Special Report - Interventional Cardiology (2015) Volume 7, Issue 6
Detection and structural characterization of lipid-core plaques with intravascular NIRS-IVUS imaging
- Corresponding Author:
- Ryan D Madder
Frederik Meijer Heart & Vascular Institute, Spectrum Health, Grand Rapids, MI 49503, USA
E-mail: madder@spectrumhealth.org
Intracoronary near-infrared spectroscopy (NIRS) combined with intravascular ultrasound is a novel diagnostic tool for the characterization of coronary plaque structure and composition. The principle advantage of NIRS is its ability to directly and reliably identify chemical composition rather than infer composition from signals that fundamentally have little inherent chemical specificity. NIRS has been prospectively validated in autopsy studies and approved by the US FDA to detect lipid-core plaque in the coronary arteries. The accurate detection of lipid-core plaques is likely to be of clinical importance because of the close association of lipid-core plaques and coronary events. Prospective studies are in progress to test the hypothesis that combined NIRS and intravascular ultrasound imaging can detect vulnerable coronary plaques and guide preventive therapy.
Article submitted: 26 May 2015; Article accepted for publication: 14 August 2015; Published online: 12 November 2015
Abstract
Intracoronary near-infrared spectroscopy (NIRS) combined with intravascular ultrasound is a novel diagnostic tool for the characterization of coronary plaque structure and composition. The principle advantage of NIRS is its ability to directly and reliably identify chemical composition rather than infer composition from signals that fundamentally have little inherent chemical specificity. NIRS has been prospectively validated in autopsy studies and approved by the US FDA to detect lipid-core plaque in the coronary arteries. The accurate detection of lipid-core plaques is likely to be of clinical importance because of the close association of lipid-core plaques and coronary events. Prospective studies are in progress to test the hypothesis that combined NIRS and intravascular ultrasound imaging can detect vulnerable coronary plaques and guide preventive therapy.
Keywords
intracoronary imaging, intravascular ultrasound, IVUS, lipid-core plaque, near-infrared spectroscopy, NIRS, vulnerable plaque
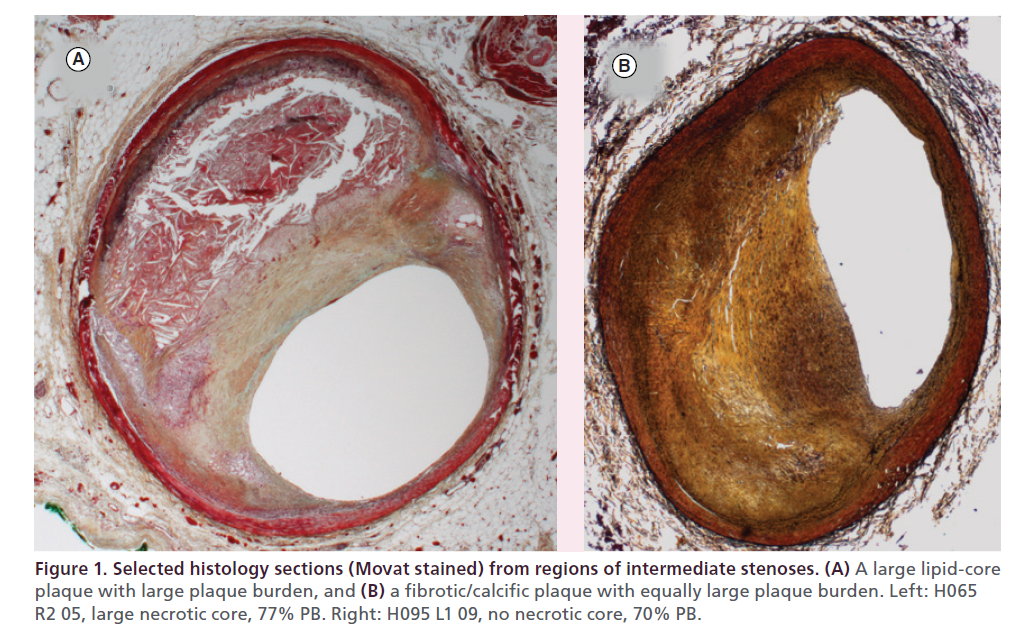
Numerous imaging methods for characterization of the structure of coronary plaques have been available for decades and continue to be refined to provide greater ease of use, increased accuracy, higher resolution and faster sampling speeds. Reliable methods for measurement of plaque composition, however, have only recently become available. As illustrated in Figure 1, two plaques can have very similar luminal dimensions and plaque burden yet contain vastly different underlying pathology.
Figure 1. Selected histology sections (Movat stained) from regions of intermediate stenoses. (A) A large lipid-core plaque with large plaque burden, and (B) a fibrotic/calcific plaque with equally large plaque burden. Left: H065 R2 05, large necrotic core, 77% PB. Right: H095 L1 09, no necrotic core, 70% PB.
Histopathological studies have repeatedly demonstrated that large lipid-core plaques (LCP) with thin or weak caps are closely associated with susceptibility to rupture and resultant thrombosis, leading to myocardial infarction and sudden cardiac death [1,2]. Many imaging modalities are under study for their ability to measure analogous plaque characteristics in vivo. This would permit prospective identification of vulnerable plaques in patients and help guide preventive therapy. Histopathologic studies of plaques that have caused coronary events have led to consensus definitions of plaques that are considered to be high risk or vulnerable. Most coronary events have been found to be associated with a thin-cap fibroatheroma (TCFA) as defined by a thin section, stains with certain chemical specificities and careful expert morphometric interpretation. in vivo imaging techniques are needed to identify TCFAs and define their features.
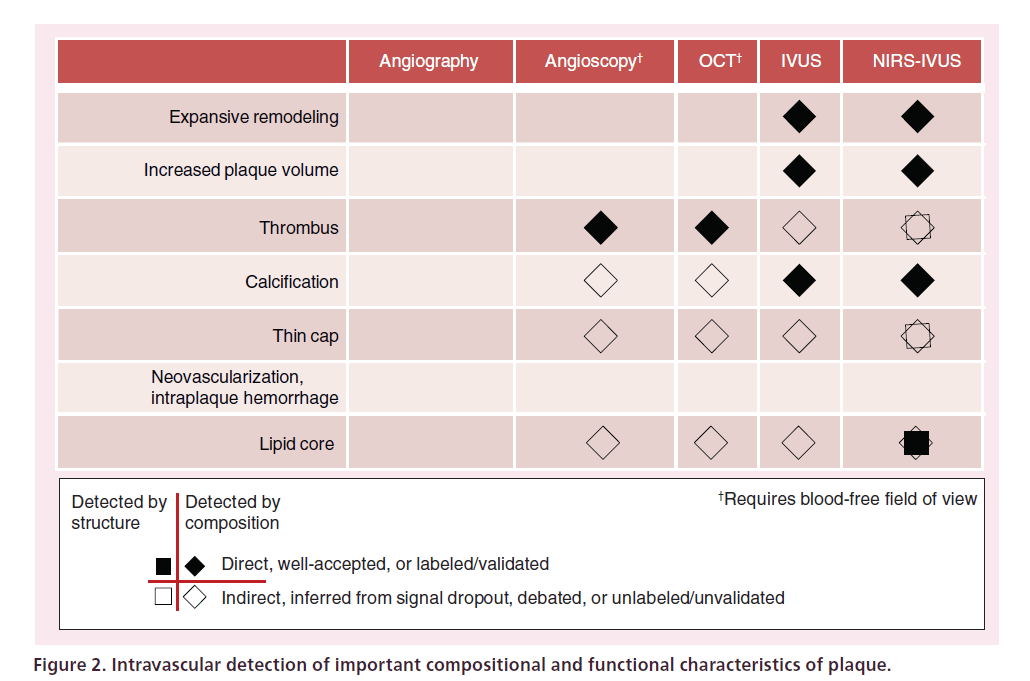
Certain features of plaques suspected to be high risk that may be of clinical relevance are shown in the rows of Figure 2. In the ideal case, a single imaging modality would be able to directly detect all of these features. The table presents the ability of current in vivo intravascular imaging techniques to identify plaque features of interest. Angiography, the most widely used invasive coronary diagnostic method, is of limited use for plaque characterization since its primary result is an image of the lumen containing contrast-loaded blood and does not image into the arterial wall. The ability of angiography to predict new events by detection of a stenosis is limited, since a large number of events occur at sites that did not demonstrate critical luminal narrowing prior to the event [3]. Imaging of a complex multifaceted concept such as inflammation is likely to require the use of an injectable agent to amplify the signal and provide specific molecular imaging. As indicated in Figure 2, most intravascular imaging techniques measure structural characteristics of a vulnerable plaque. The detection of plaque composition is then attempted based on the physical characteristics of the plaque. Detection of lipid by this method is based on a pattern of signal loss of an IVUS, optical coherence tomography (OCT) or computed tomography signal. However, signal loss due to lipid competes with multiple other causes of signal attenuation, such as overlying calcification, which significantly affect the accuracy of the measurement. In addition, the detection of lipid by structural techniques requires image interpretation, which is prone to subjective influences. For these reasons, methods that attempt compositional analysis based on structural information are inherently more limited for accurately determining lipid composition than technologies with chemical specificity.
The presence of a lipid core (also called necrotic core) is one of the most important compositional characteristics of a vulnerable plaque. LCP, which are known to be closely associated with coronary events, have not been reliably detectable in vivo with traditional intravascular imaging methods [4]. Intracoronary near-infrared spectroscopy (NIRS) was developed to accurately detect the presence of LCPs during cardiac catheterization.
A multimodality in vivo imaging approach can detect a greater number of important plaque characteristics than can a single modality approach. At present, the combined NIRS-intravascular ultrasound (IVUS) system is the only multimodality intracoronary imaging system in general clinical use. In this instrument, IVUS provides high-resolution structural images, while NIRS imaging accurately identifies lipid-core plaque. Both methods function through blood, and the dual, coregistered information is acquired during a single pullback. Imaging modalities that can detect a greater number of vulnerable plaque characteristics from Figure 2 will be of great benefit in the future.
Near-infrared spectroscopy
Fundamental principles
Spectroscopy can be broadly defined as the measurement of the wavelength-dependent interaction of electromagnetic radiation with matter (Figure 3). In diffuse-reflectance NIRS, a sample of interest is irradiated with unfocused near-infrared light and a detector measures the proportion of diffusely reflected light returned as a function of wavelength. Two different processes determine the amount of light that returns to the detector – scattering and absorption. Scattering in biological tissues, for example, is a consequence of localized difference in the refractive index of cellular and extracellular components. Absorption, on the other hand, occurs when light energy is absorbed by chemical bonds of the constituent molecules. Absorbed light is mainly transformed into molecular vibrational energy in the form of oscillations of atoms within their chemical bonds. Since the light is not focused, and the distance the photons travel before returning to a sensor cannot be known accurately (as in the case of intracoronary NIRS), the depth of the structure producing the signal cannot be known exactly. While lack of determination of precise location of the source of the signal is a limitation, the diffusely reflected signal is not as easily corrupted by a small obstruction (large calcified particle, stent strut, etc.) as it is with methods requiring a straight-path photon maintaining coherence, such as OCT.
Since scattering and absorption properties vary for different substances at different wavelengths, they can provide unique spectroscopic signatures that can be exploited for material identification. Diffuse nearinfrared (NIR) reflectance spectroscopy provides direct, rapid, nondestructive and accurate measurements in challenging environments with little or no sample preparation. For these reasons, NIRS has been widely used in the fields of food, agriculture, petroleum, astronomy, pharmaceuticals and medicine. A well-known medical application of NIRS in common use is the measurement of deoxyhemoglobin and oxyhemoglobin to determine oxygen saturation in tissues.
NIRS is well suited for analysis of LCP in coronary tissue since NIR light can adequately penetrate blood; diffusely reflected light can be collected from several millimeters into the tissue; NIR measurements can be made in milliseconds with an ultrafast swept laser, overcoming the problem of cardiac motion; scanning speeds permit acquisition of tens of thousands of spatial measurements required to survey the entire artery; and NIRS provides a positive and specific chemical signature of the lipid-core target of interest. As noted above, NIRS does not rely on dropout of signal or interpretation of a structural measurement to infer the presence of a lipid core as do IVUS and OCT.
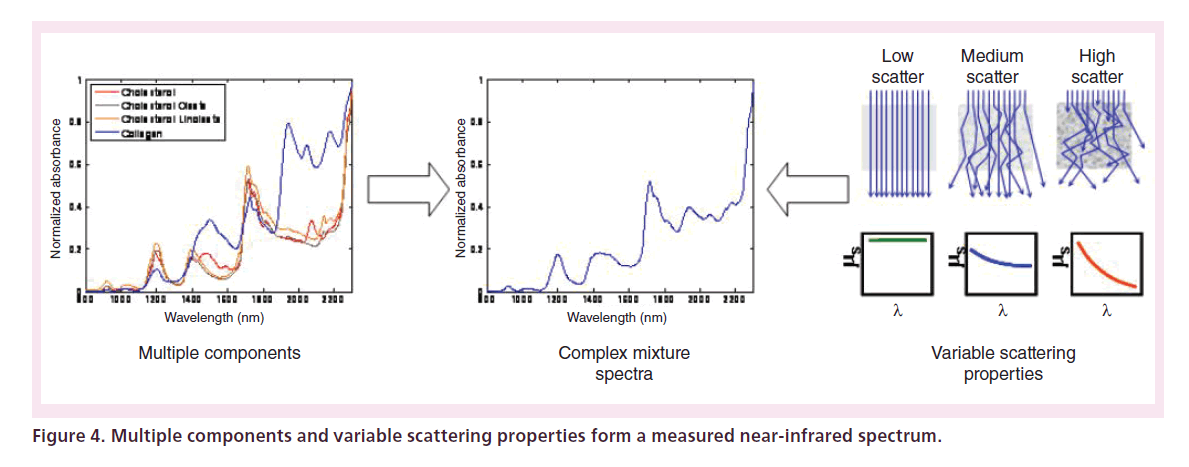
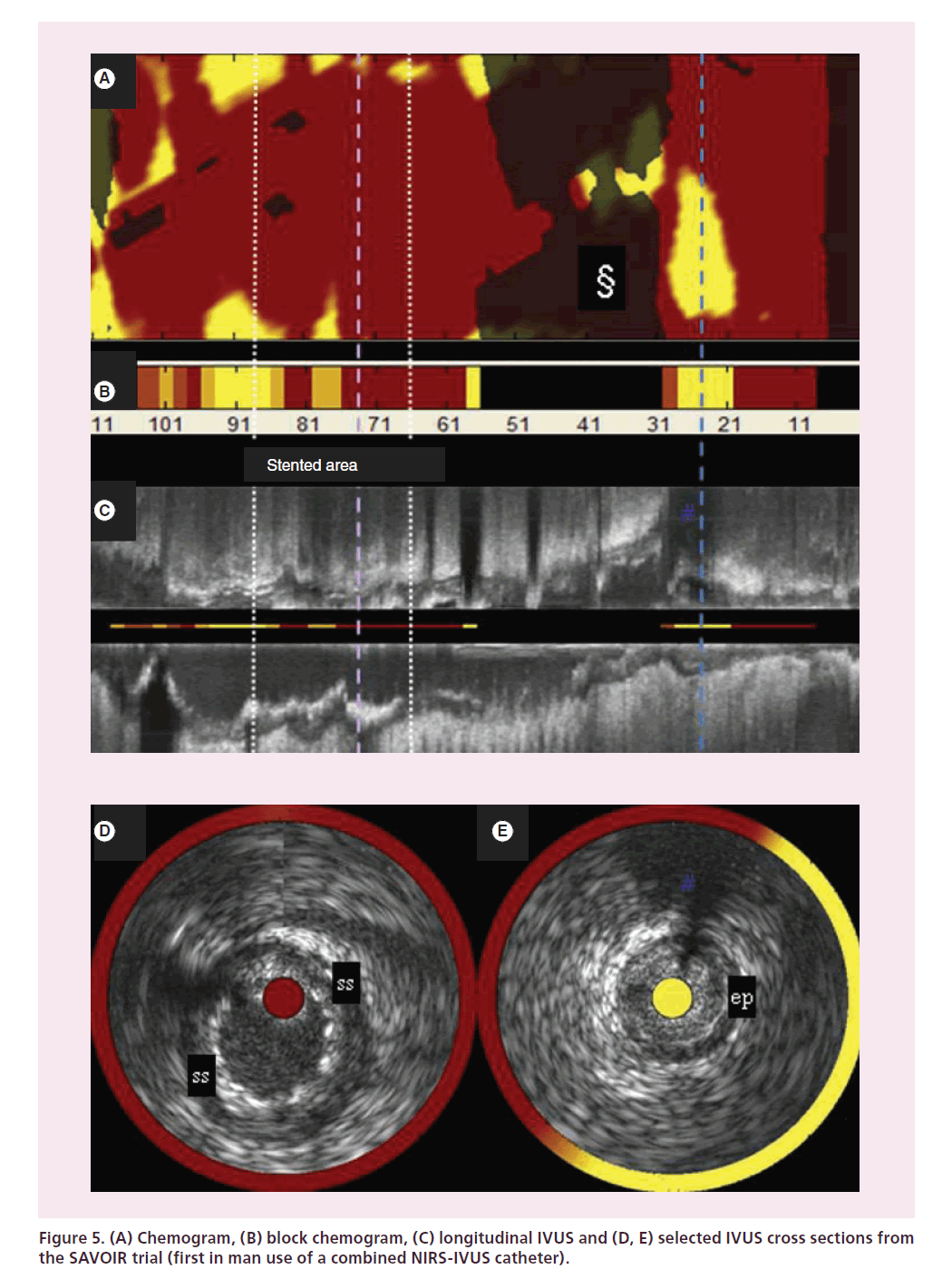
In a multicomponent sample, the measured spectrum is a composite of spectral contributions and scattering properties from all the constituents of the sample (Figure 4). The broad-featured NIRS absorbance spectra from mixed samples in scattering media are generally difficult to visually interpret directly and typically require multivariate mathematical methods of analysis to extract information. Furthermore, in a clinical application, the physician should ideally be presented with a simple and easy image to interpret rather than any raw spectroscopic information. Thus, the current commercially available NIRS employs an algorithm to automatically transform the underlying spectral information into a probability of the presence of lipid core. The methodology that was employed in the algorithm development process is derived from the family of techniques called ‘chemometrics’, a branch of analytical chemistry studying the application of mathematical and statistical techniques for chemical data analysis [5]. In commercial intracoronary NIRS, the result of the chemometric algorithm is a probability of the presence of lipid-core plaque for each spatial position of the sensor over the course of a rotating pullback. This spatial map of probabilities is color-coded and presented as a 2D image called as ‘chemogram’ [6]. From the chemogram image, a vertical summary metric was developed, termed as ‘block chemogram’, and is computed by taking the 90th percentile value of all pixels in each 2 mm section. An example of this can be observed in Figure 5B. A measurement from the chemogram is the lipid-core burden index (LCBI), which is a quantitative summary metric of the total LCP detected in the wall of the artery relative to the total length of the pullback [6–8]. A further use of the LCBI metric in the clinic is often the max LCBI4mm, which is defined as the maximum LCBI of any 4 mm segment in a region of interest [9].
Validation for lipid-core plaque
To validate a specific label claim for the detection of lipid-core plaque by NIRS, an extensive ex vivo human coronary autopsy study was conducted, and a concurrent clinical study was carried out.
NIRS spectra and histological data were collected in human autopsy coronaries to build a calibration model capable of recognizing the NIRS spectral shapes unique to LCPs. Arteries were mounted in a tissue fixture and connected to a blood circulation system with physiologic pressure, temperature and flow. After scanning, the arterial segments were cut into 2 mm thick cross-sectional blocks, thin sections were prepared from the blocks and histology was analyzed with both H&E and Movat staining (Figure 1). Detailed qualitative and quantitative histology parameters were assessed and recorded in each section using well-accepted criteria from the American Heart Association [1]. The resulting set of NIRS spectra and corresponding histology data were used to train an LCP detection algorithm, which was finalized and locked-down prior to validation. The algorithm was then prospectively tested in doubleblinded fashion using a new set of validation hearts. A total of 33 hearts and 86 coronary segments were used for calibration, and 51 hearts and 126 segments were used for validation. The chemogram was validated by comparing each 2 mm block chemogram value to its corresponding histology truth for lipidcore plaque presence (binary presence or absence of LCP in each 2 mm block) in a receiver operating characteristic curve analysis of diagnostic accuracy. Prospective validation of the system for detection of LCP in nearly 2000 individual blocks yielded an area under the receiver operating characteristic curve of 0.80 (95% CI: 0.76–0.85) [6].
Concurrently, the SPECTroscopic Assessment of Coronary Lipid (SPECTACL) pivotal clinical study [10] was conducted to determine if the spectra recorded in patients (in whom tissue is not available for histology), were equivalent to the spectra recorded in autopsy specimens (in which tissue was available for histological validation) [11]. Spectral similarity was prospectively analyzed using common measures of multivariate distance and calibration model residual. A success rate of 0.83 (95% CI: 0.70–0.93) was observed, satisfying the primary end point of the study [10].
The system was subsequently given a specific US FDA label claim for the detection of lipid-core plaque; this is the first and to our knowledge the only label claim of this kind.
Relationship to other technologies
Optical coherence tomography/optical frequency domain imaging (OFDI)
Optical coherence tomography is the optical analog of IVUS [12]. It is an interferometric technique utilizing directly reflected laser light that can portray the 3D microarchitecture of plaques. OCT achieves the highest spatial resolution of existing intravascular imaging modalities (10–20 microns vs 100–200 microns for conventional IVUS) and is currently the only structural imaging method with adequate spatial resolution to measure the fibrous cap thickness overlying LCP [13–15].
Since intracoronary OCT usually utilizes NIR light for its favorable penetration depth into tissue, it is sometimes confused with NIRS. In contrast to methods analyzing wavelength-dependent absorptions or emissions, such as NIRS, OCT is better described as ‘optical IVUS’ due to the analogous measurement of backscattering as a function of time delay. However, unlike NIRS or IVUS, the light absorption and scattering of blood substantially limits imaging depth leading to a requirement for flushes (usually contrast injection) during OCT imaging [16]. To limit the number of flushes during imaging, second generation OCT systems utilize a faster acquisition speed.
The use of OCT photons for imaging also requires the maintaining of coherence. Coherence is disrupted by scattering media, which can limit the useful imaging depth of OCT. This is often called a lack of ‘depth of penetration’ or ‘shadowing’. At the laser powers typically used for OCT, the light can travel up to several millimeters into the vessel wall and still return to the sensor at detectable levels. Rather than actual lack of light penetration or return, the problem is that beyond a certain depth, the probability that the photons have encountered a coherence-disrupting scattering event approaches 100%. This limiting depth almost always precludes the imaging of the external elastic membrane of the artery and hence the measurement of plaque burden.
Purported OCT determination of plaque composition relies on subjective interpretation of image characteristics that are inherently structural in nature. The use of OCT for LCP detection is based on expert interpretation of multiple cross-sectional views and is time consuming. Definitions for lipid core almost always involve patterns of signal loss that are in competition with other instrumental and biological reasons for signal loss that are unrelated to lipid content [17]. For example, OCT-detected lipid may be confused with calcification in clinical practice, as both lipid and calcium within a plaque will generate a signal-poor region on the OCT image [18]. Additionally, superficial inflammatory cells tend to attenuate the OCT signal, causing a signal loss behind the superficial layer, which may be confused as lipid necrotic core formation.
Recently, a multimodality system combining OCT and NIRS has been demonstrated. Using the same light source, a dual modality system was developed to obtain coregistered OCT images and NIRS information. Custom made optical components, using double- clad fiber, were developed to simultaneously capture OCT and diffusely reflected NIRS. The system was used to image ex vivo human arteries. Information provided by NIRS allowed the discrimination of two plaques that looked similar by OCT but differed in the presence of lipid [19]. Similar to combining NIRS and IVUS, this demonstrates the advantage of combining complementary techniques: NIRS for chemical assessment and OCT for microstructural information. However, this system has currently only been demonstrated as a prototype and is not available for commercial use.
Angioscopy
Angioscopy is similar in principle to NIRS, but it utilizes light in the visible wavelengths to interrogate the vessel, while NIRS utilizes NIR light. Rather than fine resolution of wavelengths, angioscopy generally relies on the broad color distinctions of the human vision system for characterizing and categorizing plaques [20,21]. The advantages of NIR over visible light for plaque characterization are that the NIR can penetrate blood (avoiding the need for occlusion or flushing of the vessel required with angioscopy), it has greater depth of penetration into tissue and NIR interactions with molecules provide much more specific chemical information than do visible light interactions. Angioscopy has not gained widespread use in clinical practice for a number of reasons, including a paucity of commercially available instrumentation, the necessity for vessel occlusion or saline flushing [22], device size and catheter deliverability and maneuverability. Recent data have shown a good correlation between detection of yellow plaque by angioscopy and NIRS [23]. However, angioscopy requires operator interpretation of the results and may not detect plaques that are located deeper than the lumen surface. Recent advances in angioscopy have used color fluorescence to characterize the presence of collagen fibers, oxidized low-density lipoprotein and further deposition of lipid [24]. These capabilities and refinement of the hardware may allow angioscopy to aid in the determination of plaque vulnerability.
Raman spectroscopy
Raman spectroscopy is a powerful analytical technique closely related to diffuse reflectance NIRS. However, in Raman spectroscopy, samples are illuminated with monochromatic laser light and photons return to the detector at wavelengths shifted from the source light. Raman wavelength shifts correspond to vibrational modes both overlapping and complementary to those observed in infrared spectroscopy. Raman spectroscopy is similarly powerful for positive identification of organic compounds due to the high specificity of spectral patterns. Raman shifts can be observed using a variety of source wavelengths and thus the laser source can be chosen based on practical criteria favoring a particular measurement such as tissue penetration depth, available laser powers, optical materials and detectors. Raman spectroscopy, however, has been extremely difficult to implement in the coronaries due to weak strength of the signal, interfering background fluorescence and a dependence of Raman intensity on the fourth power of the laser frequency [25,26]. Raman methods have also been difficult for coronary application because of the need for the sensor to be in contact with the tissue. Commercialization of intravascular Raman spectroscopy was previously attempted by Prescient Medical.
Photoacoustic imaging
Intravascular photoacoustic (IVPA) imaging combines high-spatial resolution imaging with chemicallyspecific molecular absorptions akin to those exploited in NIRS. Nanosecond laser pulses irradiate tissue, the energy absorbed by the tissue is converted into sound and the ultrasound wave is detected by a highly sensitive ultrasound transducer. The acquired signal is a depth-resolved intensity map of the vessel tissue that is correlated to the optical absorption properties of the tissue [27].
The potential benefit of intravascular photoacoustic over NIRS is that the photoacoustic signal is both depth-resolved and chemically specific, allowing for coronary images to provide a depth-measured location of the compositional information such as lipid [27]. Several catheter prototypes have been developed to demonstrate the feasibility of intravascular imaging of lipid [28]. However, commercialization of photoacoustic technology is still in its early phases. Some of the drawbacks that hinder this technology involve the high cost and custom nature of instrumentation, especially the laser sources necessary to generate a spectroscopic photoacoustic image in vivo.
Molecular imaging
Molecular imaging enables the visualization of cellular and molecular functions in vivo [29,30]. This emerging technology is envisioned to allow early detection of presymptomatic states when disease is still in progress or to elucidate subtle biochemical processes not currently detectable as bulk structural or compositional manifestations. The term ‘molecular imaging’ is a rapidly developing field in which NIRS and other technologies can be modified to identify a broad range of individual molecular constituents; but the general use of the term involves designing a specific biomarker for a disease target or pathway in order to increase contrast within an area of interest. Important to note is that the biochemical sensitivity and specificity to a certain disease characteristic is determined by the mechanism of action of the contrast agent, while the in vivo detection is usually a nonspecific imaging parameter (fluorescence band, increase in imaging density from uptake of dense nanoparticles, etc.). While this technology is still in its early research stages, recent advances have been made for detecting various stages and components of atherosclerosis, including adhesion molecules, such as VCAM-1 [31], macrophages [32], matrix metalloproteases [33] and angiogenesis factors. Development of various forms of molecular imaging as viable routine methods is complex, since it involves not only all of the challenges common to instrumental in vivo imaging methods but also the challenges more akin to approval of a pharmaceutical treatment such as toxicity, delivery dose, residence time and clearance.
Clinical use
Technology demonstration/validation
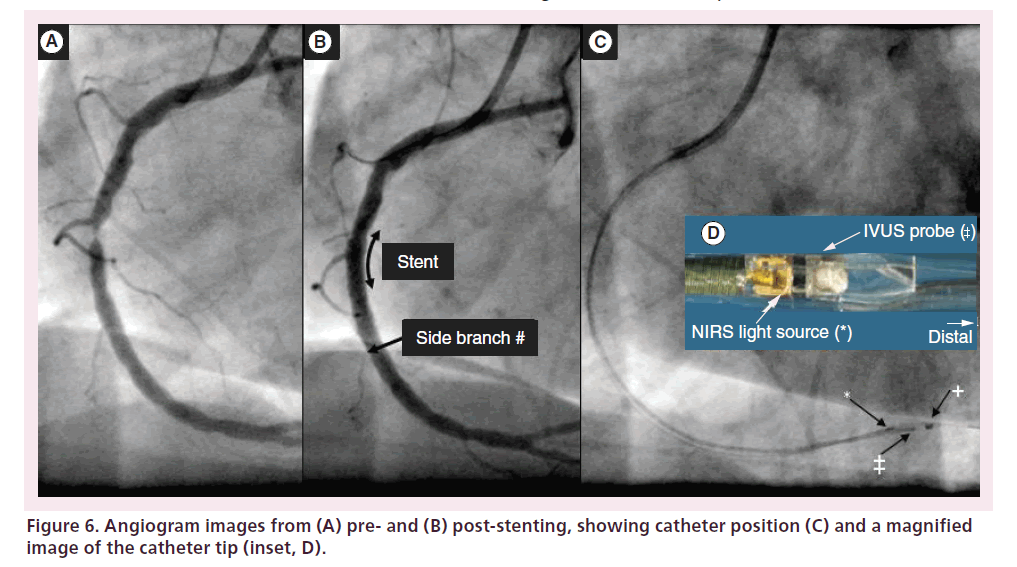
The NIRS system was clinically validated and approved by the FDA in 2008; an IVUS capability was added to the system and approved by the FDA in 2010. This combined NIRS-IVUS catheter was first tested in ten patients by investigators at the Thoraxcenter in Rotterdam in the SAVOIR trial (Figure 6) [34]. The complementary nature of the NIRS compositional and IVUS structural information collected in this trial allowed much more complete characterization of coronary plaques than had previously been possible. For example, NIRS may confirm LCP presence where high plaque burden and hypoechoic regions are found by IVUS.
Figure 6. Angiogram images from (A) pre- and (B) post-stenting, showing catheter position (C) and a magnified image of the catheter tip (inset, D).
Figure 5 shows data acquired with a prototype version of the NIRS-IVUS instrument. The prototype user interface shown here reveals a chemogram and block chemogram (Figure 5A & B) with chemogram information overlaid on coregistered IVUS below (Figure 5C, D & E). Down the center of the longitudinal IVUS view, the block chemogram is redisplayed as a thin line (Figure 5C). And for each IVUS cross section, the chemogram information is overlaid in a halo while the block chemogram color value is portrayed in the central catheter artifact (Figure 5D & E).
Further validation studies have continued to bring this new NIRS-IVUS technology to mainstream interventional cardiology as well [35]. Madder et al. utilized the commercial system establishing practical usage of the multimodality technology in the USA [36]. Furthermore, the new NIRS-IVUS technology has been frequently compared with alternative cardiovascular imaging technologies. Brugaletta et al. tested NIRS-IVUS against virtual histology (VH) and standalone IVUS. While both NIRS and VH-IVUS were independently correlated with larger plaque area by standalone IVUS, there was a poor correlation between the findings of virtual histology, which previously failed a validation test in a porcine autopsy model and NIRS [37].
Improved Characterization of Early Atherosclerosis NIRS has been used to assess the effect of early stages of atherosclerotic disease on the coronary circulation. In vitro and animal studies have shown that early atherosclerosis can produce endothelial dysfunction by limiting the production of vasoactive molecules [38–41]. Choi et al. used NIRS to assess the degree of lipid-rich plaque presence in patients with coronary endothelial dysfunction without significant coronary stenoses [42]. Patients with early coronary artery disease (CAD) (defined as stenosis <30% throughout entire arteries by angiography) underwent coronary endothelial function assessment and NIRS of the proximal left anterior descending artery (LAD). NIRS characteristics were compared between patients with dysfunctional and normal epicardial artery endothelial function. Significant correlation was found between LCBI, LCBI/ length of scanned artery and maxLCBI4mm versus the degree of endothelial dysfunction [42].
NIRS to optimize stenting procedures
While coronary stenting has improved over time, periprocedural myocardial infarction (MI) still occurs in 10–30% of cases. Early users of intracoronary NIRS observed an increase in peri-stenting MI in cases in which an LCP of high angular extent was present at a stenotic site requiring balloon dilation. Balloon inflation at the LCP site resulted in angiographic no-reflow and associated periprocedural MI [43,44]. This finding is in agreement with studies using other imaging technologies showing that dilation of a stenotic plaque that was lipid-rich was more likely to cause a periprocedural infarct.
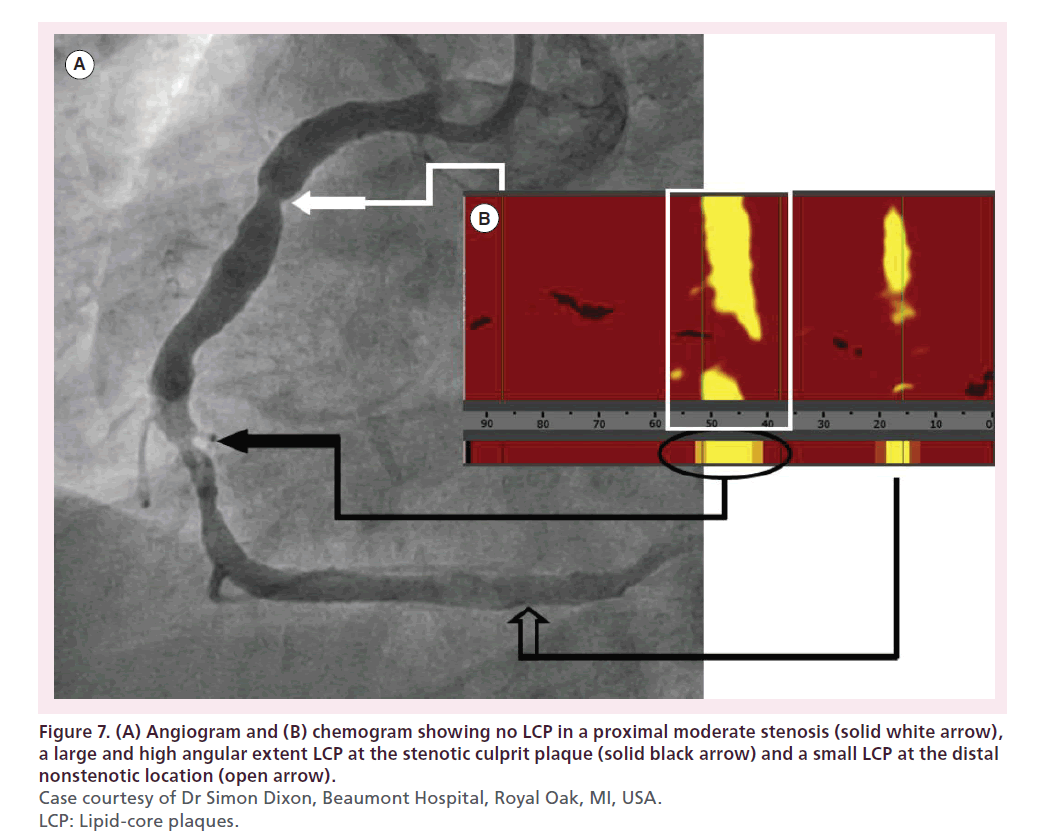
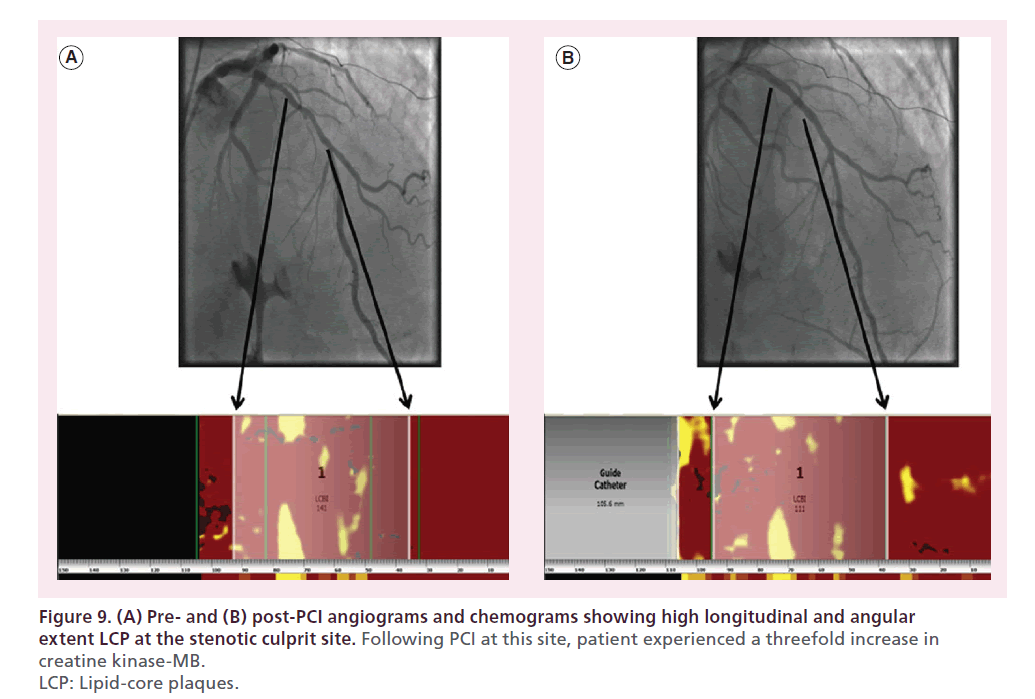
Figure 7 shows the angiogram and chemogram from a patient in whom NIRS indicated a large LCP at the stenotic culprit plaque. Balloon angioplasty at this site led to no-reflow associated with severe bradyarrhythmia and profound hypotension (Figure 8). Following cardiopulmonary resuscitation (CPR), pharmacological support and stenting, the patient’s rhythm, blood pressure and angiographic outcome improved; however, the patient experienced a periprocedural MI and required 1 day of additional hospital care [43,45–46]. Figure 9 shows a similar case in which percutaneous coronary intervention (PCI) of a large LCP resulted in no-reflow and a threefold increase in creatine kinase- MB [47].
Figure 7. (A) Angiogram and (B) chemogram showing no LCP in a proximal moderate stenosis (solid white arrow), a large and high angular extent LCP at the stenotic culprit plaque (solid black arrow) and a small LCP at the distal nonstenotic location (open arrow). Case courtesy of Dr Simon Dixon, Beaumont Hospital, Royal Oak, MI, USA. LCP: Lipid-core plaques.
In the COLOR registry, among 62 cases with CAD in whom adequate enzyme measurements were available to detect a periprocedural MI, nine MIs were observed (Figure 10). A max LCBI4mm > 500 identified a 12-fold relative risk of periprocedural MI [48]. The ability of NIRS to provide an easy-to-use, accurate predictor of the likelihood of peri-stenting MI is welldocumented and at present is the greatest contribution of NIRS imaging to optimization of PCI.
Figure 10. Representative chemograms from 62 stable angina patients. Patients experiencing a periprocedural myocardial infarction (MI) are in the leftmost column.
The ability to predict periprocedural MI led to a study of use of a distal protection filter which was tested in the CANARY Study. The trial was halted early due to difficulty in identifying an adequate number of patients with anatomy suitable for the trial and apparent ineffectiveness of the treatment, but the predictive ability of the LCBI for periprocedural infarction was confirmed [48].
NIRS has been used to assist in decisions regarding the length of artery to be stented [45]. Identifying lesion length by NIRS may be important, as LCP has been shown to extend beyond the margins of the angiographic stenosis in 16% of cases [49]. The identification of LCP located outside the margins of the angiographic stenosis may be clinically relevant, as autopsy studies have documented cases in which the termination of a stent in an LCP was associated with stent thrombosis [50]. NIRS makes it possible to avoid placement of the ends of a stent in an LCP by accurately and precisely revealing the location of LCPs. By imaging the target lesion prior to stent placement, the selected stent length can be altered to ensure placement of the stent edges into segments of vessel free of LCP [51]. While intuitive and supported by autopsy studies, long-term studies are required to determine whether NIRS-informed stent length decisions result in better clinical outcomes.
In addition to using NIRS-IVUS to determine optimal stent length, investigations are underway to determine whether detection of LCP presence near vessel bifurcations can facilitate prevention of side-branch compromise during PCI procedures. When a stent is placed near a bifurcation, plaque redistribution or ‘plaque-shift’ can occur across the carina of a bifurcation and compromise the side branch. While compromise of a side branch can be limited with placement of a wire in the side branch, the maneuver adds complexity and may not be needed when side branch occlusion is unlikely due to absence of lipid in the area being dilated.
There is emerging evidence that LCP presence may lead to different results in bare metal versus drug-eluting (DES) stent placement. Joner et al. reported that DES strut penetration into a necrotic core was associated with late stent thrombosis [52]. Angioscopy studies by Oyabu et al. revealed that placement of a DES over yellow (presumed lipid-core) plaque resulted in a greater frequency of thrombosis than that observed when the DES was located over a non-yellow (presumed fibrous) plaque [53]. Farb et al. showed increased acute inflammation when stent struts penetrated an LCP [54]. Lipid presence may also affect DES drug distribution and absorption, strut contact with the vessel wall and the relative difference in endothelialization between bare metal stent and DES [55].
Large LCP, as determined by NIRS, has also been associated with intrastent thrombus as detected by OCT. In this study, 33% of patients with large LCP developed periprocedural MI. Additionally, two out of three (66%) patients with a large LCP developed intrastent thrombus after stent implantation compared with none in patients without large LCP [56].
NIRS for assessment of treatment of LCP
The IBIS-3 (Integrated Biomarker and Imaging Study) trial examined the effect of intensive rosuvastatin therapy on LCP status of nonintervened coronary plaques, as measured by NIRS. A baseline chemogram was compared with a 1 year follow-up NIRS chemogram to assess treatment-induced changes in LCP. The intense statin therapy, which does not eliminate all future events in clinical outcomes trials, had relatively little effect on the lipid deposition as measured by NIRS [57]. The YELLOW trial sought to determine the impact of short-term, intensive statin therapy compared with standard lower dose statin therapy, on the lipid content of coronary plaques. In this trial, 87 patients were randomized to intensive or standard dose statin therapy. Unfortunately, there was a pretreatment imbalance in LCP frequency between the treatment groups, with more LCP in the group assigned to receive intensive statin therapy. After 7 weeks, a significant reduction was observed in LCBI within the intensive versus standard treatment group [58]. Further work in this area to identify the effect of the use of statins and other agents to treat high-risk plaques is ongoing, with increased patient numbers and longer follow-up periods [59].
As noted below, there is also evidence that NIRS imaging can identify vulnerable patients [60,61]. This permits identification of very high-risk groups in whom trials of new pharmacological agents can be conducted with conventional major adverse cardiovascular event (MACE) end points, and small sample sizes.
Association of plaque properties with ongoing events
NIRS-IVUS has been used to investigate culprit plaque properties in patients with an ongoing ST-segment elevation myocardial infarction (STEMI). It has been found that the STEMI culprit lesions have a highly specific strong yellow signal by NIRS and large plaque burden by IVUS indicating the presence of a large lipid core [9,60]. The IBIS-3 data cited above indicate that the yellow NIRS signals are relatively stable over a year of observation. Hence, the yellow signals observed at the STEMI culprit sites were likely to be present a year or more prior to the event, and could, therefore, serve as a target for preventive treatment.
Some prior studies utilizing IVUS-based virtual histology have described a ‘dynamic nature’ of atherosclerosis, with plaque properties coming and going on a short timescale. However in this study, the major transition was to and from thin and thick-capped fibroatheroma, and not between fibroatheroma and no fibroatheroms. Serial studies in animals and autopsy evidence indicate that atherosclerotic plaques take decades to form. Given the high reproducibility of NIRS imaging [61], the high likelihood of plaque properties being slow-developing processes [62], and the notion that an acute trigger of plaque progression/rupture is the nidus for thrombus formation [63], the finding of an NIRSIVUS signature at STEMI culprit sites detected after the event suggests that such a signature may be useful for the prospective detection of vulnerable plaque.
NIRS-IVUS imaging for the detection of vulnerable plaques
Multiple studies have documented that large plaque burden, as detected by IVUS is a sign of plaque vulnerability. The largest study to demonstrate that sites with a large plaque burden by IVUS are vulnerable was PROSPECT I (Providing Regional Observations to Study Predictors of Events in the Coronary Tree). It was found that nonculprit, nonstenotic sites with plaque burden >70% (as measured by IVUS cross section) were five-times more likely to cause a future coronary event in a 3-year follow-up period than sites without a large plaque burden [64]. While this was a significant finding, it did not change medical practice because it had low specificity. It is expected that with combined NIRS-IVUS imaging, the detection of LCP by NIRS will add to the predictive value of plaque burden detected by IVUS alone.
A new study entitled PROSPECT II has now been initiated in Scandinavia [65]. It differs from PROSPECT I in two features: PROSPECT II will utilize NIRS in addition to IVUS imaging, and it will include a substudy of treatment of the vulnerable plaques identified by PROSPECT I criteria [63]. A subset of patients with nonflow limiting lesions with a plaque burden by IVUS of >70% will be randomized to treatment with a bioresorbable vascular scaffold. A post-randomization stratification will be performed to determine the plaques rich in lipid as detected by NIRS. It is expected that the greatest benefit of the scaffold will be detected in this lipid-rich subset.
A second study testing the prediction of coronary events by NIRS-IVUS imaging is the Lipid-Rich Plaque (LRP) Study [66]. The LRP Study is a large, multicenter, prospective study of 2000 patients with stable ischemic heart disease or stabilized acute coronary syndrome (ACS) undergoing PCI. The patients undergo routine therapy for their index event. NIRS-IVUS scanning is then performed in two or more arteries to characterize the types of plaques present in the coronary circulation.
Patients are then followed over a period of 2 years to determine the occurrence of new coronary events. The location of the new culprit site will be determined by follow-up angiography and compared with the baseline NIRS findings. The study will test the ability of multivessel scanning by NIRS in nonculprit segments to predict coronary events caused by a new culprit lesion [64]. As of May 2015, over 1100 patients had been enrolled in LRP in 44 hospitals. It is expected that results of the LRP study will be available in late 2015.
ORACLE-NIRS (Lipid core plaque association with clinical events) is a trial of similar design as the LRP Study to be conducted in the VA healthcare system [67].
NIRS-IVUS imaging for the detection of vulnerable patients
Two studies indicate that NIRS detection of LCP is helpful in identifying vulnerable patients, a different goal than detection of vulnerable plaque [61]. Madder et al., performed NIRS-IVUS imaging to identify large lipid-core plaques, defined as maxLCBI4mm ≥ 500 in the segment of the culprit artery that was not stented [60]. Patients with and without large lipid cores were followed for subsequent secondary coronary and cerebrovascular events over 2 years. Secondary events occurred in 58.3% of patients with maxLCBI4mm ≥ 500 compared with 6.4% of those with maxLCBI4mm < 500.
A study in the Netherlands investigated NIRS identification of vulnerable patients by imaging a nonculprit artery. The ATHEROREMO-NIRS substudy was conducted in patients undergoing PCI for stable angina or an ACS. An NIRS scan approximately 60 mm in length was obtained in anon-culprit and nonstenotic artery. Patients were assessed for incidence of a second event defined as all-cause mortality, nonfatal ACS, stroke or unplanned coronary revascularization over a 1 year period caused by a new culprit lesion. Patients with a high LCBI, defined as LCBI ≥ median (LCB1 = 43), had a fourfold increase of developing a major adverse cardiac and cerebrovascular event within the year post imaging [61].
As noted above, the successful identification by NIRS of very high-risk group of patients will be most helpful for the design of studies to evaluate novel pharmacologic and antithrombotic agents.
Conclusion
The association between LCP and coronary events has been known for decades. However, traditional coronary imaging techniques have a limited ability to provide accurate, easily obtainable information regarding the presence of LCP. NIRS is a reliable method for detecting LCP in the coronary arteries, and the technology has been rigorously validated against histology specifically for this purpose. Early NIRS studies have demonstrated an association between NIRS-detected LCP and the occurrence of angiographic no-reflow and associated periprocedural MI. Additional studies have suggested a role for NIRS imaging in identifying vulnerable patients at increased risk of patient-level events. While NIRS provides compositional information about the coronary arteries, its combination with IVUS, which provides structural, morphological information, provides added value. Long-term, largescale prospective studies are in progress to assess the ability of combined NIRS-IVUS imaging to detect vulnerable plaque and vulnerable patients.
Future perspective
Coronary intravascular imaging is a broad field with several competing technologies pursuing the goal of detecting plaque vulnerability. Among these techniques, NIRS-IVUS imaging provides a unique set of information regarding plaque vulnerability, and is under intensive study for the ability to predict second coronary events in patients already undergoing PCI. If vulnerable plaques can be detected, there are many treatments that are likely to be effective such as stents, drug coated balloons, scaffolds and novel pharmacologic agents.
Once successful treatment of vulnerable plaques is proven in patients post PCI, it will be important to study the use of NIRS-IVUS imaging as part of a multistep primary prevention strategy. As the accuracy and prevalence of noninvasive diagnostics continue to increase, and as biomarker accuracy improves, noninvasively obtained vulnerable plaque predictors could justify a diagnostic catheterization before the first coronary event, and NIRS-IVUS could be used as an invasive confirmatory step.
Encouraging data from other competing imaging technologies exist showing the potential of directly or indirectly detecting various components of coronary plaques. While only intracoronary NIRS imaging has an FDA label claim specific to the detection of LCP, the lack of prospective clinical data for predicting future events by NIRS currently prevents its widespread adoption in contemporary practice. As clinical trials, such as the Lipid-Rich Plaque study and PROSPECT ABSORB, report out in the next few years, their results may drive the technology and its clinical usage forward. There is also the possibility that the potential of NIRSIVUS to predict coronary events can be increased by the identification of even more plaque features associated with risk of rupture and thrombosis. For instance, LCP is not always associated with a thin cap. Large plaques that are associated with a thick cap may in fact be stable and at low risk of rupture. Since NIRS is not depthresolved, the current state of the technology does not allow differentiation between thin and thick capped fibroatheromas. It may be possible to utilize NIRS imaging to identify the collagen content of plaque caps, and identify plaques with a cap low in collagen, and therefore weak and prone to rupture. Greater integration of the IVUS and NIRS could also be used in the future to combine the compositional measurement of NIRS with the structural information of IVUS to provide a risk assessment of plaque vulnerability.
Financial & competing interests disclosure
JL Su, SJ Grainger, CA Greiner, SP Madden, ST Sum and JE Muller are employees or contractors of Infraredx, Inc. RD Madder has received research support from Infraredx, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
• Vulnerable plaques are a subset of coronary plaques thought to have a high risk of rupture and resultant thrombosis. Plaques having a large plaque burden, expansive remodeling, inflammation from macrophage activity, a large necrotic lipid core and a thin/weak cap are suspected to be vulnerable plaques.
• Plaque composition is an important feature of coronary pathology that is distinct from plaque structure. However, traditional coronary imaging techniques have been limited in their ability to provide accurate information regarding the presence and quantity of lipid.
Intravascular near-infrared spectroscopy
• Provides a positive and specific chemical measure of the lipid-core plaque (LCP) of interest.
• Intravascular NIRS is the only technology that is validated with a specific label claim for the detection of lipidcore plaque.
Clinical benefits
• The complementary combined near-infrared spectroscopy (NIRS) and intravascular ultrasound (IVUS) imaging allows for more complete characterization of coronary plaques.
• Using NIRS to properly place stent edges outside of an LCP region may reduce the risk of stent thrombosis, but requires confirmation in a prospective study.
• Patients with a high max LCBI4mm were found to have a higher risk of periprocedural myocardial infarction.
Prospective prediction of vulnerable plaques
• PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) II-ABSORB, The Lipid-Rich Plaque Study and ORACLE-NIRS are large clinical trials initiated to prospectively study the association of large lipid-core plaques detected by NIRS with future site-specific coronary events.
• The PROSPECT ABSORB arm of PROSPECT II will test the possibility of treating currently nonflow limiting plaques showing signs of vulnerability using a bioresorbable vascular scaffold.
Summary
• Detection of lipid-core plaques is important for assessing the vulnerability of plaques to rapid progression or rupture. Large lipid-core plaques can be detected through the use of combined NIRS-IVUS imaging.
• Chemogram and lipid-core burden index results can detect vulnerable patients and are under study for the detection of vulnerable plaques.
• Studies are underway to test the hypothesis that NIRS-IVUS imaging can identify both vulnerable patients and vulnerable plaques.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20(5), 1262–1275 (2000).
- Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 47(8, Suppl. C), C13–C18 (2006).
- Ambrose JA, Tannenbaum MA, Alexopoulos D et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J. Am. Coll. Cardiol. 12(1), 56–62 (1988).
- Naghavi M, Libby P, Falk E et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 108(14), 1664–1672 (2003).
- Mark H, Workman J. Chemometrics in Spectroscopy. Academic Press, Elsevier, NY, USA (2007).
- Gardner CM, Tan H, Hull EL et al. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. JACC Cardiovasc. Imaging 1(5), 638–648 (2008).
- Caplan JD, Waxman S, Nesto RW, Muller JE. Near-infrared spectroscopy for the detection of vulnerable coronary artery plaques. J. Am. Coll. Cardiol. 47(8, Suppl. 1), C92–C96 (2006).
- US Food and Drug Administration. Coronary artery plaque imaging device cleared by FDA (2008). www.fda. gov/newsevents/newsroom/pressannouncements/2008/ ucm116888.htm.
- Erlinge D, Harnek J, Gonçalves I, Götberg M, Muller JE, Madder RD. Coronary liposuction during percutaneous coronary intervention: evidence by near-infrared spectroscopy that aspiration reduces culprit lesion lipid content prior to stent placement. Eur. Heart J. Cardiovasc. Imaging 16(3), 316–324 (2014).
- Waxman S, Dixon SR, L’Allier P et al. In vivo validation of a catheter-based near-infrared spectroscopy system for detection of lipid core coronary plaques: initial results of the SPECTACL study. J. Am. Coll. Cardiol. Cardiovasc. Imaging 2(7), 858–868 (2009).
- Clinical trials database: NCT00330928. http://clinicaltrials. gov/ct2/show/NCT00330928
- Jang I-K, Bouma BE, Kang D-H et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J. Am. Coll. Cardiol. 39(4), 604–609 (2002).
- Brezinski ME. Optical coherence tomography for identifying unstable coronary plaque. Int. J. Cardiol. 107(2), 154–165 (2006).
- Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary optical coherence tomography: a comprehensive review. JACC Cardiovasc. Interv. 2(11), 1035–1046 (2009).
- Prati F, Regar E, Mintz GS et al. Expert’s OCT Review Document. Expert review document on methodology, terminology, clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 31(4), 401–415 (2010).
- Yun S. H., Tearney G. J., Vakoc B. J. et al. Comprehensive volumetric optical microscopy in vivo. Nat. Med. 12(12), 1429–1433 (2007).
- Yabushita H, Bouma BE, Houser SL et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 106(13), 1640–1645 (2002).
- Yonetsu T, Suh W, Abtahian F et al. Comparison of near-infrared spectroscopy and optical coherence tomography for detection of lipid. Catheter Cardiovasc. Interv. 84(5), 710–717 (2014).
- Fard AM, Vacas-Jacques P, Hamidi E et al. Optical coherence tomography – near infrared spectroscopy system and catheter for intravascular imaging. Opt. Express 21(25), 30849–30858 (2013).
- Uchida Y, Nakamura F, Tomaru T et al. Prediction of acute coronary syndromes by percutaneous coronary angioscopy in patients with stable angina. Am. Heart J. 130(2), 195–203 (1995).
- Ohtani T, Ueda Y, Mizote I et al. Number of yellow plaques detected in a coronary artery is associated with future risk of acute coronary syndrome: detection of vulnerable patients by angioscopy. J. Am. Coll. Cardiol. 47(11), 2194–2200 (2006).
- Ishibashi F, Aziz K, Abela GS, Waxman S. Update on coronary angioscopy: review of a 20 year experience and potential application for detection of vulnerable plaque. J. Intervent. Cardiol. 19(1), 17–25 (2006).
- Ueda Y, Matsuo K, Nishimoto Y et al. Detection of angioscopic yellow plaque by intracoronary near-infrared spectroscopy. JACC Cardiovasc. Interv. 7(6), e49–e50 (2014).
- Uchida Y, Uchida Y, Kawai S et al. Detection of vulnerable coronary plaques by color fluorescent angioscopy. JACC Cardiovasc. Imaging 3(4), 398–408 (2010).
- Schaar JA, Mastik F, Regar E et al. Current diagnostic modalities for vulnerable plaque detection. Curr. Pharm. Des. 13(10), 995–1001 (2007).
- Van de Poll SWE, Römer TJ, Puppels GJ, Van der Laarse A. Imaging of atherosclerosis. Raman spectroscopy of atherosclerosis. J. Cardiovasc. Risk 9(5), 255–261 (2002).
- Sethuraman S, Amirian JH, Litovsky SH, Smalling RW, Emelianov SY. Spectroscopic intravascular photoacoustic imaging to differentiate atherosclerotic plaques. Opt. Express 16(5), 3362 (2008).
- Jansen K, Springeling G, Lancee C et al. An intravascular photoacoustic imaging catheter. IEEE Ultrasonics Symposium (IUS) 378–381 (2010).
- Libby P, Nahrendorf M, Weissleder R. Molecular imaging of atherosclerosis. Tex. Heart Inst. J. 37(3), 324–327 (2010).
- Osborn EA, Jaffer FA. The advancing clinical impact of molecular imaging in CVD. JACC Cardiovasc. Imaging 6(12), 1327–1341 (2013).
- Nahrendorf M, Keliher E, Panizzi P et al. 18F-4V for PET–CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc. Imaging 2(10), 1213–1222 (2009).
- Nahrendorf M, Zhang H, Hembrador S et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation 117(3), 379–387 (2008).
- Deguchi J, Aikawa M, Tung C-H et al. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation 114(1), 55–62 (2006).
- Clinical trials database: NCT00901446. http://clinicaltrials. gov/ct2/show/NCT00901446
- Clinical trials database: NCT02154295. http://clinicaltrials. gov/ct2/show/NCT02154295
- Madder RD, Abbas AE, Safian RD. First-in-man use of intravascular near-infrared spectroscopy in the carotid arteries to characterize atherosclerotic plaque prior to carotid stenting. JACC Cardiovasc. Interv. 8(2), e29–e31 (2015).
- Brugaletta S, Garcia-Garcia HM, Serruys PW et al. NIRS and IVUS for characterization of atherosclerosis in patients undergoing coronary angiography. JACC Cardiovasc. Imaging 4(6), 647–655 (2011).
- Qiang L, Tsuchiya K, Kim-Muller J-Y, Lin HV, Welch C, Accili D. Increased atherosclerosis and endothelial dysfunction in mice bearing constitutively deacetylated alleles of Foxo1 gene. J. Biol. Chem. 287(17), 13944–13951 (2012).
- Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 84(4), 1381–1478 (2004).
- De Caterina R, Libby P, Peng HB et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Invest. 96(1), 60–68 (1995).
- Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 109(23, Suppl. 1), III27–III32 (2004).
- Choi B-J, Prasad A, Gulati R et al. Coronary endothelial dysfunction in patients with early coronary artery disease is associated with the increase in intravascular lipid core plaque. Eur. Heart J. 34(27), 2047–2054 (2013).
- Goldstein JA, Grines C, Fischell T et al. Coronary embolization following balloon dilation of lipid-core plaques. JACC Cardiovasc. Imaging 2(12), 1420–1424 (2009).
- Schultz C, Serruys PW, van der Ent M et al. Prospective identification of a large lipid-core plaque with a novel near-infrared spectroscopy and intravascular ultrasound (NIR-IVUS) catheter: infarction following stenting possibly due to distal embolization of plaque contents. JACCdoi: 10.1016/j. jacc.2009.10.090 (2010).
- Dixon S. Detecting vulnerable plaque. Cath. Lab. Digest 17(3), (2009). www.cathlabdigest.com/articles/Detecting-Vulnerable-Plaque, accessed 4/15/2015
- Naghavi M. Preventive cardiology: the SHAPE of the future. A synopsis from the Screening for Heart Attack Prevention and Education (SHAPE) task force report. Herz 32(5), 356–361 (2007).
- Raghunathan D, Abdel-Karim A-RR, Papayannis AC et al. Relation between the presence and extent ofcoronary lipid core plaques detected by near-infrared spectroscopy with postpercutaneous coronary intervention myocardial infarction. Am. J. Cardiol. 107(11), 1613–1618 (2011).
- Goldstein JA, Maini B, Dixon SR et al. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ. Cardiovasc. Interv. 4(5), 429–437 (2011).
- Dixon SR, Grines CL, Munir A et al. Analysis of target lesion length before coronary artery stenting using angiography and near-infrared spectroscopy versus angiography alone. Am. J. Cardiol. 109(1), 60–66 (2012).
- Liu X, Tsujita K, Maehara A et al. Intravascular ultrasound assessment of the incidence and predictors of edge dissections after drug-eluting stent implantation. JACC Cardiovasc. Interv. 2(10), 997–1004 (2009).
- Marshik B, Tan H, Tang J, Lindquist A, Zuluaga A. Detection of thin-capped fibroatheromas in human aorta tissue with near infrared spectroscopy through blood. J. Am. Coll. Cardiol. 41(6, Suppl. 1), 42 (2003).
- Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193–202 (2006).
- Oyabu J, Ueda Y, Ogasawara N, Okada K, Hirayama A, Kodama K. Angioscopic evaluation of neointima coverage: sirolimus drug-eluting stent versus bare metal stent. Am. Heart J. 152(6), 1168–1174 (2006).
- Farb A, Sangiorgi G, Carter AJ et al. Pathology of acute and chronic coronary stenting in humans. Circulation 99(1), 44–52 (1999).
- Finn AV, Nakazawa G, Joner M et al. Vascular responses to drug eluting stents. Arterioscler. Thromb. Vasc. Biol. 27(7), 1500–1510 (2007).
- Papayannis AC, Abdel-Karim A-RR, Mahmood A et al. Association of coronary lipid core plaque with intrastent thrombus formation: a near-infrared spectroscopy and optical coherence tomography study. Catheter. Cardiovasc. Interv. 81(3), 488–493 (2013).
- Simsek C, Garcia-Garcia HM, Van Geuns R-J et al. Integrated Biomarker and Imaging Study-3 investigators. The ability of high dose rosuvastatin to improve plaque composition in non-intervened coronary arteries: rationale and design of the Integrated Biomarker and Imaging Study-3 (IBIS-3). EuroIntervention 8(2), 235–241 (2012).
- Kini AS, Moreno P, Kovacic J et al. Does aggressive statin therapy reduce coronary atherosclerotic plaque lipid content? Results from: Reduction in Yellow Plaque by Aggressive Lipid Lowering Therapy (yellow) Trial. J. Am. Coll. Cardiol. 59(13, Suppl. S), E304 (2012).
- Clinical trials database: NCT01837823. http://clinicaltrials. gov/ct2/show/NCT01837823
- Madder RD, Husaini M, Davis AT et al. TCT-398 identification of vulnerable patients by intracoronary near-infrared spectroscopy. J. Am. Coll. Cardiol. DOI: 10.1016/j. jacc.2014.07.447 (2014) (Epub ahead of print).
- Oemrawsingh RM, Cheng JM, García-García HM et al. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J. Am. Coll. Cardiol. 64(23), 2510–2518 (2014).
- Virmani R, Narula J, Leon MB, Willerson JT. The Vulnerable Atherosclerotic Plaque: Strategies for Diagnosis and Management (1st edition). Wiley-Blackwell, MA, USA (2006).
- Falk E. Why do plaques rupture? Circulation 86(Suppl. 6), III30–III42 (1992).
- Stone GW, Maehara A, Lansky AJ et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 364(3), 226–235 (2011).
- Clinical trials database: NCT02171065. http://clinicaltrials. gov/ct2/show/NCT02171065
- Clinical trials database: NCT02033694. http://clinicaltrials. gov/ct2/show/NCT02033694
- Clinical trials database: NCT02265146. http://clinicaltrials. gov/ct2/show/NCT02265146
- Stone G. Coronary Assessment by Near-infrared of Atherosclerotic Rupture-prone Yellow (CANARY Trial). TCT (2014).
•• This paper details the design and results of the rigorous prospective study for development of the near-infrared spectroscopy algorithm for detecting lipid-core plaques, which resulted in the first, still only, label claim for lipidcore plaque detection.
• Longitudinal study is the first to demonstrate a correlation between near-infrared spectroscopy imaging and future adverse cardiovascular events.