Research Article - International Journal of Clinical Rheumatology (2022) Volume 17, Issue 1
Depression in children with systemic lupus Erythematosus in correlation with disease activity
- *Corresponding Author:
- Heba Taher Osman
Department of Pediatrics, Faculty of Medicine, Cairo University, Egypt
E-mail: drhebataherosman@gmail.com
Received: 01-Jan-2022, Manuscript No. fmijcr-22-53203; Editor assigned: 07-Jan-2022, PreQC No. fmijcr-22-53203(PQ); Reviewed: 21-Jan-2022, QC No. fmijcr-22-53203; Revised: 24-Jan-2022, Manuscript No. fmijcr-22-53203(R); Published: 30-Jan-2022, DOI: 10.37532/1758-4272.2022.17(1).016-025
Abstract
Introduction
Systemic lupus erythematosus (SLE) is a chronic, multisystem, autoimmune disease characterized by periods of increased disease activity caused by inflammation of blood vessels and connective tissue. Pediatric patients with SLE have a more severe clinical course in comparison with their adult counterparts. Compared with adults, children with SLE have more widespread organ involvement [1]. Approximately 20% of all patients who have SLE are diagnosed in childhood. The onset of SLE is rare in those younger than 5 years of age; most pediatric patients are diagnosed in adolescence [2].
SLE has a more severe clinical course than that seen in adults, with a higher prevalence of lupus nephritis, hematologic anomalies, photosensitivity, neuropsychiatric, and mucocutaneous involvement [3]. Because SLE can present with a number of signs and symptoms, the diagnosis often is considered in children who have prolonged unexplained complaints [2]. Glucocorticoids are the mainstay of pharmacological treatment in patients with pSLE with or without major organ involvement. Glucocorticoids are given mainly as oral prednisone, prednisolone, or intravenous high-dose methylprednisolone. Daily doses of glucocorticoids can range from 0.5 to 2 mg/kg/day. The initial dose is decided by the extent of disease severity and organ involvement [4].
The relationship between chronic physical disease and mental illness is bidirectional. Understanding the relationship between mental and physical health is of utmost importance in pediatric populations, in which both poor physical and mental health outcomes can affect development and lead to long-lasting consequences [5]. The prevalence of depressive symptoms is higher in children with SLE (20%) when compared to their healthy peers (8%) and the general adolescent population (13%). Depression may be the first presentation of juvenile SLE. They may present with sadness, decreased interest in activities, poor communication, and absences from school, fatigue and irritable moods [6]. However, very young children tend not to look depressed but they may present rather with insomnia, weight loss, and increased or new onset of `anxiety symptoms. Anhedonia and social withdrawal are also significant symptoms and are considered a sign of severe illness in a younger child [7]. Depression can have significant lasting effects when diagnosed in childhood and adolescence, and has been associated with later interpersonal difficulties, early parenthood, impaired school performance, unemployment, and other mental disorders and substance use disorders [8] as well as high risk of suicide [9].
The aim of this current study was to evaluate the prevalence and severity of depression among pediatric patients with SLE and its relation to disease activity. The correlation between the use of corticosteroids and disease activity was also studied. It was hypothesized that patients with more disease activity would be more depressed.
Methods
This study was a cross-sectional, analytical clinical study conducted on 30 SLE patients in activity and another 30 SLE patients not in activity all within an age range of 8 to 12 years (inclusive of 49 females and 11 males). They were recruited from the outpatient clinic of the collagen vascular unit in the Specialized Children Hospital, Cairo University during their regular follow up visit over a 6-month period from April 2019 to October 2020. The required sample size has been calculated using the Sample Size Calculator for Prevalence Studies (SSCPS) version 1.0.01, which is based on the formula described by Daniel [10] that is as follows: n= Z*P 1-Pd2Where n = sample size, Z = Z statistic for the desired level of confidence, P =expected prevalence, and d = level of precision.
The study design and methodology were approved by the scientific research committee of the Department of Pediatrics, Faculty of Medicine, Cairo University and also by the Local Ethics Committee of Scientific Research, Faculty of Medicine, Cairo University. Before the enrollment in this study, an informed assent from each participant was taken and informed consents from their caregivers were obtained.
Clinical history was obtained from the patients that included demographic data as well as Clinical manifestations of SLE including arthritis, malar rash, nephritis, central nervous system (CNS) disease, fever, fatigue, weight loss, hair loss, stomach pain, headaches, easy bruising and painful joints. Also, history of prescribed medications including doses like corticosteroids, hydroxychloroquine, immunosuppressants and pain killers was recorded. Systemic lupus erythematosus disease activity index (SLEDAI) was used to assess disease activity [11]. It is based on 24 questions assessing the clinical manifestations of SLE which measures manifestations over the past 10 days, including physical findings and laboratory values weighted across organ systems. The final score ranges between 0 and 105. The higher the score, the more significant the degree of disease activity. Scores of 6 and above are considered to be consistent with active disease requiring therapy. General examination of all participants was performed, and their weight, height, BMI and blood pressure were recorded. A detailed chest, cardiac and abdominal examination was also performed. Laboratory tests in the form of CBC, ESR, C3, C4, ANA test, double stranded DNA, Anti double stranded DNA , Anti –smith antibody , CRP, Antiphospholipid antibodies and Circulating lupus anticoagulant were done to all participants.
Depressive symptoms and their severity were assessed using the Children’s Depression Inventory (CDI) (Arabic version) (2nd edition) [12] which is a selfrated, symptom-oriented scale consisting of 27 groups of statements suitable for assessing depression in youths aged 7 to 17 years old. The statements are related to depressive symptoms including sadness, pessimism, self-depreciation, anhedonia, misbehavior, academic decrement, worrying, self-hate and blame, interpersonal problems, irritability, crying spells and suicidal ideation. On administrating Children’s Depression Inventory (CDI), the participants were asked to choose one statement out of the three statements in each group that best described him or her in the past two weeks. It took around 10 minutes to complete.
Statistical analysis
Data were subjected to computer assisted statistical analysis using statistical package for social science â SPSSâ VERSION 18. Nominal data were expressed as frequency and percentage and were compared using Chi-square test. Numerical data were expressed as mean +/- standard deviation and were compared using T test. Nonparametric data were expressed as median âinter quartile range âand were compared using Mann Whitney u test. Associations between numerical variables were studied using Pearson’s correlation. p values less than 0.05 were considered significant. Charts and graphs were prepared using Excel or SPSS programs.
Results
Among the 60 patients included in this study 49 patients (81.7%) were females while 11 patients (18.3%) were males (Tables 1-3).
| Demographic data of the studied patients | Total no. = 60 | |
|---|---|---|
| Age (years) | Mean ± SD | 10.98 ± 1.30 |
| Range | 8-12 | |
| Sex | Female | 49 (81.7%) |
| Male | 11 (18.3%) | |
| Residence | Rural | 34 (56.7%) |
| Urban | 26(43.3%) | |
Table 1. The demographic data of the studied patients.
| Clinical manifestations at the onset of the disease | ||
|---|---|---|
| Rash | No | 29 (48.3%) |
| Yes | 31 (51.7%) | |
| Arthralgia | No | 12 (20.0%) |
| Yes | 48 (80.0%) | |
| Fever | No | 31 (51.7%) |
| Yes | 29 (48.3%) | |
| Hair loss | No | 48 (80.0%) |
| Yes | 12 (20.0%) | |
| Seizures | No | 52 (86.7%) |
| Yes | 8 (13.3%) | |
| Serositis | No | 56 (93.3%) |
| Yes | 4 (6.7%) | |
| Nephritis | No | 40 (66.7%) |
| Yes | 20 (33.3%) | |
| Photosensitivity | No | 56 (93.3%) |
| Yes | 4 (6.7%) | |
| Vasculitis | No | 56 (93.3%) |
| Yes | 4 (6.7%) | |
| Oral ulcer | No | 56 (93.3%) |
| Yes | 4 (6.7%) | |
Table 2. The clinical manifestations of the studied patients at the onset of the disease.
| During the course of the disease | Total no. = 60 | ||
|---|---|---|---|
| Rash | No | 20 (33.3%) | |
| Yes | 40 (66.7%) | ||
| Arthralgia | No | 7 (11.7%) | |
| Yes | 53 (88.3%) | ||
| Fever | No | 23 (38.3%) | |
| Yes | 37 (61.7%) | ||
| Hair loss | No | 31 (51.7%) | |
| Yes | 29 (48.3%) | ||
| Seizures | No | 49 (81.7%) | |
| Yes | 11 (18.3%) | ||
| Serositis | No | 53 (88.3%) | |
| Yes | 7 (11.7%) | ||
| Nephritis | No | 33 (55.0%) | |
| Yes | 27 (45.0%) | ||
| Photosensitivity | No | 52 (86.7%) | |
| Yes | 8 (13.3%) | ||
| Vasculitis | No | 52 (86.7%) | |
| Yes | 8 (13.3%) | ||
| Oral ulcer | No | 56 (93.3%) | |
| Yes | 4 (6.7%) | ||
Table 3. The clinical manifestations of the studied patients during the course of the disease.
Patients were divided into 2 groups: 30 patients in activity and 30 patients not in activity according to SLEDAI.
Regarding steroid therapy, 52 patients were receiving steroids, 34 patients (65.4%) were on high dose steroids while 18 patients(34.6%) were on low dose steroids (0.25-1 mg /kg).
CDI score revealed no depression in 27 patients (45%), mild depression in 12 patients (20%),moderate depression in 11 patients (18.3%) and severe depression in 10 patients.
There was no statistically significant relation difference found between active group and inactive group regarding age (10.9 ± 1.4 vs 11.07 ± 1.2 with p-value = 0.622). Also, there was no statistically significant difference found between active group and inactive group regarding sex of the studied patients with p-value = 0.317 (Table 4).
| Laboratory findings at the studied group | Total no. = 60 | |
|---|---|---|
| Hb | Normal | 47 (78.3%) |
| Anemia | 13 (21.7%) | |
| Hb | Mean ± SD | 11.63 ± 1.99 |
| Range | 5.5 - 14.4 | |
| TLC | Normal | 45 (75.0%) |
| Neutrophilia | 2 (3.3%) | |
| Leukopenia | 13 (21.7%) | |
| TLC | Mean ± SD | 6.70 ± 2.49 |
| Range | 2.2 - 17.2 | |
| PLTs | Normal | 53 (88.3%) |
| Thrombocytosis | 2 (3.3%) | |
| Thrombocytopen ia | 5 (8.3%) | |
| PLTs | Mean ± SD | 272.67 ± 100.72 |
| Range | 23 -615 | |
| C3 | Normal | 28 (46.7%) |
| Consumed | 32 (53.3%) | |
| C4 | Normal | 30 (50.0%) |
| Consumed | 30 (50.0%) | |
| ESR | Normal | 19 (31.7%) |
| Elevated | 41 (68.3%) | |
| CRP | Negative | 51 (85.0%) |
| Positive | 9 (15.0%) | |
Table 4. The laboratory investigations of the studied patients.
No statistically significant relation was found between activity of the studied patients and Clinical manifestations at the onset of the disease except hair loss, serositis and vasculitis were found significantly associated with activity among the studied patients with p value = 0.001, 0.038 and 0.038 respectively (Table 5).
| Laboratory findings in the studied group | Total no. = 60 | |
|---|---|---|
| LFTs | Normal | 52 (86.7%) |
| Elevated | 8 (13.3%) | |
| KFTs | Normal | 52 (86.7%) |
| Elevated | 8 (13.3%) | |
| Urine analysis | Free | 34 (56.7%) |
| Affected | 26 (43.3%) | |
| Urine analysis finding | Free | 34 (56.7%) |
| Proteinuria | 6 (10.0%) | |
| Pyuria | 10 (16.7%) | |
| Casts | 2 (3.3%) | |
| Hematuria | 1 (1.7%) | |
| UTI | 1 (1.7%) | |
| Ca oxalates +++ | 1 (1.7%) | |
| Proteinuria, pyuria | 3 (5.0%) | |
| Pyuria, casts | 1 (1.7%) | |
| Pus cells over I 00, protei nuria | 1 (1.7%) | |
| ANA | Negative | 15 (25.0%) |
| Positive | 45 (75.0%) | |
| Anti ds DNA | Negative | 21 (35.0%) |
| Positive | 39 (65.0%) | |
Table 5. The laboratory investigations of the studied patients.
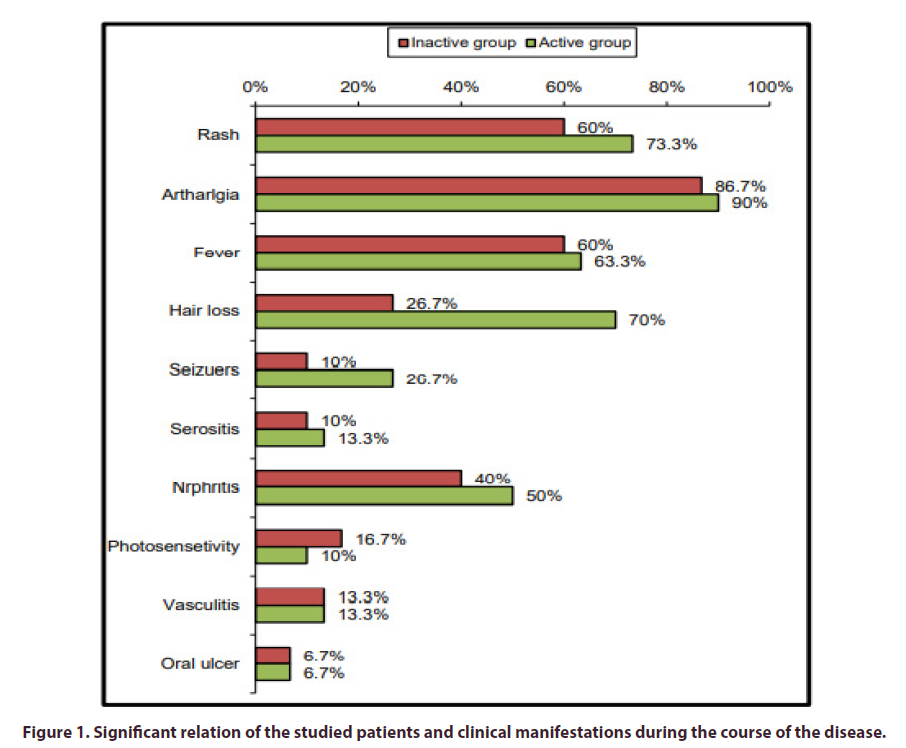
No statistically significant relation was found between activity of the studied patients and clinical manifestations during the course of the disease except hair loss was found significantly associated with activity among the studied patients with p-value = 0.001 (Figure 1).
There was no statistically significant relation between activity of the studied patients and medications used in treatment of SLE except steroids and the dose of steroids used, which were found to be significantly associated with activity among the studied patients with p-value = 0.001, 0.003 respectively.
Table 6 shows a statistically significant difference between the SLEDAI score in both groups where all patients in disease activity had scores reflecting severe activity while patients classified not in activity had scores reflecting no or mild activity (p value 0.000).
| Inactive group | Active group | Test value | P-value | Sig. | ||
|---|---|---|---|---|---|---|
| No. = 30 | No. = 30 | |||||
| Age (years) | Mean ± SD | 10.90 ± 1.40 | 11.07 ± 1.20 | -0.495• | 0.622 | NS |
| Range | 08-12 | 08-12 | ||||
| Sex | Female | 23 (76.7%) | 26 (86.7%) | 1.002* | 0.317 | NS |
| Male | 7 (23.3%) | 4 (13.3%) |
P-value > 0.05: Non significant; P-value < 0.05: Significant; P-value < 0.01: Highly significant
*:Chi-square test;•:Independent I-test
Table 6. Statistically significant relation difference found between active group and inactive group regarding age (10.9 ± 1.4 vs 11.07 ± 1.2 with p-value = 0.622).
It also shows that the active group showed more depression than the inactive group as shown by the statistically significant difference between the mean CDI scores of both groups (p value=0.006). The severity of depression was strongly related to the disease activity, as (33.3) % of the active group showed severe depression while none of the inactive group was severely depressed with a statistically significant difference between both groups By logistic regression shown in Table 7 there was highly statistically significant association between activity of the studied cases and the hair loss at the onset and during the course of the disease. Anemia, consumption in C3 & C4 and elevation in ESR, LFTs, KFTs also shows a strong relation with activity plus affection in the urine analysis and sensitivity of ANA. Degree of depression and the CDI score more than 18 shows strong association with activity (Tables 8 and 9).
| Clinical manifestations at the onset of the disease | Inactive group | Active group | Test value | P-value | Sig. | |
|---|---|---|---|---|---|---|
| No. = 30 | No. = 30 | |||||
| Rash | No | 13 (43.3%) | 16 (53.3%) | 0.601 | 0.438 | NS |
| Yes | 17 (56.7%) | 14 (46.7%) | ||||
| Arthralgia | No | 7 (23.3%) | 5 (16.7%) | 0.417 | 0.519 | NS |
| Yes | 23 (76.7%) | 25 (83.3%) | ||||
| Fever | No | 14 (46. 7%) | 17 (56.7%) | 0.601 | 0.438 | NS |
| Yes | 16 (53.3%) | 13 (43.3%) | ||||
| Hair loss | No | 29 (96.7%) | 19 (63.3%) | 10.417 | 0.001 | HS |
| Yes | 1 (3.3%) | 11 (36.7%) | ||||
| Seizures | No | 27 (90.0%) | 25 (83.3%) | 0.577 | 0.448 | NS |
| Yes | 3 (10.0%) | 5 (16.7%) | ||||
| Serositis | No | 26 (86.7%) | 30 (100.0%) | 4.286 | 0.038 | S |
| Yes | 4 (13.3%) | 0 (0.0%) | ||||
| Nephritis | No | 22 (73.3%) | 18 (60.0%) | 1.2 | 0.273 | NS |
| Yes | 8 (26.7%) | 12 (40.0%) | ||||
| Photosensitivity | No | 27 (90.0%) | 29 (96.7%) | 1.071 | 0.301 | NS |
| Yes | 3 (10.0%) | 1 (3.3%) | ||||
| Vasculitis | No | 26 (86. 7%) | 30 (100.0%) | 4.286 | 0.038 | S |
| Yes | 4 (13.3%) | 0 (0.0%) | ||||
| Oral ulcer | No | 29 (96.7%) | 27 (90.0%) | 1.071 | 0.301 | NS |
| Yes | 1 (3.3%) | 3 (10.0%) | ||||
P-value >0.05: Non significant; P-value <0.05: Significant; P-value <0.0I : Highly significant
Table 7. Significant relation between activity of the studied patients and Clinical manifestations at the onset of the disease.
| Inactive group | Active group | Test value | P-value | Sig. | ||
|---|---|---|---|---|---|---|
| No. = 30 | No. = 30 | |||||
| SLEDAl score | No activity | 27 (90.0%) | 0 (0.0%) | 60.000* | 0 | HS |
| Mild | 3 (10.0%) | 0 (0.0%) | ||||
| Severe | 0 (0.0%) | 30 (100.0%) | ||||
| CDI score | Mean ± SD | 15.97 ± 5.80 | 21.63 ± 9.19 | -2.856• | 0.006 | HS |
| Range | 10-29 | 9-34 | ||||
| Degree of depression | None | 16 (53.3%) | 11 (36.7%) | 12.350* | 0.006 | HS |
| Mild | 8 (26.7%) | 4 (13.3%) | ||||
| Moderate | 6 (20.0%) | 5 (16.7%) | ||||
| Severe | 0 (0.0%) | 10 (33.3%) |
P-value >0.05: Non significant;P-value < 0.05: Significant; P-value < 0.0I: Highly significant
*:Chi-square test; •: Independent t-test
Table 8. Statistically significant difference between the mean CDI scores of both groups (p value=0.006).
| B | S.E. | Wald | P-value | Odds ratio | 95% C.l. for OR | ||
|---|---|---|---|---|---|---|---|
| (OR) | Lower | Upper | |||||
| Hair loss at onset of the disease | 2.821 | J.085 | 6.754 | 0.009 | 16.789 | 2.001 | 140.898 |
| Hair loss at the course of the disease | 1.859 | 0.574 | 10.497 | 0.001 | 6.417 | 2.084 | 19.755 |
| Hb (anemia) | 2.093 | 0.824 | 6.446 | 0.011 | 8.105 | 1.612 | 40.766 |
| C3 | 3.807 | 0.781 | 23.741 | 0 | 45 | 9.732 | 208.077 |
| C4 | 4.394 | 0.861 | 26.07 | 0 | 81 | 14.993 | 437.605 |
| ESR | 3.773 | J.083 | 12. 131 | 0 | 43.5 | 5.205 | 363.522 |
| LITs | 2.178 | 1.105 | 3.885 | 0.049 | 8.826 | 1.012 | 76.96 |
| KFTs | 2.178 | 1.105 | 3.885 | 0.049 | 8.826 | 1.012 | 76.96 |
| Urine analysis | 2.079 | 0.599 | 12.067 | 0.001 | 8 | 2.475 | 25.86 |
| ANA | 1.792 | 0.714 | 6.304 | 0.012 | 6 | 1.482 | 24.299 |
| Anti ds DN A | 2.603 | 0.714 | 13.302 | 0 | 13.5 | 3.333 | 54.673 |
| CDI score > 18 | 1.458 | 0.568 | 6.599 | 0.01 | 4.297 | 1.413 | 13.068 |
| Degree of depression | 0.67 | 0.257 | 6.81 | 0.009 | 1.955 | 1.182 | 3.235 |
Table 9. Significant association between activity of the studied cases and the hair loss at the onset and during the course of the disease.
Discussion
The current study showed that, there was no statistically significant relation difference found between active group and inactive group regarding age (10.9 ± 1.4 vs 11.07 ± 1.2 with p-value = 0.622) and regarding the sex of the studied patients with p-value = 0.317. Similarly in study, activity was measured by the SLEDAI. At the time of diagnosis, there was a trend, but not a statistically significant difference, between both gender in the signs of disease activity although the males had less arthritis, alopecia, anti-Ro antibody, less Raynaud‘s, and more discoid lesions and thrombocytopenia [13]. While in contrast, de Carvalho et al. [14], found male patients had higher activity scores.
The current study showed that, there was no significant difference between the active and inactive group in clinical manifestations at the onset of the disease except hair loss, serositis and vasculitis were found to be significantly associated with activity among the studied patients with p-value = 0.001, 0.038 and 0.038 respectively. At the course of the disease, there was no significant difference between the active and inactive group in clinical manifestations during the course of disease except hair loss which was significantly associated with activity among the studied patients with p-value = 0.001. In agreement with us Bouaziz et al. [15], found the presence of vasculitic lesions to be strongly related to systemic disease activity. Also, in harmony with our results, Callen and Kingman reported a link between cutaneous vasculitis and the progression of disease [16]. On the other hand, Nazri et al., found oral ulcers (p=0.010) and malar rash (p=0.044) to be positively associated with an active disease [17]. Our results were against the Houman et al., finding as they found the SLEDAI score for activity at SLE diagnosis was significantly higher in patients with lupus nephritis [18].
The current study showed that, there was no significant difference between the active and inactive group in medications used in treatment of SLE except steroids uses and the dose of steroids which were found to be significantly associated with activity among the studied patients with p-value = 0.001& 0.033 respectively. In agreement with us Alsowaida et al., found no relation between medication non-adherence and disease activity [19]. This finding was also supported by the study conducted by Petri et al. [20], as none of the baseline medications evaluated, including corticosteroids, immunosuppressives (individually and as a group), antimalarials, and other concomitant medications, predicted flare on any index but their results was against us regarding steroid uses.
However, Zakeri et al. [21], did not find an association between depression and duration of steroid therapy.
The current study showed that, there was a statistically significant difference between the active and inactive group in the following laboratory findings: HB level (incidence of anemia), TLC count (Leukopenia), Consumption of C3and C4, ESR and CRP levels and urine profile with P value= 0.005, 0.00, 0.004, 0.00, 0.00, 0.00 &0.001respectively. In harmony with us, for immunological parameters, the study by Nazri et al. [17] showed elevated ESR (p=0.006), low C3 levels (p=0.008), were positively associated with SLEDAI scores. However, in our study, low C4 did not exhibit any association with disease activity, this suggests that C4 might be a less sensitive parameter of disease activity in SLE patients.
And in line with us, Fernando and Isenberg, reported that, as with rising antibodies to dsDNA, falling levels of C3 and C4 may herald a lupus flare in patients with previously documented concordance. [22]. In addition, Morrow et al. [23], concluded that, it was preferred to use complement breakdown products such as C3d or C4d, which increase when the disease is active. Also, other investigators reported no consistent association between anti-dsDNA positivity or low complement levels and risk of flare [24-27].
The current study showed that, there was statistically significant difference between the active and inactive group in laboratory findings as LFTs, KFTs, urine analysis affection, findings in urine analysis, ANA and Anti double strands DNA with P value = 0.023,0.023,0.000,0.008,0.007,0.000respectively. This was consistent with a previous study conducted by [28].
The current study showed that, there was statistically significant difference between the active and inactive group in the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score. Similarly, according to the study conducted by Alsowaida et al. [19] found the SLEDAI scores showed that 31 patients (22%) had mild-to- moderate disease activity, whereas 37 patients (24%) had severe disease activity. Also, according to the study by Houman et al. [18], The average of the SLEDAI at the time of diagnosis was 15.5 (range, 3 – 47); and after six and 12 months of treatment, it decreased respectively to 4.3 (range, 0 – 25) and 3.7 (range, 0 – 20).
In the current study, depression showed a strong relation with disease activity as 53.3 % of the inactive group showed no depression and 63.3 % of the active group suffered depression with p-value = 0.006. This finding supports our hypothesis that depression is related to SLE activity. One possible explanation for this finding is that some patients with SLE in our study may have become depressed as a result of the physical and psychological distress due to the increased SLE activity. However, an appealing interpretation for our finding is that depression can be an isolated manifestation of CNS pathology caused by the disease activity. Cerebral involvement in SLE is still poorly understood, but it is recognized to occur in patients with active disease even without overt neuropsychiatric manifestations [29].
In the current study, the degree of depression was strongly correlated to the extent of disease activity, which was concordant with the results of the study by Alsowaida et al., where patients with minimal or mild depressive symptoms (n = 114) had an average SLEDAI score of 5.16 SD, whereas patients with moderate or severe symptoms (n = 26) had an average SLEDAI score of 9.96 SD. A strong correlation between disease activity and severity of depressed mood was found (r = 0.31, p = 0.003) [19]. This finding was supported by a study by Zakeri et al. [21], which also used SLEDAI score to assess the degree of disease activity, where 43.6% of mild-type SLE patients did not suffer from depression, 51.3% had mild depression, and 5.1% had moderate depression. On the other hand, all patients with severe form of SLE achieved scores indicating depression; 42.9% reached mild depression and 57.1% reached moderate depression (P = 0.0001).
Similar findings were reported by Julian et al. and Olivera et al. [30,31] they found that the presence of depressive symptoms was strongly associated with non-adherence to therapy which precipitated the activity. In contrast, the two studies reported by Shortall et al. and Van Exel et al. found no relation between disease activity and depression [32,33]. Our finding would corroborate the assumption that depression in SLE depends on disease severity–related stress. However, it cannot be excluded that shared pathophysiological mechanisms between SLE and depression play an additional role.
Although a study conducted by Figueiredo-Braga et al. [34], had indicated that disease activity correlates with depression, it reported that there may be confounding factors that mask the effects. These could include such things as depressive symptoms lagging behind inflammatory indicators, socioeconomic factors masking or exacerbating depression, or personal psychological variables such as cognition, helplessness, or resilience that interfere with a straightforward analysis.
There are conflicting data whether active disease increases the vulnerability to depression. Miguel et al. and Utset et al [35,36]. have shown an association with active neuropsychiatric lupus but found no relations between serology and other manifestations of active disease.
Conclusion
Children with SLE in active disease have a greater risk of developing depression than those with inactive disease and the severity of depression is positively correlated with disease activity. The findings from this study confirm the importance of identifying and managing depression in SLE. The data indicate that depression may exacerbate lupus disease activity and suggest that effective treatment of depression may lead to improvements in lupus disease outcomes. Recognition of these associations may provide more appropriate management for these patients and also may bring new insights to the understanding of the underlying mechanism involved in this important clinical presentation of SLE.
Conflict of interest
None
References
- Weiss JE. Pediatric Systemic Lupus Erythematosus: More Than a Positive Antinuclear Antibody. Pediatr Rev. 33,62-74 (2012).
- Gottlieb BS, Norman TI. Systemic Lupus Erythematosus in Children and adolescents. Pediatr Rev. 27,323-330 (2006).
- Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 15,308-318 (2006).
- Guiducci C, Gong M, Xu Z et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 465,937-941 (2010).
- United States Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People. (2020).
- De Castro TCM, Hsien HC. Depression as the First Manifestation in a Young Girl With Juvenile Systemic Lupus Erythematosus. Arch Rheumatol. 33,105 (2018).
- Korczak DJ, Sheri Madigan S, Colasanto M. Children's physical activity and depression: A meta analysis. Pediatrics. 139, e20162266 (2017).
- Hammen C, Hazel NA, Brennan PA et al. Intergenerational transmission and continuity of stress and depression: depressed women and their offspring in 20 years of follow-up. Psychology Med. 42,931-941 (2012).
- Avenevolli S, Swendsen J, He J et al. Major Depression in the National Comorbidity Survey- Adolescent Supplement: Prevalence, Correlates, and Treatment. J Am Acad Child Adolesc Psychiatry. 54,37-44 (2015).
- Daniel WW. Biostatistics: A foundation for analysis in the health sciences. 7th Edition, John Wiley & Sons, Inc., Hoboken. (1999).
- Castrejon I, Tani C, Huang A et al. Indices to assess patients with systemic lupus erythematosus in clinical trials,long- term observational studies,and clinical care. Clin Exp Rheumatology. 32,S85-S95 (2014).
- Ghareeb GA, Beshai JA. Arabic Version of the Children's Depression Inventory: Reliability and Validity. Journal of Clinical Child Psychology. 18,323-326 (1989).
- Mok C, Lau C, Chan T et al. Clinical characteristics and outcome of southern Chinese males with systemic lupus erythematosus. Lupus. 8,188-196 (1999).
- De Carvalho JF, do Nascimento AD, Bonfá E. Male gender results in more severe lupus nephritis. Rheumatology International. 30,1311–1315 (2010).
- Bouaziz J, Barete S, Le Pelletier F et al. Cutaneous lesions of the digits in systemic lupus erythematosus: 50 cases. Lupus. 16,163-167 (2007).
- Callen JP, Kingman J. Cutaneous vasculitis in systemic lupus erythematosus. A poor prognostic indicator. Cutis. 32,433-6 (1983).
- Nazri S, Wong KK, Hamid W. Pediatric systemic lupus erythematosus. Retrospective analysis of clinico-laboratory parameters and their association with Systemic Lupus Erythematosus Disease Activity Index score. Saudi Med J. 39,627-631 (2018).
- Houman MH, Smiti khanfir M, Ghorbell B et al. Systemic lupus erythematosus in Tunisia: demographic and clinical analysis of 100 patients. Lupus. 13,204-11 (2004).
- Alsowaida N, Alrasheed M, Mayet A et al. Medication adherence, depression and disease activity among patients with systemic lupus erythematosus. Lupus. 27,327-332 (2018).
- Petri MA, van Vollenhoven RF, Buyon J et al. Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III belimumab trials. Arthritis Rheum. 65,2143-53 (2013).
- Zakeri Z, Shakiba M, Narouie B et al. Prevalence of depression and depressive symptoms in patients with systemic lupus erythematosus: Iranian experience. Rheumatology international. 32,1179-1187 (2012).
- Fernando MMA, Isenberg DA. How to monitor SLE in routine clinical practice. Annals of the rheumatic diseases. 64,524-527 (2005).
- Morrow W, Williams D, Ferec C et al. The use of C3d as a means of monitoring clinical activity in systemic lupus erythematosus and rheumatoid arthritis. Annals of the rheumatic diseases. 42,668-671 (1983).
- Mirzayan M, Schmidt R, Witte T. Prognostic parameters for flare in systemic lupus erythematosus. Rheumatology. 39,1316-1319 (2000).
- Petri M, Singh S, Tesfasyone H et al. Prevalence of flare and influence of demographic and serologic factors on flare risk in systemic lupus erythematosus: a prospective study. The Journal of rheumatology. 36,2476-2480 (2009).
- Ho A, Barr SG, Magder LS et al. A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus. Arthritis & Rheumatism. 44,2350-2357 (2001).
- Nikpour M, Urowitz MB, Ibañez D et al. Frequency and determinants of flare and persistently active disease in systemic lupus erythematosus. Arthritis Care & Research: Official Journal of the American College of Rheumatology. 61,1152-1158 (2009).
- O'Sullivan M, McLean-Tooke A, Loh R. Antinuclear antibody test. Australian family physician. 42,718 (2013).
- Lopez-Longo F, Caro N, Almoguera M et al. Cerebral hypoperfusion detected by SPECT in patients with systemic lupus erythematosus is related to clinical activity and cumulative tissue damage. Lupus. 12,813-819 (2003).
- Julian LJ, Yelin E, Yazdany J et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum. 61,240–6 (2009).
- Oliveira-Santos M, Verani J, Klumb E et al. Evaluation of adherence to drug treatment in patients with systemic lupus erythematosus in Brazil. Lupus. 20,320-329 (2011).
- Shortall E, Isenberg D, Newman S. Factors associated with mood and mood disorders in SLE. Lupus. 4,272-279 (1995).
- van Exel E, Jacobs J, Korswagen LA et al. Depression in systemic lupus erythematosus, dependent on or independent of severity of disease. Lupus. 22,1462-1469 (2013a).
- Figueiredo-Braga M, Cornaby C, Bernardes M et al. Correlation between physical markers and psychiatric health in a Portuguese systemic lupus erythematosus cohort: The role of suffering in chronic autoimmune disease. PloS one. 13,e0195579 (2018).
- Miguel EC, Pereira R, Pereira C et al. Psychiatric manifestations of systemic lupus erythematosus: clinical features, symptoms, and signs of central nervous system activity in 43 patients. Medicine. 73,224-232 (1994).
- Utset TO, Golden M, Siberry G et al. Depressive symptoms in patients with systemic lupus erythematosus: association with central nervous system lupus and Sjögren's syndrome. The Journal of rheumatology. 21,2039-2045 (1994).
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar CrossRef
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref