Review Article - Interventional Cardiology (2010) Volume 2, Issue 1
Different patent foramen ovale closure techniques in varying anatomies
- Corresponding Author:
- Ryan Ko
Adult Congenital Heart Disease Unit, Royal Brompton Hospital, London, SW3 6NP, UK
Tel: +44 207 351 8600
Fax: +44 207 351 8513
E-mail: r.ko@rbht.nhs.uk
Abstract
Keywords
Amplatzer®, anatomy, atrial septal aneurysm, long tunnel, morphology, patent foramen ovale, PFO closure, PFO closure device, Star flex®
A patent foramen ovale (PFO) can be found in up to a quarter of the general population [1]. In recent years, this remnant of the fetal circulatory system has been implicated in a number of pathological states, including cryptogenic strokes, transient ischemic attacks, migraine with auras, decompression sickness and severe refractory hypoxemia [2–5].
Developments in medical engineering over the past decade have made percutaneous transcatheter closure of PFO a feasible and safe treatment option. In experienced centers, this is a very low-risk procedure that can be carried out with a short procedural time [6]. Because of this favorable risk–benefit ratio, catheterization laboratories worldwide have seen an increase in the number of transcatheter PFO closures being performed [7].
Numerous devices are now available with a high procedural success rate and reported closure rates of up to 94% at 1 year [8,9]. Manufacturers continue to improve their devices in order to simplify the technical requirements and allow wider adoption. However, successful closure of PFO depends on an understanding of the variability of the anatomy of PFO and surrounding structures, as well as selecting the appropriate device and technique of closure.
PFO anatomy
An in-depth knowledge on the embryological development of the atrial septum is necessary in order to better understand the varying PFO anatomy pertinent to successful device closure [10]. A PFO is a persistent inter-atrial communication that resembles a trapdoor; the thin and compliant septum primum is analogous to the door closing against the foramen ovale on the septum secundum. A PFO is anatomically differentiated from a secundum atrial septal defect (ASD) by a complete coverage of the foramen ovale [11], which effectively separates the two atriums under normal physiological conditions. Right-to-left shunting can therefore only occur when right atrial pressure exceeds left atrial pressure, as in the release phase of a valsalva maneuver.
The entrance of a PFO from the right atrium is an oblique, slit-like defect on the anterior– superior margin of the foramen ovale. This opens into a curvilinear tunnel-like gap, walled between the septum primum on the left side and the septum secundum on the right. Finally exiting into the left atrium via the remnant of the foramen secundum in close proximity to the anterior atrial wall and the aortic root. This entry and exit pattern of the PFO tunnel is fitting to the fetal circulation in utero where oxygenated inferior vena cava blood is streamed directly into the left atrium. Based on this topography, variations in PFO anatomy can arise from different degrees of overlapping of the foramen ovale, as well as the mobility of the septum primum.
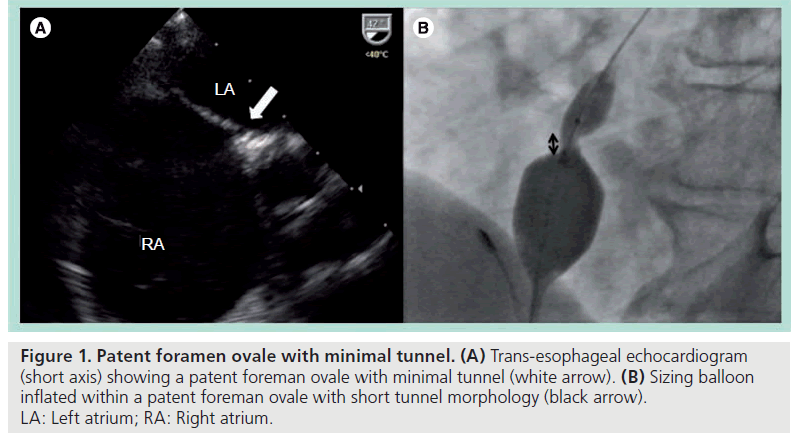
In patients, a number of variations of this complex anatomy may be found: first, PFO with minimal tunnels. This is due to minimal overlapping by the septum primum, analogous to a better-fitted trapdoor against the doorframe. The openings on either side of the atrium are therefore geometrically closer together (Figure 1).
Figure 1: Patent foramen ovale with minimal tunnel. (A) Trans-esophageal echocardiogram (short axis) showing a patent foreman ovale with minimal tunnel (white arrow). (B) Sizing balloon inflated within a patent foreman ovale with short tunnel morphology (black arrow). LA: Left atrium; RA: Right atrium.
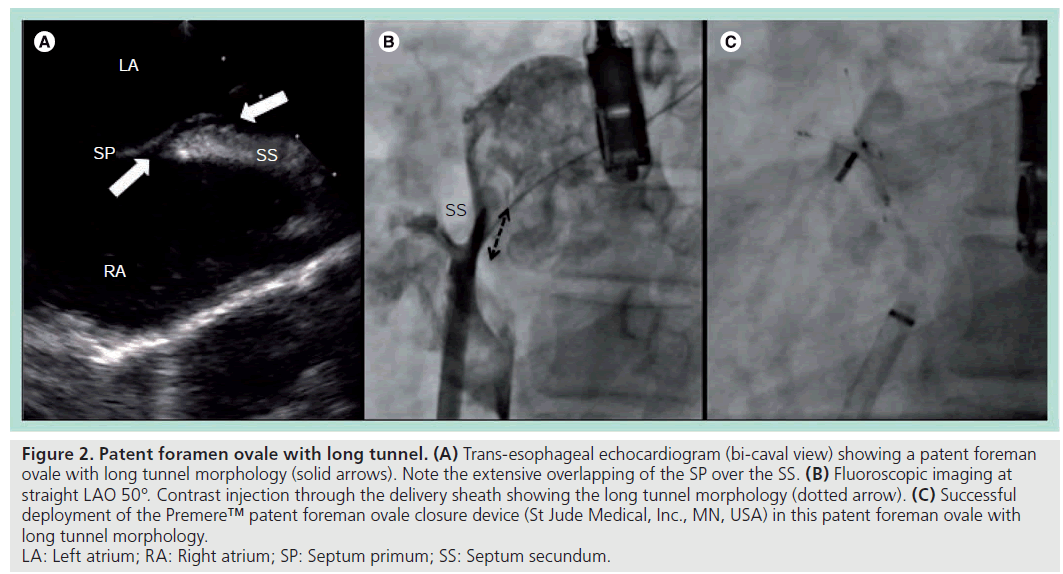
Second, PFO with long tunnels. This is due to extensive overlapping by the septum primum, giving rise to a long tunnel-like gap between the septum secundum and septum primum. The entrance and exit of this type of PFO are therefore further away from each other (Figure 2).
Figure 2: Patent foramen ovale with long tunnel. (A) Trans-esophageal echocardiogram (bi-caval view) showing a patent foreman
ovale with long tunnel morphology (solid arrows). Note the extensive overlapping of the SP over the SS. (B) Fluoroscopic imaging at
straight LAO 50°. Contrast injection through the delivery sheath showing the long tunnel morphology (dotted arrow). (C) Successful
deployment of the Premere™ patent foreman ovale closure device (St Jude Medical, Inc., MN, USA) in this patent foreman ovale with
long tunnel morphology.
LA: Left atrium; RA: Right atrium; SP: Septum primum; SS: Septum secundum.
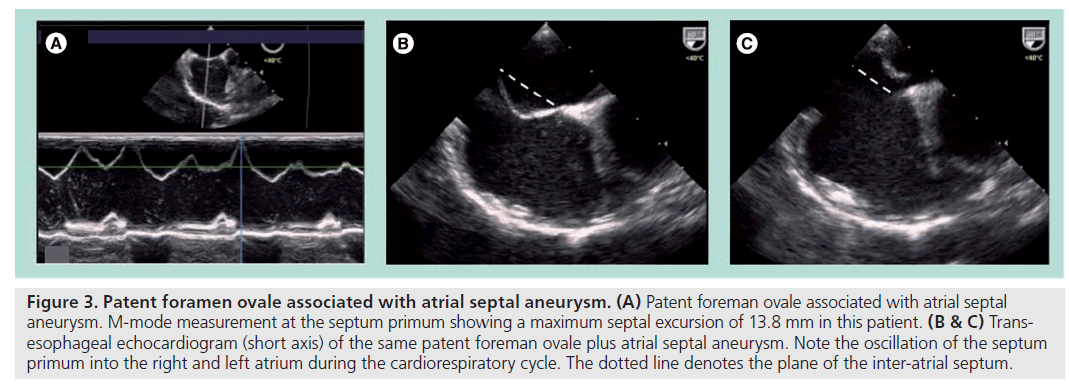
Finally, PFO associated with atrial septal aneurysm (ASA). This is due to a redundant and highly mobile septum primum that has been defined in different echocardiographic studies as a septal excursion of at least 10 mm during the cardiorespiratory cycle [12,13]. ASA can also exist alone or in association with single or multifenestrated secundum ASDs (Figure 3).
Figure 3: Patent foramen ovale associated with atrial septal aneurysm. (A) Patent foreman ovale associated with atrial septal aneurysm. M-mode measurement at the septum primum showing a maximum septal excursion of 13.8 mm in this patient. (B & C) Transesophageal echocardiogram (short axis) of the same patent foreman ovale plus atrial septal aneurysm. Note the oscillation of the septum primum into the right and left atrium during the cardiorespiratory cycle. The dotted line denotes the plane of the inter-atrial septum.
It is also possible for PFOs to coexist with multiple small secundum ASDs. These multi-fenestrated PFOs represent a distinctive entity from an interventional perspective, as they require closure of both the PFO and the ASD to effectively prevent residual shunts.
PFO closure devices
The objective of any PFO closure device is to hold the septum primum against the septum secundum; effectively keeping the trapdoor shut. Secondary closure then occurs when the device is fully endothelialized and becomes incorporated within the atrial septum; sealing off any residual shunts. Commonly used PFO closure devices predominantly consist of a double disc design with a connecting waist and rely on appositional forces between the two atrial discs to effect closure. Examples are the Amplatzer® PFO occluder (AGA Medical, MN, USA), the CardioSEAL® (NMT Medical Inc., MA, USA), the Premere™ device (St Jude Medical, Inc., MN, USA) and the Gore Hele x® Septal occluder (WL Gore and Associates, AZ, USA). The newer generation STARflex® and BioSTAR® devices (NMT Medical Inc., MA, USA), as well as the Solysafe® septal occluder (Swissimplant AG, Switzerland) have self-centering features in their design, which may help in seating of the device during deployment. The general implantation techniques are similar across different devices and will not be further described in this article.
A number of studies focusing on individual devices have been published, each reporting their own procedural success and closure rates [8,14,15]. However, there is not yet a direct head-to-head comparison between different devices with reference to specific anatomical subtypes [16]. With operators’ own experience and preference aside, it is likely that different devices will work equally well if they are correctly placed and matched to their best-suited PFO anatomy. This reiterates the need to appreciate individual patient’s anatomical variations and to tailor each device closure strategy based on these findings.
By contrast, selecting an appropriate device size is often based on operators’ intuition. Various methods using sizing balloon or echo-cardiographic measurements have been proposed to standardize the device size selection process, but none have been widely accepted [17]. In some centers, a single ‘work-horse’ size and device is used successfully for almost all PFO closures, removing the sizing issue altogether [18].
Despite the lack of a standardized protocol on device size selection, it is well recognized that inappropriate device size will result in both early and late complications. Deployment of an oversized device can lead to poor final positioning and encroachment on the aortic root. This can lead to wall erosion and cardiac perforation as reported in [19]. An undersized device increases the risk of early device embolization as well as recurrent neurological events from inadequate PFO closure and persistent residual shunts [20].
In our practice, a compliant sizing balloon together with periprocedural echocardiographic guidance (either trans-esophageal or intracardiac) is used in every patient during PFO device closure. Although slightly more laborious, this arrangement offers several advantages. First, the sizing balloon allows for a fluoroscopic estimation of the length of the tunnel as well as a roadmap for the subsequent intervention. Second, the combination of a sizing balloon and an echocardiogram allows an assessment on the size of the PFO and the septal anatomy. Finally, intraprocedural echocardiographic monitoring can help avoid trapping surrounding structures such as prominent Eustachian valves or Chiari networks, and help to assess the degree of anchorage in difficult cases, such as with large atrial septal aneurysm and the early detection of periprocedural complications [21].
PFO with minimal tunnel
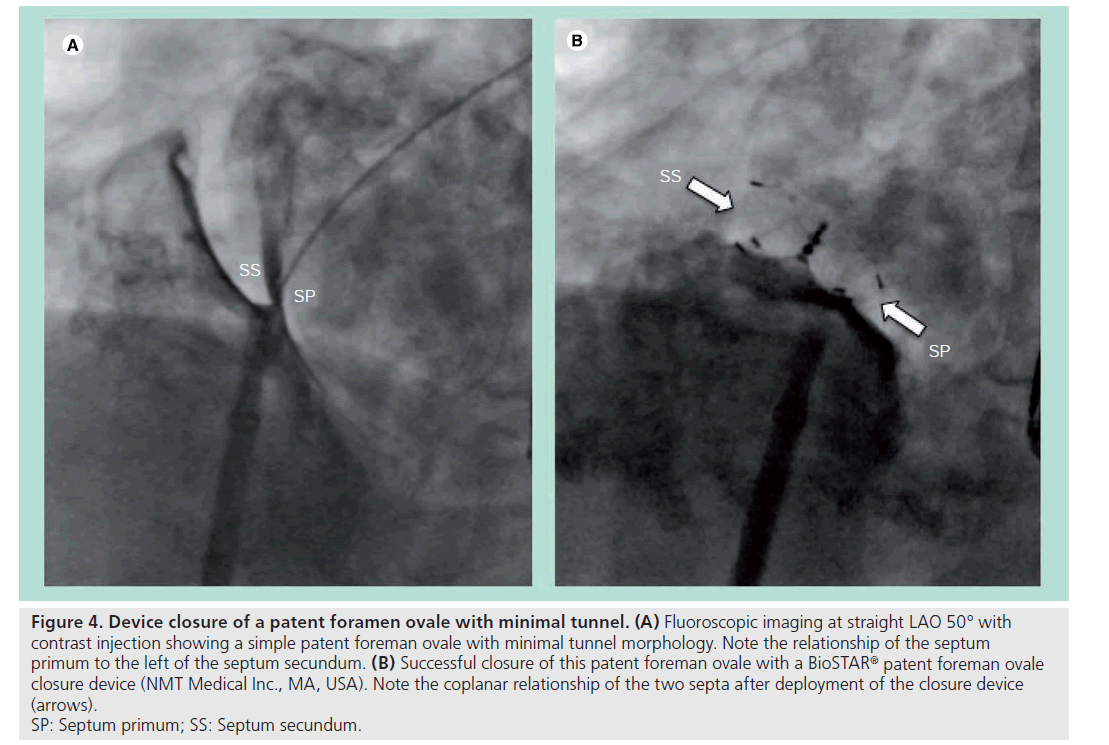
This is the most favorable type of PFO anatomy from an interventional perspective for two reasons. First, minimal overlapping of the septum primum over the foramen ovale means only a short tunnel connects the entrance and exit of the PFO. Second, the compliant nature of the thin septum primum allows it to be retracted posterior–inferiorly by closure devices during deployment. The combination of these two anatomical features in this type of PFO means radial forces generated from the closure device itself can effectively modify and abolish the short tunnel morphology to resemble the anatomy of an ASD, where the two septa become coplanar and the two atrial discs of the device can be deployed in parallel [22]. This is also why devices originally designed for secundum ASD closure have worked adequately in this situation and are widely used for treating this type of PFO closure (Figure 4).
Figure 4: Device closure of a patent foramen ovale with minimal tunnel. (A) Fluoroscopic imaging at straight LAO 50° with
contrast injection showing a simple patent foreman ovale with minimal tunnel morphology. Note the relationship of the septum
primum to the left of the septum secundum. (B) Successful closure of this patent foreman ovale with a BioSTAR® patent foreman ovale
closure device (NMT Medical Inc., MA, USA). Note the coplanar relationship of the two septa after deployment of the closure device
(arrows).
SP: Septum primum; SS: Septum secundum.
The key technique when using these standard, double-disc ASD-type devices is to maintain continuous tension on the delivery cable during the deployment procedure. This can be done once the left atrial disc is deployed and engaged onto the atrial septum. Keeping tension on the delivery cable will then cause the thin septum primum to retract back into the same plane as the septum secundum. Under this coplanar configuration, the right atrial disc can then easily be deployed in parallel to the left atrial disc, thereby achieving a well-conformed final device position. The connecting waist of the device will then be surrounded by the rigid muscular rim of the foramen ovale anterior–superiorly, and engulfed by the slightly retracted septum primum tissue in the posterior–inferior margin. Endothelialization and healing of the exposed surfaces over time will then allow complete sealing of the shunt.
PFO with long tunnel
This is the most challenging type of PFO anatomy from an interventional perspective because it sits outside the comfort zone of traditional ASD closure devices. Although the septum primum is still thin and compliant, the extensive overlapping of the foramen ovale means there are more septum primum tissues to be retracted before commonly used closure devices can be deployed. This cannot be done simply by the radial force of the closure device as described previously in PFO with minimal tunnel. Therefore, without specific techniques, there is a potential for both discs of the device to be partially deployed within the long tunnel (Figure 5). This will result in a poorly conformed final device position; leading to delayed or incomplete endothelialization and, ultimately, residual shunts.
Figure 5: Deployment of a BioST AR® patent foreman ovale closure device (NMT Medical
Inc., MA, USA) within a long tunnel. Note the right atrial disc of the device is malpositioned
within the tunnel. This will likely inhibit endothelialization and prevent complete secondary closure of
the patent foramen ovale.
SP: Septum primum; SS: Septum secundum.
There are three main ways to manage this type of PFO in order to achieve better device positioning.
▪ Tunnel modification
This involves pretreating and modifying the long tunnel morphology and is especially useful when using devices such as the CardioSEAL and STARFlex. It takes advantage of the compliant nature of the septum primum by retracting it back in the posterior–inferior direction, and effectively shortens the tunnel. But because of the large amount of excess primum septum tissue involved, an additional balloon is needed to pretreat the septum primum prior to the delivery of the standard closure device. This can be done using the sizing balloon with gentle and staged dilatation within the tunnel, as described in the detunnelization technique [23]. This is a safe, simple and controlled method that has been shown to be highly successful in a small series of patients. This method also allows for an interim assessment on the compliance and the degree of recoil of the septum primum following balloon dilatation. In cases where detunnelization procedure is suboptimal, the operator can then decide on an alternative technique.
A similar method of long tunnel modification has also been reported using the balloon pull-through technique [24]. The author suggests the use of a 2 ml balloon wedge catheter to pull the septum primum into the right atrium, thereby shortening the PFO tunnel. The major disadvantage of this technique is the lack of control over the modification procedure. As opposed to the detunnelization technique, this does not give the operator a visual-pressure feedback response to avert potential damage to the septum primum or secundum. It also does not allow for an interim assessment for the operators to change closure strategy in cases of a suboptimal result.
▪ Tunnel avoidance
If the basic concept of effective PFO device closure requires the septum primum to be held against the septum secundum, the long PFO tunnel can be completely avoided with the transseptal puncture technique [25]. This technique requires a trans-septal puncture of the septum primum and deployment of a slightly larger standard closure device through the iatrogenic hole. Because the puncture is in close proximity to the PFO entrance, the two discs deployed on either side of the atrial septum will hold the septum primum against the septum secundum and effect closure of the PFO tunnel. Provided the trans-septal puncture can be made at a good position under echocardiographic guidance, this technique will allow good device conformation by keeping the inter-atrial septum in its natural undisturbed position.
However, the major disadvantage that has prevented its widespread acceptance is the risk of trans-septal puncture itself. Although small and controlled, the trans-septal puncture causes a deliberate trauma to the septum primum. The known risk associated with a trans-septal puncture alters the risk–benefit ratio of a standard PFO device closure procedure. This is especially important when alternative low-risk techniques are available.
Furthermore, the potential benefit of transseptal technique over standard in-tunnel device deployment was not established in a case series of patients with PFO and long tunnels. The transseptal technique was successfully performed in 12 patients without complications (CardioSEAL septal occluder in ten patients and CardiaStar PFO occluder in two patients). However, the 6‑month closure rate was lower than for a similar consecutive group with standard in-tunnel device deployment (n = 108) [26]. This further adds to the unfavorable risk–benefit ratio associated with this technique.
▪ PFO-specific devices
A device with an adjustable distance between the left and right atrial disc as with the Premere device is theoretically ideal for this type of PFO anatomy. As opposed to devices with distensible connector necks, the Premere device allows an adjustable connector without affecting the original shape of each atrial disc (Figure 2C). This allows a well-conformed device through a PFO tunnel, thereby achieving PFO closure with minimal disturbance of the natural atrial septal anatomy. However, the need to adjust the distance between the two atrial discs before release can invite errors and can potentially increase the rate of residual shunts with unfamiliar operators.
In the clinical setting, PFO closure with the Premere device has been shown to be feasible and safe with a high procedural success rate [27]. The PFO closure rate reported at 6 months for a group of 67 patients was 86%; however, it should be noted that only 13 patients (19%) had tunnel tracks of more than 5 mm in length. While this is a very promising device, still more data is required on its clinical performance with respect to PFO with long tunnel morphology.
PFO associated with ASA
Depending on the definition, ASA can be found in up to 1–2% of the general population. However, the prevalence of ASA ranges from 7.9 to 15% in patients with embolic stroke, and a PFO can be found in combination with an ASA in up to 45–84% of these patients [28–31]. The combination of PFO and ASA (PFO + ASA) is an important entity as it was shown in a series of retrospective studies to be highly associated with cryptogenic stroke in young patients (less than 55 years of age) [32]. It has also been prospectively demonstrated to significantly increase the risk of recurrent strokes [31]. The mechanism is unclear and might simply reflect patients with large PFO and a greater propensity to paradoxical shunt, although the association between ASA and stroke in the absence of a PFO might suggest an independent mechanism. Possibilities include thrombus formation within the aneurysmal tissue or an association with wider left atrial dysfunction stimulating a chronic atrial fibrillation pathophysiology [33].
Relatively little has been published on the interventional aspects of PFO + ASA. Among the few studies available, percutaneous closure for this type of PFO was shown to be feasible and safe with various devices [34]. In particular, a large European series of patients (n = 144) with PFO + ASA and presumed paradoxical emboli have shown effective treatment by different closure devices [35]. Compared with a case-controlled group of patients with PFO alone (n = 220), it reported no significant differences between their procedural successes, complication rates and residual shunts at 6 months. There was also no difference in recurrent event-free survival at 4 years, which was reported at 95% for the PFO + ASA group. Two additional analyses from this study are worth noting. First, device closure of the PFO also significantly reduced ASA excursion from 16 ± 5 mm to 4 ± 3 mm in the group of patients with PFO + ASA, thereby eliminating the ASA component for this type of PFO. Second, regression analysis showed that presence of residual shunts was the only predictor of recurrent events in patients with PFO + ASA.
We believe that when managing PFO + ASA, operators should focus on closing the PFO and not on stabilizing the aneurysm. This will directly reduce residual shunts and therefore should translate into reduced recurrent embolic events. We often find by achieving PFO closure with a well-conformed device that the septal excursion is also reduced. The above study has suggested there is no need to deliberately oversize the closure device as there was no difference in the complication rates or residual shunts between different device sizes. Furthermore, there is currently no evidence to suggest that reducing septal excursion alone in PFO + ASA reduces recurrent events, reiterating that it is most important to keep the ‘trapdoor’ shut.
Multifenestrated PFO
This is anatomically more complicated and consists of both a PFO and multiple small ASD. In this context, the complete coverage of the ovale fossa as well as sealing off the PFO is necessary to prevent residual shunts. There are two interventional strategies for this type of PFO: one option is to deploy a single, large, double-disc device covering both defects. This should be deployed through the ASD to allow better device conformation; conceptually similar to that described with the trans-septal technique. With the same comparison, the major difficulty with this strategy is to ensure coverage of both defects and prevent residual shunts.
A second option is to deploy individual devices through the PFO and ASD separately. This is technically more demanding and the increased amount of device material on the inter-atrial septum raises concerns. However, the development of novel ‘device-less’ PFO closure technologies can reduce the amount of foreign material left behind in the body and can make this option more acceptable.
Conclusion
Percutaneous PFO device closure is becoming a permanent part of our interventional work. Therefore, it is important for interventional cardiologists and medical inventors alike to have a thorough understanding of the varying PFO anatomy as well as the relevant pathophysiology if we are to be agile and creative in our closure techniques. It is unlikely that a single ‘ideal device’ will emerge that is suitable for all types of PFO anatomy. More realistically, our future armamentarium will consist of a few anatomy-specific closure technologies that are minimalistic or device-less in nature.
Given the currently available evidence, skepticism on the need for PFO closure has definitely limited the speed of growth and acceptance of this field of interventional cardiology. Hopefully, results from a number of prospective, randomized, controlled trials in the near future [36] will clarify this issue and make the current playing field even more exciting.
Future perspective
In the past decade, increased awareness of the pathophysiological consequence of PFO has led to rapid developments in closure device technologies. However one thing has remained constant with these technologies; a device is always left behind in the body. Irrespective of an effective shunt closure, the device in situ forms a nidus for potential problems and this is now increasingly recognized and addressed [37,38].
Theoretically, an ideal device should mimic the natural PFO closure process by facilitating the apposition and fusion of the septum primum to the septum secundum. There should be very little foreign material left behind in the body and it should be effective, easy to use and adaptable to different PFO anatomies.
An initial approach is the development of bioabsorbable devices. An example in this category is the BioSTAR device, which is now commercially available in Canada and Europe. Building on the STARFlex platform, the original polyester material scaffold forming the two atrial discs has been replaced with a purified acellular collagen matrix. This scaffold is reabsorbed over time and replaced with native tissue through natural healing processes, leaving only the metal frame attached to the inter-atrial septum. There is a very high procedural success rate with this device and effective PFO closures are seen in up to 96% of the treated patients at 6 months [39]. Development of a totally absorbable device is currently under development [40].
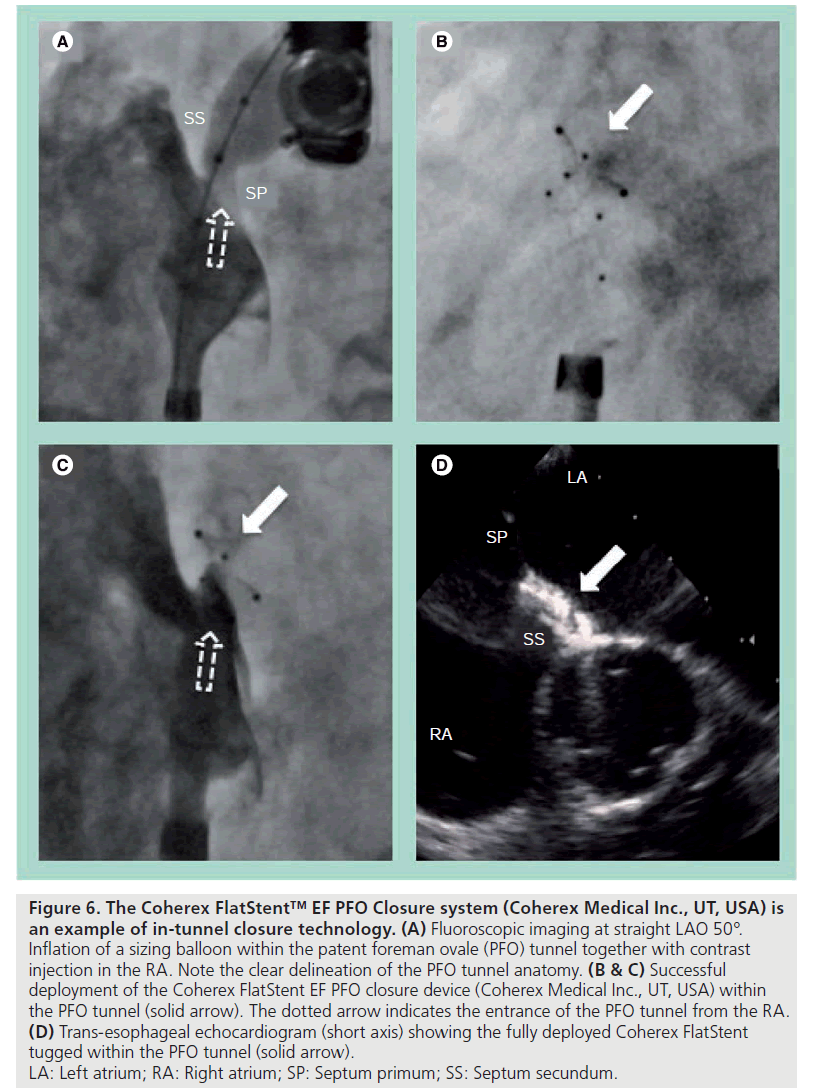
In-tunnel closure technology is another attractive option, where the septum primum is held against the septum secundum by a device that only resides within the tunnel. This technology moves away from the double atrial disc design and minimizes the disturbance to the inter-atrial septal anatomy. This also means very little of the device is exposed into the atrium. The Coherex FlatStent™ EF PFO Closure system (Coherex Medical Inc., UT, USA) is an example of this technology. It consists of a flat, self-expanding Nitinol wire frame that is stabilized within the PFO tunnel by a pair of right and left atrial anchors (Figure 6). It is delivered over a monorail system and can be easily resheathed and repositioned without losing the wire position across the PFO. Primary closure is achieved by the expanded in-tunnel stent holding the two septa together, while secondary closure occurs with tissue growth into the PFO tunnel. This later stage is facilitated by the polyurethane foam attached to the in-tunnel portion of the device. The initial experiences were promising and the device is now commercially available in Europe.
Figure 6: The Coherex FlatStent™ EF PFO Closure system (Coherex Medical Inc., UT, USA) is
an example of in-tunnel closure technology. (A) Fluoroscopic imaging at straight LAO 50°.
Inflation of a sizing balloon within the patent foreman ovale (PFO) tunnel together with contrast
injection in the RA. Note the clear delineation of the PFO tunnel anatomy. (B & C) Successful
deployment of the Coherex FlatStent EF PFO closure device (Coherex Medical Inc., UT, USA) within
the PFO tunnel (solid arrow). The dotted arrow indicates the entrance of the PFO tunnel from the RA. (D) Trans-esophageal echocardiogram (short axis) showing the fully deployed Coherex FlatStent
tugged within the PFO tunnel (solid arrow).
LA: Left atrium; RA: Right atrium; SP: Septum primum; SS: Septum secundum.
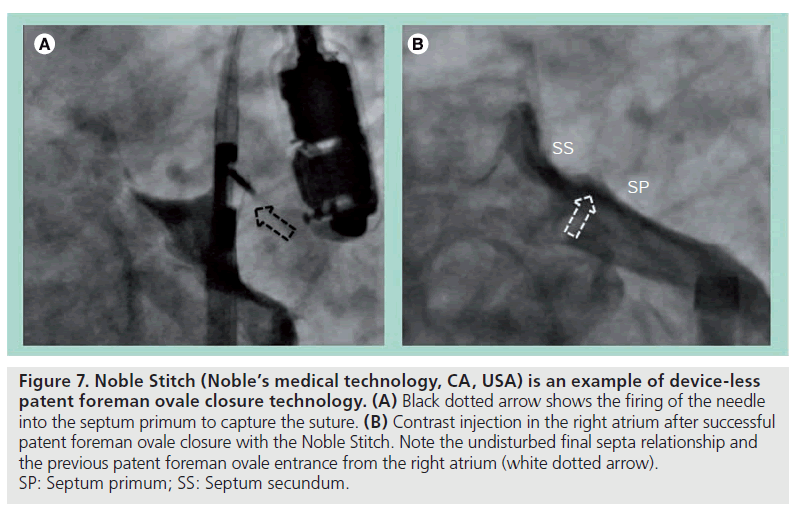
Even more conceptually appealing are the ‘device-less’ closure technologies. One such device currently under investigation is the Noble Stitch (Noble’s medical technology, CA, USA), which makes use of the transcatheter suture technology adapted from vascular access suturing devices. It involves engaging and puncturing each septum separately, where a suture is captured by the needle and pulled through the septum. Completing the suture in a figure-of-eight fashion with a polypropylene suture knot under tension will approximate the septum primum to the septum secundum, and therefore achieve PFO closure (Figure 7). This has been shown to be technically feasible [41] and has been successfully performed in a small group of PFO patients in our center.
Figure 7: Noble Stitch (Noble’s medical technology, CA, USA) is an example of device-less patent foreman ovale closure technology. (A) Black dotted arrow shows the firing of the needle into the septum primum to capture the suture. (B) Contrast injection in the right atrium after successful patent foreman ovale closure with the Noble Stitch. Note the undisturbed final septa relationship and the previous patent foreman ovale entrance from the right atrium (white dotted arrow). SP: Septum primum; SS: Septum secundum.
Another interesting ‘device-less’ development involves the use of radiofrequency to weld the septum primum and secundum together. The PFx™ Closure System was recently studied in a large multicenter series of 144 patients, where feasibility was demonstrated with an initial procedural success of up to 90% and an average procedural time of 47 min [42]. The average closure rate was reported as 55% at 6 months, although a more acceptable result was seen in the small PFO (stretched diameter <4 mm) subgroup, where the closure rate was reported to be 89%. Clearly, this technology will need further modifications and for the time being have been abandoned.
In the next decade, the demand for simple and minimalistic PFO closure technologies will continue. Further refinements on some of the current novel designs will hopefully make it effective and robust enough to take up a major share of the market in the next few years.
Executive summary
▪ Patent foreman ovale (PFO) is a remnant of the fetal circulatory system and can be found in up to a quarter of the general population.
▪ The entrance of a PFO from the right atrium is on the anterior superior margin of the foramen ovale. This leads into a tunnel-like gap between the septum primum and septum secundum before exiting into the left atrium in close proximity to the anterior atrial wall.
▪ There is significant variability with PFO anatomy, but they can be broadly classified into PFO with minimal tunnel, PFO with long tunnel and PFO associated with atrial septal aneurysm (ASA).
▪ Different percutenaous PFO closure devices have different merits and are likely to work equally well if placed in their best-suited anatomy.
▪ Periprocedural echocardiographic guidance allows a more thorough assessment on the size of the PFO and its associated septal anatomy. It can also help assess the degree of device anchorage in difficult cases and can help in the early detection of complications.
▪ PFO with minimal tunnel is the most favorable anatomy for device closure, because the compliant septum primum can be easily retracted posterior–inferiorly. The end result is a well-conformed device.
▪ PFO with long tunnel is the most challenging type of anatomy for device closure. There are three main strategies: tunnel modification, tunnel avoidance and PFO-specific devices.
▪ Tunnel modification involves shortening the PFO tunnel by taking advantage of the compliant nature of the septum primum. It can be performed either with balloon dilatation or with a balloon pull-through method.
▪ Tunnel avoidance involves creating a hole in the inter-atrial septum with a transeptal puncture. The PFO closure device is then deployed in the newly created hole, thus avoiding the long tunnel morphology. The two atrial discs of the device will hold the septum primum against the septum secundum to effect PFO closure.
▪ PFO-specific devices have an adjustable distance between the two atrial discs. This allows the device to remain well conformed despite the long PFO tunnel.
▪ With PFO plus ASA, operators should focus on closing the PFO and not on stabilizing the ASA. There is no need to deliberately oversize the closure device to reduce septal excursion.
▪ A huge amount of resources are pushing PFO closure technologies in a minimalistic direction. An ideal device should facilitate apposition and fusion of the septum primum and septum secundum. There should be minimal foreign material left behind in the body.
▪ Indications for PFO closure remain unclear. Hopefully, results from a number of randomized trials will provide much needed answers.
Financial & competing interests disclosure
Michael J Mullen has recieved a grant from NMT Medical and consultancy from Sutura Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Hagen PT, Scholz DG, Edwards WD:Incidence and size of patent foramen ovaleduring the first 10 decades of life: an autopsystudy of 965 normal hearts. Mayo Clin. Proc.59, 17–20 (1984).
- Mas JL, Arquizan C, Lamy C et al.:Recurrent cerebrovascular events associatedwith patent foramen ovale, atrial septalaneurysm, or both. N. Engl. J. Med. 345(24),1740–1746 (2001).
- Papa M, Gaspardone A, Fracasso G et al.:Usefulness of transcatheter patent foramenovale closure in migraineurs with moderate tolarge right-to-left shunt and instrumentalevidence of cerebrovascular damage. Am. J.Cardiol. 104(3), 434–439 (2009).
- Torti SR, Billinger M, Schwerzmann M et al.:Risk of decompression illness among 230divers in relation to the presence and size ofpatent foramen ovale. Eur. Heart J. 25(12),1014–1020 (2004).
- Godart F, Rey C, Prat A et al.: Atrialright-to-left shunting causing severehypoxaemia despite normal right-sidedpressures. Report of 11 consecutive casescorrected by percutaneous closure.Eur. Heart J. 21(6), 483–489 (2000).
- Meier B: Closure of patent foramenovale: technique, pitfalls, complications,and follow up. Heart 91(4), 444–448(2005).
- Opotowsky AR, Landzberg MJ, Kimmel SEet al.: Trends in the use of percutaneousclosure of patent foramen ovale and atrialseptal defect in adults, 1998–2004. JAMA299(5), 521–522 (2008).
- Braun M, Gliech V, Boscheri A et al.:Transcatheter closure of patent foramenovale (PFO) in patients with paradoxicalembolism. Periprocedural safety andmid-term follow-up results of three differentdevice occluder systems. Eur. Heart J. 25(5),424–430 (2004).
- Martín F, Sánchez PL, Doherty E et al.:Percutaneous transcatheter closure ofpatent foramen ovale in patients withparadoxical embolism. Circulation (9),1121–1126 (2002).
- Hara H, Virmani R, Ladich E et al.:Patent foramen ovale: current pathology,pathophysiology, and clinical status.J. Am. Coll. Cardiol. 46, 1768–1776 (2005).
- Ho SY, McCarthy KP, Rigby ML:Morphological features pertinent tointerventional closure of patent ovalforamen. J. Interv. Cardiol. 16(1), 33–38(2003).
- Belkin RN, Kisslo J: Atrial septal aneurysm:recognition and clinical relevance. Am. HeartJ. 120(4), 948–957 (1990).
- Ghosh S, Ghosh AK, Ghosh SK: Patentforamen ovale and atrial septal aneurysmin cryptogenic stroke. Postgrad. Med.J. 83(977), 173–177 (2007).
- Khositseth A, Cabalka AK, Sweeney JP et al.:Transcatheter Amplatzer device closure ofatrial septal defect and patent foramen ovalein patients with presumed paradoxicalembolism. Mayo Clin. Proc. 79(1), 35–41(2004).
- von Bardeleben RS, Richter C, Otto J et al.:Long term follow up after percutaneous closureof PFO in 357 patients with paradoxicalembolism: difference in occlusion systems andinfluence of atrial septum aneurysm. Int. J.Cardiol. 134(1), 33–41 (2009).
- Taaffe M, Fischer E, Baranowski A et al.:Comparison of three patent foramen ovaleclosure devices in a randomized trial(Amplatzer® versus CardioSEAL®-STARflex®versus Hele x® occluder). Am. J. Cardiol.101(9), 1353–1358 (2008).
- Alibegovic J, Bonvini RF, Sigwart U et al.:The role of the sizing balloon in selection ofthe patent foramen ovale closure device size.Exp. Clin. Cardiol. 13(1), 42–46 (2008).
- Fateh-Moghadam S, Steeg M, Dietz R et al.:Is routine ultrasound guidance reallynecessary for closure of patent foramen ovaleusing the Amplatzer® PFO occluder? CatheterCardiovasc. Interv. 73(3), 361–366 (2009).
- Lange SA, Schoen SP, Braun MU et al.:Perforation of aortic root as secondarycomplication after implantation of patentforamen ovale occlusion device in a31‑year-old woman. J. Interv. Cardiol. 19(2),166–169 (2006).
- Windecker S, Wahl A, Chatterjee T et al.:Percutaneous closure of patent foramen ovalein patients with paradoxical embolism:long-term risk of recurrent thromboembolicevents. Circulation 101(8), 893–898 (2000).
- Tobis J. What is the best way to close a PFO?Catheter Cardiovasc. Interv. 73(3), 361–366(2009).
- Marshall AC, Lock JE: Structural andcompliant anatomy of the patent foramenovale in patients undergoing transcatheterclosure. Am. Heart J. 140(2), 303–307(2000).
- Spence MS, Khan AA, Mullen MJ:Balloon assessment of patent foramen ovalemorphology and the modification of tunnelsusing a balloon detunnelisation technique.Catheter Cardiovasc. Interv. 71(2), 222–228(2008).
- Chintala K, Turner DR, Leaman S et al.:Use of balloon pull-through technique toassist in CardioSEAL® device closure ofpatent foramen ovale. Catheter Cardiovasc.Interv. 60(1), 101–106 (2003).
- McMahon CJ, El Said HG, Mullins CE:Use of the transseptal puncture intranscatheter closure of long tunnel-typepatent foramen ovale. Heart 88(2), E3(2002).
- Tande AJ, Knickelbine T, Chavez I et al.:Transseptal technique of percutaneous PFOclosure results in persistent interatrialshunting. Catheter Cardiovasc Interv. 65(2),295–300 (2005).
- Büscheck F, Sievert H, Kleber F et al.:Patent foramen ovale using the Premeredevice: the results of the CLOSEUP trial.J. Interv. Cardiol. 19(4), 328–333 (2006).
- Agmon Y, Khandheria BK, Meissner I et al.:Frequency of atrial septal aneurysms inpatients with cerebral ischemic events.Circulation 99, 1942–1944 (1999).
- Mügge A, Daniel WG, Angermann C et al.:Atrial septal aneurysm in adult patients. Amulticenter study using transthoracic andtransesophageal echocardiography.Circulation 91(11), 2785–2792 (1995).
- Pearson AC, Nagelhout D, Castello Ret al.: Atrial septal aneurysm and stroke:a transesophageal echocardiographic study.J. Am. Coll. Cardiol. 18(5), 1223–1229(1991).
- Lamy C, Giannesini C, Zuber M et al.:Clinical and imaging findings in cryptogenicstroke patients with and without patentforamen ovale: the PFO-ASA Study. Atrialseptal aneurysm. Stroke 33(3), 706–711(2002).
- Overell JR, Bone I, Lees KR. Interatrialseptal abnormalities and stroke: a meta-analysisof case–control studies. Neurology55(8), 1172–1179 (2000).
- Rigatelli G, Aggio S, Cardaioli P et al.:Left atrial dysfunction in patients withpatent foramen ovale and atrial septalaneurysm: an alternative concurrentmechanism for arterial embolism? JACCCardiovasc. Interv. 2(7), 655–662 (2009).
- Krumsdorf U, Keppeler P, Horvath Ket al.: Catheter closure of atrial septal defectsand patent foramen ovale in patients with anatrial septal aneurysm using differentdevices. J. Interv. Cardiol. 14(1), 49–55(2001).
- Wahl A, Krumsdorf U, Meier B et al.:Transcatheter treatment of atrial septalaneurysm associated with patent foramenovale for prevention of recurrent paradoxicalembolism in high-risk patients. J. Am. Coll.Cardiol. 45, 377–380 (2005).
- O’Gara PT, Messe SR, Tuzcu EM et al.:Percutaneous device closure of patentforamen ovale for secondary strokeprevention: a call for completion ofrandomized clinical trials. A scienceadvisory from the American HeartAssociation/American Stroke Associationand the American College of CardiologyFoundation. J. Am. Coll. Cardiol. 53,2014–2018 (2009).
- Krumsdorf U, Ostermayer S, Billinger Ket al.: Incidence and clinical course ofthrombus formation on atrial septal defectand patient foramen ovale closure devices in1,000 consecutive patients. J. Am. Coll.Cardiol. 43(2), 302–309 (2004).
- Spies C, Khandelwal A, Timmermanns Iet al.: Incidence of atrial fibrillation followingtranscatheter closure of atrial septal defects inadults. Am. J. Cardiol. 102(7), 902–906(2008).
- Mullen MJ, Hildick-Smith D, De Giovanni JVet al.: BioSTAR Evaluation Study (BEST), aprospective, multicenter, phase I clinical trialto evaluate the feasibility, efficacy, and safety ofthe BioSTAR bioabsorbable septal repairimplant for the closure of atrial-level shunts.Circulation 114(18), 1962–1967 (2006).
- Majunke N, Sievert H: ASD/PFO devices:what is in the pipeline? J. Interv. Cardiol.20(6), 517–523 (2007).
- Ruiz CE, Kipshidze N, Chiam PTet al.: Feasibility of patent foramen ovaleclosure with no-device left behind:first-in-man percutaneous suture closure.Catheter Cardiovasc. Interv. 71(7), 921–926(2008).
- Sievert H, Ruygrok P, Salkeld M et al.:Transcatheter closure of patent foramenovale with radiofrequency: acute andintermediate term results in 144 patients.Catheter Cardiovasc. Interv. 73(3), 368–373(2009).
▪ Valuable review article covering all aspects of patent foramen ovale (PFO) closure.
▪▪ Provides a good description on the embryological development of PFO.
▪▪ Extremely valuable article on the anatomy of a PFO.
▪ Valuable article on current development of PFO closure technologies.