabbits fed cholesterol rich diet for 24 weeks | Induced atherosclerotic plaques had increased 8-oxoG levels seen on immunohistochemistry, this was highest within the most superficial layer of the plaques, correlating with numerous macrophage derived foam cells. DNA strand breaks were higher with upregulation of XRCC1, PARP-1, p53 and phospho-p53. These breaks normalised after 4 weeks of dietary lipid lowering. |
| Martinet, et al. [61] | Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. | Circulation | Human atherosclerotic plaques (carotid endarterectomy specimens) | Increased immune reactivity against 8-oxo-dG in carotid plaques compared to adjacent inner intima and normal mammary arteries, this was present in all cell types including macrophages, vascular smooth muscle cells and endothelial cells. This was associated with concordant upregulation of PARP-1 and p53. |
| Ballinger, et al. [15] |
Mitochondrial integrity and function in atherogenesis. | Circulation | ApoE-/- murine model and human aortic samples (obtained during surgery, normal aortic tissue harvested form transplant donors at organ harvesting) | mtDNA damage directly correlated with the extent of atherosclerosis in human arterial specimens and ApoE-/- mice. Furthermore, the mtDNA damage preceded atherosclerosis formation in ApoE-/- mice. ApoE-/- mice deficient in manganese superoxide dismutase showed early mtDNA damage and accelerated atherogenesis at arterial bifurcations. |
| Brouilette, et al. [69] | White cell telomere length and risk of premature myocardial infarction. | Arteriosclerosis, Thrombosis, and Vascular Biology | Human circulating leukocytes in patients with premature myocardial infarction (<50 years age) | Age and sex adjusted mean terminal restriction length of telomeres in circulating leukocytes of patients who suffered premature myocardial infarction was significantly shorter than controls. This finding was independent of any other coronary artery disease risk factor, suggesting biological age may play a role in the aetiology of CAD. |
| Spyridopoulos, et al. [70] | Telomere gap between granulocytes and lymphocytes is a determinant for hematopoietic progenitor cell impairment in patients with previous myocardial infarction | Arteriosclerosis, Thrombosis, and Vascular Biology | Human circulating leukocytes and mononuclear bone marrow cells in patients with coronary artery disease and previous myocardial infarction | Telomere erosion was noted at the bone marrow level with age and number of affected vessels as independent predictors (P=0.025 and P=0.029 respectively). This was associated with bone marrow functionality with reduced SDF and VEGF specific migration of mononuclear bone marrow cells and reduced mean telomere lengths in patients with CAD. Lymphocytes demonstrated significant telomere length shortening between mononuclear bone marrow cells and peripheral blood in patients with CAD. |
| Mahmoudi, et al. [71] | Statins use a novel Nijmegen breakage syndrome-1 dependent pathway to accelerate DNA repair in vascular smooth muscle cells | Circulation Research | C57B1-6 mice. Male New Zealand white rabbits fed cholesterol rich diet for 9 months. Human aortic VSMC. |
Human atherosclerotic VSMCs were noted to have increased levels of double strand DNA breaks and increased activity of ATM/H2AX mediated repair pathways in vivo and in vitro. Statin treatment did not reduce oxidative stress or DNA damage but accelerated DNA repair pathways via NBS-1 and Hdm2. Furthermore, statins were noted to reduce VSMC senescence and telomere attrition, accelerate DNA repair and reduce apoptosis in vivo following irradiation. |
| Yu, et al. [78] | Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans | Circulation | ApoE-/- and PolG-/-/ApoE-/- murine model and human circulating leukocytes who underwent VH IVUS. | mtDNA damage in the vascular wall and circulating monocytes is present in ApoE-/- mice prior to the formation of atherosclerotic plaque. PolG-/-/ApoE-/- mice had increased atherosclerosis with impaired proliferation and apoptosis of vascular smooth muscle cells. Human atherosclerotic plaque showed increased mtDNA damage compared to normal vessels and higher leukocyte mtDNA damage was associated with higher-risk plaques. |
| Shah, et al. [65] | Defective base excision repair of oxidative DNA damage in vascular smooth muscle cells promotes atherosclerosis | Circulation | OGG-/- and SM22α-SIRTex4/ex4 murine model and human atherosclerotic plaques (carotid endarterectomy samples and normal aorta from patients undergoing aortic valve replacement). | Human plaque VSMC have defective nuclear 8oxoG base excision repair and reduced acetylation of OGG1. p300 and SIRT-1 were identified as the OGG1 acetyl transferase and deacetylase regulators. Reduction in oxidative damage improved OGG1 activity and thus reduces plaque development, highlighting the detrimental effects of 8oxoG on VSMC function. |
| Shah, et al. [80] | DNA damage and repair in patients with coronary artery disease: correlation with plaque morphology using optical coherence tomography (DECODE study) | Cardiovascular Revascularization Medicine | Human circulating peripheral blood mononuclear cells in those with stable angina and NSTEMI | Genes involved in double strand break repair and nucleotide excision repair differed between patients with stable angina and NSTEMI compared to controls. GTSE1, DDB1, MLH3 and XRCC1 expression were higher in patients with stable angina, and this correlated with a high degree of fibrocalcific plaque seen on OCT. In NSTEMI patients, ATM and XPA expression was strongly corelated with fibrous plaques. |
Table 1: A summary of the most notable studies addressing the evidence for DNA damage in atherosclerosis.
Therapies to Reduce DNA Damage in Atherosclerosis
Antioxidant therapy
It is well established that an attenuated response to oxidative stress agents occurs with ageing [108-110]. Collins, et al. showed the mechanism through which older low density lipoprotein receptor null (LDLR-/-) mice have accelerated vascular injury is a consequence of impaired response to oxidative stress. LDLR- /- 12-month-old male mice (middle aged) were noted to express greater aortic levels of the key antioxidant genes, glutathione peroxidase-1 and -4, catalase, superoxide dismutase-2 and uncoupling protein-2, compared to young mice. Interestingly, once young mice were fed a high fat diet the aortic expression of these genes significantly increased, but this was not replicated in middle aged LDLR-/- mice. The inability to mount an antioxidant response was due to a reduction in the vascular expression of 2 key regulatory transcriptional pathways: DJ-1 and forkhead box, subgroup O family. The group found that treatment of the mice with the antioxidant apocynin reduced oxidative stress and attenuated atherosclerosis [111]. There are many other historical studies supporting these findings, in which cholesterol rich animal models have atherosclerosis regression in response to antioxidative agents [112]. Unfortunately, to date, randomised controlled trials assessing several major dietary antioxidants including ascorbic acid, vitamin E, folic acid, selenium have shown no clinical benefit in humans and, thus, antioxidant therapy does not feature in any established guideline for the treatment of epicardial atherosclerotic coronary artery disease [113-118].
However, antioxidant therapy has shown promise in the treatment of end organ damage as a result of microvascular dysfunction. Free radical scavengers such as ascorbic acid and N-acetylcysteine yielded benefit in patients with microvascular disease typically caused by diabetes mellitus [119]. Interestingly low level endogenous Nitric Oxide (NO) in inducible Nitric Oxide Synthase (iNOS) transfected cultured B6 mouse fibroblasts resulted in protection from DNA single-strand break formation and micronuclei induction by hydrogen peroxide (H2O2) [120]. Higher concentrations of NO (>1 mM) resulted in moderate to severe endonuclease sensitive oxidative base damage. However, as a double-edged sword, NO was also noted to selectively inhibit the repair of oxidative DNA base modifications [120,121]. Modulation of NO levels may provide a therapeutic target to reduce DNA damage.
It is important to note that the evidence to suggest that antioxidant therapy can reduce atherosclerosis in animal models is predominantly in a young adult population, whereas in human randomised clinical trials the population tend to be older age groups with more mature coronary artery disease [122].
Polyphenols
Polyphenols are naturally occurring organic compounds, abundant in plants and fruit, and have been found to have atheroprotective effects in humans [123,124]. Flavonoids are a commonly occurring plant polyphenol, and epidemiological and cohort studies have shown those with a higher dietary intake of flavonoids have a lower incidence of coronary artery disease, as well as a lower cardiovascular mortality [125-128]. To explore the biological consequences of the action of flavonoids further Weisel, et al. administered anthocyanin rich juice to healthy male adult humans in comparison with polyphenol depleted juice in the control arm. They observed a decrease in oxidative DNA damage, and an increase of glutathione levels and glutathione status in the anthocyanin juice group which returned to baseline in a wash out period [129]. However, there was no reduction in nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) binding activity in those consuming anthocyanin juice, as has been previously seen in diabetic patients consuming flavonoids [130]. NF-κB DNA binding activity is a key regulatory process in the expression of many genes related to inflammation [131].
Caloric reduction, dietary modification and exercise
A causal link between nutritional status, diet and the development of atherosclerosis has been clearly established [132]. Furthermore, dietary manipulation by Caloric Restriction (CR) has been shown to exert beneficial effects on endothelial function in vivo [133,134]. This occurs primarily by the reduction in inflammation and reduction of oxidative stress through the upregulation of antioxidant expression, thus resulting in improved endothelial physiology and preservation of mitochondrial function [135,136]. Sirtuins are an important class of Nicotinamide Adenine Dinucleotide (NAD+)-dependant deacetylases and adenosine diphosphate-ribosyltransferases [64] and have a similar structure to the Saccharomyces cerevisiae Sir2 protein [137]. In B6D2F1 mice models, CR increased sirtuin-1 (SIRT1), corresponding to an improvement of endothelial function [136]. Dietary supplementation with compounds mimicking the effects of CR, without sacrificing balanced nutrition, may also have therapeutic benefits.
SIRT1 inducers such as resveratrol, quercetin, berberine, curcumin and fisetin are important in the treatment of cardiovascular disease acting to improve DNA repair by higher oxidative stress responses though amplified mitochondrial function [138,139]. Resveratrol (a stilbenoid), is a naturally occurring phenol found in the skin of grapes, raspberries, and peanuts and can increase SIRT1 expression [140]. Resveratrol has been shown to increase aortic elasticity and reduce endothelial apoptosis in C57BL/6NIA mice and closely mimics dietary or caloric restriction [137]. Human studies have replicated this finding, so that both CR and resveratrol both increased plasma concentration of SIRT1, although any biological/clinical consequences of this pathway are yet to be observed in humans [141]. Conversely, Sirtuin inhibitors such as cambinol and Ex-527 may interfere with and corrupt CVD prevention therapeutics [142-144]. Typically, the diet of developed populations is much higher in Sirtuin inducers, with the diet of developing nations higher in Sirtuin inhibitors [145], this presents a public health challenge that if addressed may have an impact of the global burden of CVD [146].
Physical activity and aerobic exercise are potentially powerful contributors in the primary prevention of cardiovascular disease [147]. High intensity exercise has been proven to not only reduce oxidative stress through upregulation of key antioxidants such as superoxide dismutase, but also to be directly anti-atherogenic [148,149]. Recently, in a mtDNA mutator mouse model, forced endurance exercise mice were found to have high levels of full length mtDNA content and fewer mtDNA point mutations in comparison to sedentary controls. Furthermore, increased mitochondrial oxidative capacity and lower levels of DNA fragmentation were noted in several tissues including myocardial specimens, suggesting the presence of lower levels of caspase driven apoptosis in forced endurance exercise mice [150]. In elite athletes, intermittent hypoxic exposure, using a GO2 altitude hypoxiactor, in combination with physical activity, was found to increase NO and Heat Shock Protein 27 (HSP27), enhancing NO bioavailability and endothelial function [151].
Statins
The role of 3-Hydroxy-3-methylglutaryl coenzyme A (HMGCo- A) reductase inhibitors, known as statins, has been well established in the primary and secondary prevention of coronary artery disease [152,153]. Outside of their main mechanisms of actions (inhibiting cholesterol synthesis and increasing low density lipoprotein uptake), the pleotropic effects of statins are wide ranging and include: Maintenance of atherosclerotic plaque stability [154], modulation of inflammatory response [155], reduction of TNFα- induced apoptosis [156], and improvement of endothelial function by restoration of nitric oxide availability [157]. Statins have been shown to be instrumental in the prevention of DNA damage and stabilisation of cellular genetic material and reduction of oxidative stress through down regulation of NAD(P)H oxidase subunits and upregulation of catalase expression [158-160]. Statins have also been shown to provide increased telomere protection by an increase in the expression of the telomere capping protein TRF2 [74] in human Peripheral Blood Mononuclear Cells (PBMC). A cross-sectional study of individuals on statin therapy revealed increased telomerase activity and increased telomere length in the PBMCs of subjects compared to controls not taking statin therapy [161]. Lovastatin, whilst not commonly used in clinical practice in many countries, was found to protect primary human endothelial cells from ionising radiation-induced DNA damage by increased p53 expression and ATM/ATR-regulated activation of Chk1 [162]. A further protective mechanism of statins against DSB was identified by Mahmoudi et al., who reported atorvastatin accelerated DNA repair via Hdm2 phosphorylation, NBS1 stabilisation and faster ATM and H2A histone family member X (H2AX) phosphorylation, attenuating DNA damage, telomere length reduction and senescence, suggesting an atherosclerotic plaque stabilisation effect [62].
PCSK9 inhibitors
Pro-protein Convertase Subtilisin/Kexin type 9 (PCSK9) inhibitors have been shown to be effective at lowering of LDL in patients already taking statin therapy, and have shown promise in the secondary prevention of future events in those with previous acute coronary syndromes [163,164], and also the prevention of the development of complex coronary disease requiring high risk intervention [165]. Wu et al. found that knockdown of PCSK9 in human umbilical vein endothelial cells resulted in reduced oxidised Low-Density Lipoprotein (ox-LDL)-induced apoptosis, thus suggesting PCSK9 can impair endothelial cell survival [166]. The myriad of typical inflammatory stimuli found within atherosclerotic plaque, such as TNFα, oxLDL and lipopolysaccharides, stimulated PCSK9 via the NF-κB signalling pathway [167]. The same study found that lectin-like ox-LDL receptor-1 (LOX-1), one of the major scavenger receptors responsible for the removal of ox-LDL [168] stimulates PCSK9 and vice versa, and thereby suggested that their cross-talk is accentuated in the inflammatory state. This suggests that PCSK9 inhibitors may inhibit atherogenesis in hypercholesterolaemic states by altering LOX-1 expression [167]. Further research into the molecular consequences of PCSK9 inhibition and its effect on DNA integrity and stability is now needed.
ACE inhibitors
Angiotensin Converting Enzyme-Inhibitors (ACE-I) exert their cardiovascular effects by inhibiting the conversion of angiotensin I to angiotensin II within the renin-angiotensin-aldosterone system (RASS), thus reducing the degradation of bradykinin, a potent vasodilator. Angiotensin II has several deleterious effects, including the production of ROS from VSMCs resulting in DNA damage [169]. The ROS formed as result of angiotensin II cause Stress- Induced Premature Senescence (SIPS) and premature replicative senescence due to accelerated telomere attrition [170,171]. It is likely that both SIPS and replicative senescence occur simultaneously in vascular cells, and furthermore they also share common signalling and termination pathways involving stabilisation of p53, increased p21 expression and resultant hypophosphorylation of Rb protein mediating growth arrest [171,172]. Higher levels of bradykinin, as a result of ACE inhibition, reduces superoxide-induced endothelial cell senescence, subsequently reducing DNA damage in VSMCs via bradykininB2 receptor mediated nitric oxide release [173,174]. Previous studies have shown that ACEI and ARB are consistently associated with improvement in endothelial function. Both ACE-I and ARB cause a reduction in DNA damage inducing ROS and therefore they can directly modulate the pathogenesis of atherosclerosis [175,176].
Future Perspectives
The role of DNA damage in the development and progression of coronary artery atherosclerosis is increasingly well understood. There is a firm evidence base for the existence of DNA damage within both nuclear and mitochondrial DNA, and that the damage itself perpetuates the cycle of the development of atherosclerosis. The consequences of DNA damage range from successful repair via DSBR, BER, NER and mismatch repair or, by contrast, result in cellular senescence, apoptosis and inflammation which further drives the pathogenesis of atherosclerotic plaques. However, the exact mechanisms by which DNA damage drives this process are not fully understood.
The development of a novel biomarker (outside of the assessment of conventional risk factors for the development of atherosclerosis) that could highlight those at higher risk would facilitate the initiation of appropriate evidence-based lifestyle modification interventions and targeted primary prevention therapeutics proven to reduce the likelihood of adverse cardiovascular events and mortality in the future [5]. The DDR is a candidate pathway to yield such a biomarker.
Alteration of the DNA damage response pathway in mouse models has been shown to reverse or stall the process of atherosclerosis, importantly modulating high risk features such as a thin capped fibrous and fragmented cap [177].
Conclusion
Currently, there is limited human data exploring DNA damage and gene expression in atherosclerosis. The prevention of DNA damage, and the augmentation of the DDR, are important targets for future therapeutics. Statins have proven benefit in reducing DNA damage and newer treatments, such as PCSK9 inhibitors, may have similar beneficial effects. Successful modulation of the DDR may help reduce the morbidity and mortality, together with the global economic burden associated with atherosclerotic coronary artery disease. The potential clinical and financial benefits of appropriate biomarkers and then therapeutic tools that could identify the high risk and modify their subsequent trajectory are enormous.
References
- Moran AE, Forouzanfar MH, Roth GA, et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: The global burden of disease 2010 study. Circulation. 129(14): 1483-92 (2014).
- Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet. 396(10258): 1204-22 (2020).
- De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 367(11): 991-1001 (2012).
- Ali ZA, Maehara A, Généreux P, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): A randomised controlled trial. Lancet. 388(10060): 2618-28 (2016).
- Boden WE, O’Rourke RA, Koon KT, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 356(15): 1503-16 (2007).
- Demirbag R, Yilmaz R, Kocyigit A. Relationship between DNA damage, total antioxidant capacity and coronary artery disease. Mutat Res. 570(2): 197-203 (2005).
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 365(22): 2078-87 (2011).
- Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 358(15): 1547-59 (2008).
- Helena JM, Joubert AM, Grobbelaar S, et al. Deoxyribonucleic acid damage and repair: Capitalizing on our understanding of the mechanisms of maintaining genomic integrity for therapeutic purposes. Int J Mol Sci. 19(4): 1148 (2018).
- Kawanishi S, Hiraku Y, Pinlaor S, et al. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 387(4): 365-72 (2006).
- Vagbo CB, Slupphaug G. RNA in DNA repair. DNA Repair (Amst). 95: 102927 (2020).
- Zhao RZ, Jiang S, Zhang L, et al. Mitochondrial electron transport chain, ROS generation and uncoupling. Int J Mol Med. 44(1): 3-15 (2019).
- Irani K. Oxidant signaling in vascular cell growth, death, and survival: A review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 87(3): 179-83 (2000).
- Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 85(8): 753-66 (1999).
- Ballinger SW, Patterson C, Knight-Lozano CA, et al. Mitochondrial integrity and function in atherogenesis. Circulation. 106(5): 544-9 (2002).
- Uryga A, Gray K, Bennett M. DNA damage and repair in vascular disease. Annu Rev Physiol. 78: 45-66 (2016).
- Jomova K, Jenisova Z, Feszterova M, et al. Arsenic: Toxicity, oxidative stress and human disease. J Appl Toxicol. 31(2): 95-107 (2011).
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 461(7267): 1071-8 (2009).
- Beard WA, Horton JK, Prasad R, et al. Eukaryotic base excision repair: New approaches shine light on mechanism. Annu Rev Biochem. 88: 137-62 (2019).
- Rzeszutek I, Betlej G. The Role of small noncoding RNA in DNA double-strand break repair. Int J Mol Sci. 21(21): 8039 (2020).
- Durik M, Kavousi M, van der Pluijm I, et al. Nucleotide excision DNA repair is associated with age-related vascular dysfunction. Circulation. 126(4): 468-78 (2012).
- Kong M, Beckwitt EC, Van Houten B. Dynamic action of DNA repair proteins as revealed by single molecule techniques: Seeing is believing. DNA Repair (Amst). 93:102909 (2020).
- Ijsselsteijn R, Jansen JG, de Wind N. DNA mismatch repair-dependent DNA damage responses and cancer. DNA Repair (Amst). 93: 102923 (2020).
- Tobias F, Lob D, Lengert N, et al. Spatiotemporal dynamics of early DNA damage response proteins on complex DNA lesions. PLoS One. 8(2): e57953 (2013).
- Dluzen DF, Kim Y, Bastian P, et al. MicroRNAs modulate oxidative stress in hypertension through PARP-1 regulation. Oxid Med Cell Longev. 2017: 3984280 (2017).
- Ting NS, Lee WH. The DNA double-strand break response pathway: Becoming more BRCAish than ever. DNA Repair (Amst). 3(8-9): 935-44 (2004).
- Mahmoudi M, Mercer J, Bennett M. DNA damage and repair in atherosclerosis. Cardiovasc Res. 71(2): 259-68 (2006).
- Chatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen. 58(5): 235-63 (2017).
- Odell ID, Wallace SS, Pederson DS. Rules of engagement for base excision repair in chromatin. J Cell Physiol. 228(2): 258-66 (2013).
- Jacobs AL, Schar P. DNA glycosylases: In DNA repair and beyond. Chromosoma. 121(1): 1-20 (2012).
- Fleming AM, Ding Y, Burrows CJ. Oxidative DNA damage is epigenetic by regulating gene transcription via base excision repair. Proc Natl Acad Sci U S A. 114(10): 2604-9 (2017).
- Almeida KH, Sobol RW. A unified view of base excision repair: Lesion-dependent protein complexes regulated by post-translational modification. DNA Repair (Amst). 6(6): 695-711 (2007).
- Dianov GL, Hubscher U. Mammalian base excision repair: The forgotten archangel. Nucleic Acids Res. 41(6): 3483-90 (2013).
- Frosina G, Fortini P, Rossi O, et al. Two pathways for base excision repair in mammalian cells. J Biol Chem. 271(16): 9573-8 (1996).
- Ferguson DO, Alt FW. DNA double strand break repair and chromosomal translocation: Lessons from animal models. Oncogene. 20(40): 5572-9 (2001).
- Panier S, Boulton SJ. Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol. 15(1): 7-18 (2014).
- Mari PO, Florea BI, Persengiev SP, et al. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc Natl Acad Sci U S A. 103(49): 18597-602 (2006).
- Pang D, Yoo S, Dynan WS, et al. Ku proteins join DNA fragments as shown by atomic force microscopy. Cancer Res. 57(8): 1412-5 (1997).
- Hammel M, Rey M, Yu Y, et al. XRCC4 protein interactions with XRCC4-Like Factor (XLF) create an extended grooved scaffold for DNA ligation and double strand break repair. J Biol Chem. 286(37): 32638-50 (2011).
- Roberts SA, Strande N, Burkhalter MD, et al. Ku is a 5'-dRP/AP lyase that excises nucleotide damage near broken ends. Nature. 464(7292): 1214-7 (2010).
- Grawunder U, Wilm M, Wu X, et al. Activity of DNA ligase IV stimulated by complex formation with XRCC4 protein in mammalian cells. Nature. 388(6641): 492-5 (1997).
- Li X, Heyer WD. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 18(1): 99-113 (2008).
- Sun Y, Jiang X, Chen S, et al. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci U S A. 102(37): 13182-7 (2005).
- Sebesta M, Burkovics P, Juhasz S, et al. Role of PCNA and TLS polymerases in D-loop extension during homologous recombination in humans. DNA Repair (Amst). 12(9): 691-8 (2013).
- Sung P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science. 265(5176): 1241-3 (1994).
- de Latt WL, Jaspers NGJ, Hoeijmakers JH. Molecular mechanism of nucleotide excision repair. Genes Dev. 13(7): 768-85 (1999).
- Marteijn JA, Lans H, Vermeulen W, et al. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol. 15(7): 465-81 (2014).
- Fousteri M, Vermeulen W, van Zeeland AA, et al. Cockayne syndrome A and B proteins differentially regulate recruitment of chromatin remodeling and repair factors to stalled RNA polymerase II in vivo. Mol Cell. 23(4): 471-82 (2006).
- Nishi R, Okuda Y, Watanabe E, et al. Centrin 2 stimulates nucleotide excision repair by interacting with xeroderma pigmentosum group C protein. Mol Cell Biol. 25(13): 5664-74 (2005).
- Ogi T, Limsirichaikul S, Overmeer RM, et al. Three DNA polymerases, recruited by different mechanisms, carry out NER repair synthesis in human cells. Mol Cell. 37(5): 714-27 (2010).
- Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 7(5): 335-46 (2006).
- Chatterjee N, Lin Y, Wilson JH. Mismatch repair enhances convergent transcription-induced cell death at trinucleotide repeats by activating ATR. DNA Repair (Amst). 42: 26-32 (2016).
- Li F, Mao G, Tong D, et al. The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSalpha. Cell. 153(3): 590-600 (2013).
- Sachadyn P. Conservation and diversity of MutS proteins. Mutat Res. 694(1-2): 20-30 (2010).
- Kadyrov FA, Dzantiev L, Constantin N, et al. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 126(2): 297-308 (2006).
- Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 481(7381): 287-94 (2012).
- Biorender. DNA repair mechanisms. (2021).
- Andreassi MG, Botto N. DNA damage as a new emerging risk factor in atherosclerosis. Trends Cardiovasc Med. 13(7): 270-5 (2003).
- Botto N, Rizza A, Colombo AM, et al. Evidence for DNA damage in patients with coronary artery disease. Mutat Res. 493(1-2): 23-30 (001).
- Martinet W, Knaapen MW, De Meyer GR, et al. Oxidative DNA damage and repair in experimental atherosclerosis are reversed by dietary lipid lowering. Circ Res. 88(7): 733-9 (2001).
- Martinet W, Knaapen MW, De Meyer GR, et al. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 106(8): 927-32 (2002).
- Mahmoudi M, Gorenne I, Mercer J, et al. Statins use a novel Nijmegen breakage syndrome-1-dependent pathway to accelerate DNA repair in vascular smooth muscle cells. Circ Res. 103(7): 717-25 (2008).
- Neeley WL, Essigmann JM. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem Res Toxicol. 19(4): 491-505 (2006).
- Shah AV, Bennett MR. DNA damage-dependent mechanisms of ageing and disease in the macro- and microvasculature. Eur J Pharmacol. 816: 116-28 (2017).
- Shah A, Gray K, Figg N, et al. Defective base excision repair of oxidative DNA damage in vascular smooth muscle cells promotes atherosclerosis. Circulation. 138(14): 1446-62 (2018).
- Matthews C, Gorenne I, Scott S, et al. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. Circ Res. 99(2): 156-64 (2006).
- Minamino T, Miyauchi H, Yoshida T, et al. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation. 105(13): 1541-4 (2002).
- Satoh M, Ishikawa Y, Takahashi Y, et al. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 198(2): 347-53 (2008).
- Brouilette S, Singh RK, Thompson JR, et al. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 23(5): 842-6 (2003).
- Spyridopoulos I, Erben Y, Brummendorf TH, et al. Telomere gap between granulocytes and lymphocytes is a determinant for hematopoetic progenitor cell impairment in patients with previous myocardial infarction. Arterioscler Thromb Vasc Biol. 28(5): 968-74 (2008).
- Spyridopoulos I, Hoffmann J, Aicher A, et al. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: Role of cytomegalovirus seropositivity. Circulation. 120(14): 1364-72 (2009).
- Panayiotou AG, Nicolaides AN, Griffin M, et al. Leukocyte telomere length is associated with measures of subclinical atherosclerosis. Atherosclerosis. 211(1): 176-81 (2010).
- Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: A nested case-control study. Lancet. 369(9556): 107-14 (2007).
- Spyridopoulos I, Haendeler J, Urbich C, et al. Statins enhance migratory capacity by upregulation of the telomere repeat-binding factor TRF2 in endothelial progenitor cells. Circulation. 110(19): 3136-42 (2004).
- James AM, Murphy MP. How mitochondrial damage affects cell function. J Biomed Sci. 9(6 Pt 1): 475-87 (2002).
- Botto N, Berti S, Manfredi S, et al. Detection of mtDNA with 4977 bp deletion in blood cells and atherosclerotic lesions of patients with coronary artery disease. Mutat Res. 570(1): 81-8 (2005).
- Topper JN, Cia J, Falb D, et al. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: Cyclooxygenase-2, manganese superoxide dismutase, and endothelial call nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 93(19): 10417-22 (1996).
- Yu E, Calvert PA, Mercer JR, et al. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation. 128(7): 702-12 (2013).
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 364(3): 226-35 (2011).
- Shah N, Meira LB, Elliott RM, et al. DNA damage and repair in patients with coronary artery disease: Correlation with plaque morphology using optical coherence tomography (DECODE Study). Cardiovasc Revasc Med. 20(9): 812-8 (2019).
- Burke AP, Kolodgie FD, Farb A, et al. Healed plaque ruptures and sudden coronary death evidence that subclinical rupture has a role in plaque progression. Circulation. 103(7): 934-40 (2001).
- Otsuka F, Joner M, Prati F, et al. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol. 11(7): 379-89 (2014).
- Feng L, Chen J. The E3 ligase RNF8 regulates KU80 removal and NHEJ repair. Nat Struct Mol Biol. 19(2): 201-6 (2012).
- Chen F, Peterson SR, Story MD, et al. Disruption of DNA-PK in Ku80 mutant xrs-6 and the implications in DNA double-strand break repair. Mutat Res. 362(1): 9-19 (1996).
- Merkle D, Douglas P, Moorhead GB, et al. The DNA-dependent protein kinase interacts with DNA to form a protein-DNA complex that is disrupted by phosphorylation. Biochemistry. 41(42): 12706-14 (2002).
- You Z, Bailis JM. DNA damage and decisions: CtIP coordinates DNA repair and cell cycle checkpoints. Trends Cell Biol. 20(7): 402-9 (2010).
- Yu X, Chen J. DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains. Mol Cell Biol. 24(21): 9478-86 (2004).
- Zimmermann M, Lottersberger F, Buonomo SB, et al. 53BP1 regulates DSB repair using Rif1 to control 5’ end resection. Science. 339(6120): 700-4 (2013).
- Bartova E, Legartova S, Dundr M, et al. A role of the 53BP1 protein in genome protection: Structural and functional characteristics of 53BP1-dependent DNA repair. Aging. 11(8): 2488-2511 (2019).
- Fouquin A, Guirouilh-Barbat J, Lopez B, et al. PARP2 controls double-strand break repair pathway choice by limiting 53BP1 accumulation at DNA damage sites and promoting end-resection. Nucleic Acids Res. 45(21): 12325-39 (2017).
- Campisi J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 120(4): 513-22 (2005).
- Di Leonardo A, Linke SP, Clarkin K, et al. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 8(21): 2540-51 (1994).
- Dimiri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vitro. Proc Natl Acad Sci U S A. 92(20): 9363-7 (1995).
- Coppe JP, Desprez PY, Krtolica A, et al. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu Rev Pathol. 5: 99-118 (2010).
- Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 75: 685-705 (2013).
- Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 10(5): 274-83 (2013).
- Acosta JC, Banito A, Wuestefeld T, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 15(8): 978-90 (2013).
- Acosta JC, O'Loghlen A, Banito A, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 133(6): 1006-18 (2008).
- Katsuda S, Kaji T. Atherosclerosis and extracellular matrix. J Atheroscler Thromb. 10(5): 267-74 (2003).
- Gray K, Bennett M. Role of DNA damage in atherosclerosis-bystander or participant? Biochem Pharmacol. 82(7): 693-700 (2011).
- Olsson A, Manzl C, Strasser A, et al. How important are post-translational modifications in p53 for selectivity in target-gene transcription and tumour suppression? Cell Death Differ. 14(9): 1561-75 (2007).
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 5(4): a008656 (2013).
- Bennett M. Apoptosis in the cardiovascular system. Heart. 87(5): 480-7 (2002).
- Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 45(3): 528-37 (2000).
- Clarke MC, Littlewood TD, Figg N, et al. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res. 102(12): 1529-38 (2008).
- Clarke M, Figg N, Davenport AP, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 12(9): 1075-80 (2006).
- Oh J, Lee YD, Wagers AJ. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat Med. 20(8): 870-80 (2014).
- Najjar SS, Scuteri A, Lakatta EG. Arterial aging: Is it an immutable cardiovascular risk factor? Hypertension. 46(3): 454-62 (2005).
- Bidault G, Garcia M, Capeau J, et al. Progerin expression induces inflammation, oxidative stress and senescence in human coronary endothelial cells. Cells. 9(5): 1201 (2020).
- Csiszar A, Labinskyy N, Zhao X, et al. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 6(6): 783-97 (2007).
- Collins AR, Lyon CJ, Xia X, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 104(6): e42-54 (2009).
- Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 105(17): 2107-11 (2002).
- Bleys J, Miller ER, Pastor-Barriuso R, et al. Vitamin-mineral supplementation and the progression of atherosclerosis: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 84(4): 880-7 (2006).
- Dietrich M, Jacques PF, Pencina MJ, et al. Vitamin E supplement use and the incidence of cardiovascular disease and all-cause mortality in the Framingham heart study: Does the underlying health status play a role? Atherosclerosis. 205(2): 549-53 (2009).
- Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 106(4): 517-27 (2010).
- Katsiki N, Manes C. Is there a role for supplemented antioxidants in the prevention of atherosclerosis? Clin Nutr. 28(1): 3-9 (2008).
- Ravn-Haren G, Bugel S, Krath BN, et al. A short-term intervention trial with selenate, selenium-enriched yeast and selenium-enriched milk: Effects on oxidative defence regulation. Br J Nutr. 99(4): 883-92 (2008).
- Yusuf S, Dagenais G, Pogue J, et al. Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med. 342(3): 154-160 (2000).
- Radomaska-Lesniewska DM, Sadowska AM, Van Overveld FJ, et al. Influence of N-acetylcysteine on ICAM-1 expression and IL-8 release from endothelial and epithelial cells. J Physiol Pharmacol. 57: 325-34 (2006).
- Phoa N, Epe B. Influence of nitric oxide on the generation and repair of oxidative DNA damage in mammalian cells. Carcinogenesis. 23(3): 469-75 (2002).
- Jaiswal M, LaRusso NF, Shapiro RA, et al. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology. 120(1): 190-9 (2001).
- Ozkanlar S, Akcay F. Antioxidant vitamins in atherosclerosis-animal experiments and clinical studies. Adv Clin Exp Med. 21(1): 115-23 (2012).
- Joshipura KJ, Hu F, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Int Med. 134(12): 1104-14 (2001).
- Bazzano LA, He J, Ogden LG, et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: The first national health and nutrition examination survey epidemiologic follow-up study. Am J Clin Nutr. 76(1): 93-9 (2002).
- Hertog MGL, Feskens EJM, Hollman PCH, et al. Dietary antioxidant flaonoids and risk of coronary heart disease: The zutphen elderly study. Lancet. 342(8878): 1007-11 (1993).
- Hertog MGL, Kromhout D, Aravanis C, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 155(4): 381-6 (1995).
- Knekt P, Jarvinen R, Reunanen A, et al. Flavonoid intake and coronary mortaility in Finland: A cohort study. BMJ. 312(7029): 478-81 (1996).
- Yochum L, Kushi LH, Meyer K, et al. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. 149(10): 943-9 (1999).
- Weisel T, Baum M, Eisenbrand G, et al. An anthocyanin/polyphenolic-rich fruit juice reduces oxidative DNA damage and increases glutathione level in healthy probands. Biotechnol J. 1(4): 388-97 (2006).
- Tsai S, Liang Y, Lin-Shiau S, et al. Suppression of TNFa-Mediated NFkB activity by myricetin and other flavonoids through downregulating the activity of IKK in ECV304 cells. J Cell Biochem. 74(4): 606-15 (1999).
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 107(2): 135-42 (2001).
- Fontana L, Vinciguerra M, Longo VD. Growth factors, nutrient signaling, and cardiovascular aging. Circ Res. 110(8): 1139-50 (2012).
- Guo Z, Yang H, Hamilton ML, et al. Effects of age and food restriction on oxidative DNA damage and antioxidant enzyme activities in the mouse aorta. Mech Ageing Dev. 122(15): 1771-86 (2001).
- Zanetti M, Cappellari GG, Burekovic I, et al. Caloric restriction improves endothelial dysfunction during vascular aging: Effects on nitric oxide synthase isoforms and oxidative stress in rat aorta. Exp Gerontol. 45(11): 848-55 (2010).
- Ungvari Z, Parrado-Fernandez C, Csiszar A, et al. Mechanisms underlying caloric restriction and lifespan regulation: Implications for vascular aging. Circ Res. 102(5): 519-28 (2008).
- Rippe C, Lesniewski L, Connell M, et al. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 9(3): 304-12 (2010).
- Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 8(2): 157-68 (2008).
- Iside C, Scafuro M, Nebbioso A, et al. SIRT1 activation by natural phytochemicals: An overview. Front Pharmacol. 11: 1225 (2020).
- Dai H, Sinclair DA, Ellis JL, et al. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol Ther. 188: 140-54 (2018).
- Zemel MB. Modulation of energy sensing by leucine synergy with natural sirtuin activators: Effects on health span. J Med Food. 23(11): 1129-35 (2020).
- Mansur AP, Roggerio A, Goes MFS, et al. Serum concentrations and gene expression of sirtuin 1 in healthy and slightly overweight subjects after caloric restriction or resveratrol supplementation: A randomized trial. Int J Cardiol. 227: 788-94 (2017).
- Gertz M, Steegborn C. Using mitochondrial sirtuins as drug targets: Disease implications and available compounds. Cell Mol Life Sci. 73(15): 2871-96 (2016).
- Grozinger CM, Chao ED, Blackwell HE, et al. Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem. 276(42): 38837-43 (2001).
- Napper AD, Hixon J, McDonagh T, et al. Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J Med Chem. 48(25): 8045-54 (2005).
- Martins IJ. Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. J Clin Epig. 3(3): 24 (2017).
- Martins IJ. Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Adv Aging Res. 5(1): 9-26 (2016).
- Barry VW, Baruth M, Beets MW, et al. Fitness vs. fatness on all-cause mortality: A meta-analysis. Prog Cardiovasc Dis. 56(4): 382-90 (2014).
- Miyazaki H, Oh-ishi S, Ookawara T, et al. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol. 84(1-2): 1-6 (2001).
- Fukai T, Siegfried MR, Ushio-Fukai M, et al. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 105(11): 1631-9 (2000).
- Safdar A, Bourgeois JM, Ogborne DI, et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 108(10): 4135-40 (2011).
- Zembron-Lacny A, Tylutka A, Wacka E, et al. Intermittent hypoxic exposure reduces endothelial dysfunction. Biomed Res Int. 2020: 6479630 (2020).
- Pedersen TR, Kjekshus J, Berg K, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavia n Simvastatin Survival Study (4S). Lancet. 344(8934): 1383-9 (1994).
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive vs. moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 350: 1495-504 (2004).
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis. JAMA. 295(13): 1556-65 (2006).
- Ridker PM, Danielson E, Fonesca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-Reactive Protein (CRP). N Engl J Med. 359(21): 2195-207 (2008).
- Du G, Song Y, Zhang T, et al. Simvastatin attenuates TNFalphainduced apoptosis in endothelial progenitor cells via the upregulation of SIRT1. Int J Mol Med. 34(1): 177-82 (2014).
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 23(2): 168-75 (2003).
- Wassmann S, Laufs U, Muller K, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 22(2): 300-5 (2002).
- Girona J, La Ville AE, Sola R, et al. Simvastatin decreases aldehyde production derived from lipoprotein oxidation. Am J Cardiol. 83(6): 846-51 (1999).
- Kwok JM, Ma CC, Ma S. Recent development in the effects of statins on cardiovascular disease through Rac1 and NADPH oxidase. Vascul Pharmacol. 58(1-2): 21-30 (2013).
- Boccardi V, Barbieri M, Rizzo MR, et al. A new pleiotropic effect of statins in elderly: Modulation of telomerase activity. FASEB J. 27(9): 3879-85 (2013).
- Nubel T, Damrot J, Roos WP, et al. Lovastatin protects human endothelial cells from killing by ionizing radiation without impairing induction and repair of DNA double-strand breaks. Clin Cancer Res. 12(3 Pt 1): 933-9 (2006).
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 379(22): 2097-107 (2018).
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 376(18): 1713-22 (2017).
- Oyama K, Furtado RHM, Fagundes A, et al. Effect of evolocumab on complex coronary disease requiring revascularization. J Am Coll Cardiol. 77(3): 259-67 (2021).
- Wu CY, Tang ZH, Jiang L, et al. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol Cell Biochem. 359(1-2): 347-58 (2012).
- Ding Z, Liu S, Wang X, et al. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc Res. 107(4): 556-67 (2015).
- Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 386: 73-7 (1997).
- Herbert KE, Mistry Y, Hastings R, et al. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 102(2): 201-8 (2008).
- Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of Stress-Induced Premature Senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 35(8): 927-45 (2000).
- Minamino T, Yoshida T, Tateno K, et al. Ras induces vascular smooth muscle cell senescence and inflammation in human atherosclerosis. Circulation. 108(18): 2264-9 (2003).
- Gorenne I, Kavurma M, Scott S, et al. Vascular smooth muscle cell senescence in atherosclerosis. Cardiovasc Res. 72(1): 9-17 (2006).
- Shah NR, Mahmoudi M. The role of DNA damage and repair in atherosclerosis: A review. J Mol Cell Cardiol. 86: 147-57 (2015).
- Oeseburg H, Iusuf D, van der Harst P, et al. Bradykinin protects against oxidative stress-induced endothelial cell senescence. Hypertension. 53(2): 417-22 (2009).
- Khaper N, Singal PK. Modulation of oxidative stress by a selective inhibition of angiotensin II type 1 receptors in MI rats. J Am Coll Cardiol. 37(5): 1461-6 (2001).
- Fiordaliso F, Cuccovillo I, Bianchi R, et al. Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes. Life Sci. 79(2): 121-9 (2006).