Research Article - Diabetes Management (2023) Volume 13, Issue 0
Differential Rescue Effects of Choline Chloride and Soy Isolate on Neuronal Metabolic Dysfunction in Immature Central Nervous System Neurons: Relevance to Fetal Alcohol Spectrum Disorder
- Corresponding Author:
- Suzanne M. de la Monte
Departments of Pathology and Laboratory Medicine, Rhode Island Hospital, Providence, USA
E-mail: Suzanne_DeLaMonte_MD@Brown.edu
Received: 24-Mar-2023, Manuscript No. FMDM-23-92638; Editor assigned: 27-Mar-2023, PreQC No. FMDM-23-92638 (PQ); Reviewed: 12-Apr-2023, QC No. FMDM-23-92638; Revised: 19-Apr-2023, Manuscript No. FMDM-23-92638 (R); Published: 27-Apr-2023, DOI: 10.37532/1758-1907.2023.13(S1).107-118
Abstract
Background: Central Nervous System (CNS) abnormalities with insulin resistance and mediated by developmental exposures to ethanol can be avoided or remediated by consumption of dietary soy, which has insulin-sensitizing as well as antioxidant effects. However, choline supplementation has been shown to diminish Fetal Alcohol Spectrum Disorder (FASD) pathologies, and dietary soy contains abundant choline. This study was designed to determine if the therapeutic effects of soy were mediated by or independent of choline. Methods: Human PNET2 cells exposed to 0 mM or 100 mM ethanol for 48 hours were seeded into 96-well or 12-well plates and treated with vehicle, choline chloride (75 µM), or 1 µM Daidzein+1 µM Genistein (D+G) for 24 h. The cells were then analyzed for viability (Hoechst 33342), mitochondrial function (MTT), and GAPDH, Tau, Acetyl Cholinesterase (AChE), Choline Acetyl Transferase (ChAT), and Aspartyl-Asparaginyl-β-Hydroxylase (ASPH) immunoreactivity. Results: Choline and D+G significantly increased MTT activity (mitochondrial function) corrected for cell number relative to vehicle in control and ethanol-exposed cultures. Both choline and D+G prevented the ethanol-induced inhibition of GAPDH and ChAT and increased cellular accumulations of Tau. However, D+G significantly increased ASPH expression relative to vehicle and Choline. Conclusion: Choline and D+G differentially modulated the expression of neuronal proteins, mitochondrial function, and ASPH. Importantly, the prominently increased expression of ASPH by D+G corresponds with the insulin-sensitizer actions of soy isoflavones since ASPH is an insulin-responsive molecule. The findings further suggest that dietary soy may be more effective than choline for reducing ethanol-impaired neuronal migration linked to ASPH inhibition in FASD.
Keywords
▪ Ethanol ▪ Soy ▪ Choline ▪ Insulin ▪ Mitochondria ▪ Lipids
Introduction
Fetal Alcohol Spectrum Disorder (FASD) is associated with excessive chronic or binge consumption of ethanol during pregnancy [1,2] and is the most preventable etiology of human neurodevelopmental deficits [3,4]. FASD includes a collection of notable pathologies such as increased fetal demise, malformations of the Central Nervous System (CNS), skeletal and craniofacial, impaired intrauterine growth, cognitive and motor deficits, defects and Attention Deficit Hyperactivity Disorder (ADHD) [5-7]. In the USA, FASD broadly afflicts 0.2-1.5 per 1000 live births but can reach 7 or 9/1000, particularly with binge drinking [8,9]. CNS structural pathologies in FASD [10- 13] are characteristically distributed in the corpus callosum, prefrontal region, temporal lobe, and cerebellum [10,14], accounting for performance deficits on cerebellar motor and spatial learning and memory tasks that persist and adversely impact function through adolescence [15,16] and beyond [7,17]. Consequently, the economic and social burdens of FASD are high [18].
The mechanisms of ethanol-mediated neurodevelopmental defects have been parsed through systematic studies of experimental models. In vitro and in vivo experiments have shown that ethanol’s adverse effects on CNS cells and tissues include neurotoxicity, stress oxidative injury, inflammation, and metabolic dysfunction [19-23]. Consequences include impairments in neuronal function and viability tied to compromised insulin and insulin-like growth factor type 1 (IGF-1) signaling through Akt and its downstream pathways [19, 24-31]. Associated reductions in signaling through Insulin Receptor Substrate (IRS) proteins and Akt increase glycogen synthase kinase 3β (GSK- 3β) activation [25,28,32-35], which inhibits neuronal function, metabolism, and survival [24,29,31,36,37]. Consequences include reduced expression of target proteins needed for various neuronal functions [25,38-43].
One such target of ethanol that is also regulated by signaling through insulin/IGF-1 pathways is Aspartyl-asparaginyl-β-Hydroxylase (ASPH) [24,36,44,45]. ASPH is an ~ 86 kD type 2 transmembrane protein that mediates cell adhesion, motility/migration, and growth [24,36,46-51]. ASPH’s effects on cell motility are mediated by Notch pathway activation via its C-terminal catalytic domain [52,53]. Corresponding with its inhibitory effects on insulin/IGF-1 signaling, ethanol reduces ASPH expression and function [24,36,45]. For example, in FASD, ethanol-induced dose-dependent reductions in ASPH correlate with the severity of impaired cerebellar neuron migration [36].
A major goal underlying mechanistic studies of FASD is to identify preventive or harm- reduction therapeutic strategies. Given the importance of insulin/IGF-1 signaling in cerebellar development and the impact that gestational alcohol exposure has on these critical pathways, one potential therapeutic approach for preventing FASD could be to fortify the function of relevant pathways with insulin sensitizers. Previous studies showed that Peroxisome- Proliferator-Activated Receptor (PPAR) agonists, which have both insulin-sensitizing and anti- oxidant effects [54-56] can abrogate the adverse effects of ethanol exposure in the CNS [57] and liver [58-62]. PPAR agonist treatments abrogated neurobehavioral dysfunction and alterations in neuroglial expression of insulin/ IGF-1 regulated brain genes and proteins in a range of experimental models, including those utilizing ethanol exposures [57,63-65]. However, despite encouraging results, the potential for translating PPAR agonist research data to human studies is restricted due to unidentified risks to the maternal-placental-fetal unit. This concern led us to consider an alternative natural insulin- sensitizer and antioxidant food, namely dietary soy. Isoflavones are among the most important constituents of soy isolate protein. Isoflavones support insulin responsiveness and help resolve states of insulin-resistance that lead to disease [66-71].
In recent reports we showed that dietary soy could prevent neurobehavioral dysfunction caused by chronic ethanol feeding in a rat model [15], and that maternal consumption of dietary soy during pregnancy prevented placental and craniofacial phenotypic pathologies in experimental FASD [72]. However, soy isolate contains substantial amounts of choline [67,68] and choline is known to benefit neurocognitive function following developmental exposures to alcohol, both in experimental models [73-75] and humans [76,77]. On the other hand, the main advantage of incorporating soy isolate over choline into the diet is that soy is a natural whole food product that provides healthful protein, which is often needed to correct nutritional deficiencies that accompany inadequate choline as well as other micronutrients intake during pregnancy in socioeconomically challenged environments. Despite these considerations, we were left with the goal of determining whether the therapeutic effects of soy were due to or the result of those of choline’s inclusion in the diet as a constituent of soy isolate. This study formally compares the therapeutic effects of dietary soy with those of choline in PNET2 human CNS-derived cerebellar neuronal cells that have been used to investigate neurotoxic and metabolic effects of ethanol [26,49,78]. The short-term experimental design compared the degrees to which the soy isolate bioactive constituents, Daidzein and Genistein, or choline chloride could support control and ethanol-exposed immature neuronal viability, function, and ASPH expression.
Materials and Methods
▪ Materials
Invitrogen (Carlsbad, CA, USA) was the source of Dulbecco’s Modified Eagle Medium (DMEM), Amplex UltraRed, and 4-methylumbelliferyl phosphate (4-MUP). Vector Laboratories (Burlingame, CA, USA) was the source of Alkaline Phosphatase Conjugated to Streptavidin. Table 1 lists the supplements for cell culture and Table 2 lists the antibodies used and their sources. All other fine chemicals were purchased from either Sigma-Aldrich (St. Louis, MO, USA) or CalBiochem (Carlsbad, CA, USA).
| Compound | Source | Catalog # | M.W. | Solvent | Concentration |
|---|---|---|---|---|---|
| Choline Chloride | Fisher Scientific | AC1102950 | 139.62 | DMSO | 75 µM |
| Daidzein (>98%)* | Sigma-Aldrich | D7802 | 254.2 | DMSO | 1 µM |
| Genistein (>95%)** | Sigma-Aldrich | G0897 | 432.4 | DMSO | 1 µM |
Note: *Daidzein was a >98% pure synthetic molecule. **Genistein was from Glycine max (soybean) and determined to be >95% pure by HPLC. Vehicle control cultures were treated with DMSO
Table 1: Choline chloride and Soy Isolate Constituents Used for Culture Supplementation.
| Antibody | Source | Company | Type | Concentration/Dilution | RRID# |
|---|---|---|---|---|---|
| Glucose-6-Phosphate Dehydrogenase (GAPDH) | Mouse | Santa Cruz, Dallas, TX | Monoclonal | 0.2 µg/ml | AB_10847862 |
| Tau | Rabbit | Agilent/Dako, Santa Clara, CA | Polyclonal | 6.2 µg/ml | AB_10013724 |
| Choline Acetyltransferase (ChAT) | Rabbit | Abcam, Waltham, MA | Polyclonal | 2.125 | AB_2244866 |
| Acetylcholinesterase (AChE) | Mouse | Abcam, Waltham, MA | Monoclonal | 0.25 µg/ml | AB_303316 |
| Aspartyl-asparaginyl β-hydroxylase (ASPH); A85E6 and A85G6 | Mouse | 21st Century Biochemicals, Marlborough, MA | Monoclonal | 1.4305556 | -1 |
| Large acidic ribosomal protein (RPLPO) | Mouse | Santa Cruz, Dallas TX | Monoclonal | 0.1 µg/ml | (2-4) |
Table 2: Antibodies used for duplex ELISA studies.
▪ Cell culture
Human PNET2 primitive neuroectodermal tumor cerebellar neuronal cells were cultured in DMEM containing 10% heat-inactivated 10% Fetal Bovine Serum (FBS), 4.5 g/L glucose, and 4 mM L-glutamine in a standard 5% CO2 cell culture incubator (37°C). For the ethanol exposure model, sub-confluent cultures, freshly seeded in 75 cm2 flasks, were treated with 0 mM or 100 mM ethanol for 48 hours, and then after re-seeding into 96-well (1.4 × 104 viable cells/ well) or 12-well plates (1.35 × 105 viable cells/ well), the cultures were treated for 24 h with vehicle (DMSO), 75 μM Choline Chloride (CC), or 1 μM each of Daidzein and Genistein (D+G). Cell viability was assessed by Trypan blue exclusion. Cultures were analyzed for viability, mitochondrial function, and protein expression.
▪ Protein extraction
The 12-well cultures were harvested in 5.0 volumes of buffer that contained 50 mM Tris- HCl, pH 7.5, 150 mM NaCl, 50 mM NaF, 5 mM EDTA, pH 8.0, 0.1% Triton X-100, phosphatase inhibitor (10 mM Na3VO4), and protease inhibitors (1 mM PMSF, 0.1 mM TPCK, 2 μg/ml aprotinin, 2 μg/ml pepstatin A, 1 μg/ml leupeptin). The supernatants obtained by centrifuging the culture homogenates at 14000 rpm for 15 min at 4°C were used to measure immunoreactivity by Enzyme-Linked Immuno Sorbent Assay (ELISA). The Bicinchoninic Acid (BCA) assay was used to measure protein concentration.
Duplex ELISAs were used to quantify immunoreactivity corresponding to Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH), Tau, Choline Acetyl Transferase (ChAT), Acetylcholinesterase (AChE), and Aspartyl-asparaginyl-β-Hydroxylase (ASPH) such that the results were corrected/normalized to large acidic Ribosomal Protein (RPLPO) as previously described [79,80]. In brief, duplicate 50 μl aliquots containing 50 and ng of protein were allowed to adsorb to the bottoms of 96- well MaxiSorp plates by overnight incubation at 4°C. Superblock (TBS) Blocking Buffer was used to mask non-specific sites. Primary antibodies diluted to 0.2–5.0 μg/ml, were incubated with the proteins overnight at 4°C. HRP-conjugated secondary antibody and the Amplex UltraRed soluble fluorophore were used to detect and quantify immunoreactivity. Fluorescence intensities were measured in a SpectraMax (Ex 530 nm/Em 590 nm). Then, RPLPO immunoreactivity, a loading control, was measured by incubating the same samples with biotinylated anti-RPLPO, the binding of which was detected with streptavidin-conjugated alkaline phosphatase and 4-MUP (Ex 360 nm/ Em 450 nm). The ratios of target protein to RPLPO fluorescence were used for inter-group comparisons. N=6 replicate cultures per group.
▪ Statistical analysis
Results were analyzed by two-way analysis of variance (ANOVA). Post hoc Tukey multiple comparisons tests compared individual differences (GraphPad Prism 9.4, San Diego, CA). Significant (P<0.05) and trend-wise (0.05<P<0.10) differences are tabulated. Significant differences are marked in the graphs.
Results
▪ MTT activity
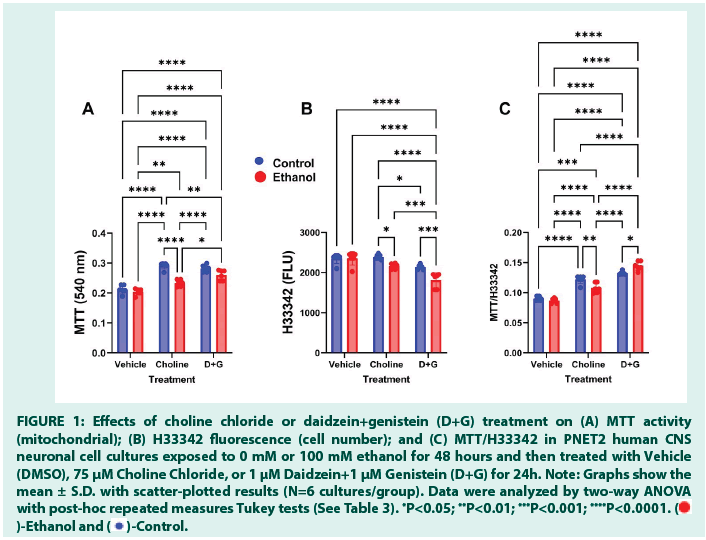
MTT activity was lowest in vehicle-treated cultures with no effects of ethanol. Choline and D+G significantly increased MTT activity relative to vehicle, although ethanol muted that response in choline-treated cultures (Figure 1A). Significant effects of ethanol, treatment, and ethanol x treatment interactions were detected by two-way ANOVA (all P<0.0001) (Table 3).
| Protein | Ethanol Factor | Treatment Factor | Ethanol x Treatment Interaction | |||
|---|---|---|---|---|---|---|
| F-Ratio | P-value | F-Ratio | P-Value | F-Ratio | P-Value | |
| MTT | 46.54 | <0.0001 | 84.7 | <0.0001 | 11.94 | 0.0002 |
| H33342 | 20.53 | <0.0001 | 32.04 | <0.0001 | 5.588 | 0.0087 |
| MTT/H33342 | 0.667 | N.S. | 217.5 | <0.0001 | 15.46 | <0.0001 |
| GAPDH | 5.097 | 0.033 | 1.802 | N.S. | 1.601 | N.S. |
| Tau | 6.237 | 0.02 | 0.338 | N.S. | 2.394 | N.S. |
| ChAT | 0.089 | N.S. | 1.252 | N.S. | 4.822 | 0.017 |
| AChE | 0.604 | N.S. | 5.08 | 0.014 | 0.337 | N.S. |
| ASPH-A85E6 | 0.026 | N.S. | 3.379 | 0.05 | 2.6 | 0.095 |
| ASPH-A85G6 | 0.039 | N.S. | 5.147 | 0.014 | 4.473 | 0.022 |
Note: N.S=Not significant.
Note: Two-way ANOVA Results for MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) activity, H33342 (Hoechst H33342 stain for nuclei of living cells) fluorescence, MTT/H33342 (relative MTT activity corrected for cell number in the cultures) and GAPDH, Tau, ChAT, AChE, ASPH-A85E6 and ASPH-A85G6 immunoreactivities (measured by Duplex ELISA) (N=6/group). Degrees of freedom: F (1, 30) for ethanol or treatment versus vehicle effects; F (2, 30) for ethanol x treatment interactive effects. Bold font marks significant P-values. Bold italics font marks P-values with statistical trends (0.05≤P≤0.10).
Table 3: Summary of Ethanol and Treatment (Choline or Soy Isoflavone) Effects on Markers of Neuronal Function.
▪ Hoechst H33342
The levels of H33342 fluorescence were similar in the vehicle- (control and ethanol-exposed), and choline-treated control cultures, whereas the lowest levels were measured in D+G-treated cultures (Figure 1B). In addition, H33342 was significantly reduced by ethanol in choline- and D+G-treated cultures. The mean level of H33342 in ethanol-exposed, D+G-treated cultures was significantly lower than in all other groups. Two-way ANOVA revealed significant effects of ethanol (P<0.0001), treatment (P<0.0001), and ethanol × treatment interactions (P=0.0087) (Table 3).
▪ MTT/H33342
Mitochondrial activity corrected for cell number is reflected by this ratio. The mean levels of MTT/H33342 increased from vehicle to Choline to D+G, without ethanol effects in the vehicle-treated cultures. In contrast, significant effects of ethanol with choline (reduced) or D+G (increased) occurred relative to the corresponding controls (Figure 1C). The mean levels of MTT/ H33342 were significantly higher in Choline- versus vehicle-treated, and in D+G-treated versus all other groups. Two-way ANOVA demonstrated significant effects of treatment (P<0.0001) and ethanol × treatment interactions (P<0.0001), but not ethanol alone (Table 3).
Figure 1: Effects of choline chloride or daidzein+genistein (D+G) treatment on (A) MTT activity
(mitochondrial); (B) H33342 fluorescence (cell number); and (C) MTT/H33342 in PNET2 human CNS
neuronal cell cultures exposed to 0 mM or 100 mM ethanol for 48 hours and then treated with Vehicle
(DMSO), 75 μM Choline Chloride, or 1 μM Daidzein+1 μM Genistein (D+G) for 24h. Note: Graphs show the
mean ± S.D. with scatter-plotted results (N=6 cultures/group). Data were analyzed by two-way ANOVA
with post-hoc repeated measures Tukey tests (See Table 3). *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Note: ( )-Ethanol and (
)-Ethanol and ( )-Control.
)-Control.
▪ Neuronal function markers
Duplex ELISAs were used to compare the effects of choline and D+G on GAPDH, Tau, ChAT, AChE, and ASPH immunoreactivity in control and ethanol-exposed PNET2 cells. The data were analyzed by two-way ANOVA tests (Table 3) and depicted graphically in Figures 2 and 3.
▪ GAPDH
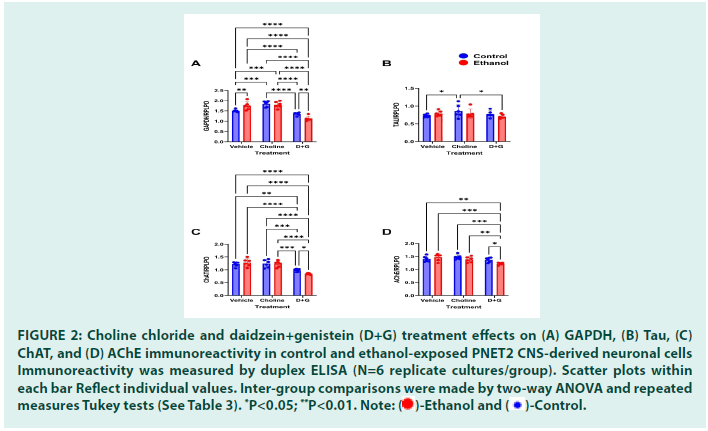
Ethanol significantly reduced GAPDH expression in vehicle-treated but not Choline- or D+G-treated cultures (Figure 2A). The mean levels of GAPDH/ RPLPO were significantly lower in the Ethanol- Vehicle relative to all other groups except Ethanol- D+G. Correspondingly, the two-way ANOVA test demonstrated a significant effect of ethanol, but not treatment with choline or D+G, or an ethanol x treatment interaction (Table 3).
▪ Tau
Ethanol significantly increased cellular Tau expression in vehicle-treated, but not Choline- or D+G-treated cultures (Figure 2B). The mean level of Tau/RPLPO was significantly elevated relative to Control-Vehicle and Control-D+G. Twoway ANOVA detected a significant effect of ethanol, but not treatment with choline or D+G, or an ethanol x treatment interaction (Table 3).
▪ ChAT
Ethanol significantly decreased neuronal ChAT expression in vehicle-treated cultures. However, the levels of ChAT were similarly reduced in Control-Choline, and Control- D+G relative to Control-Vehicle (Figure 2C). In contrast, in the Ethanol-Choline and Ethanol-D+G cultures, ChAT was elevated and not significantly different from Control- Vehicle. Two-way ANOVA detected significant ethanol x treatment interaction effects but no significant effects of ethanol or choline/D+G treatment (Table 3).
▪ AChE
The mean levels of AChE were similar across all culture exposures and treatments. However, modest tight reductions in AChE expression in the Ethanol-D+G cultures rendered the differences statistically significant relative to the Control- and Ethanol-Choline (Figure 2D). Two-way ANOVA detected a significant effect of Choline/D+G treatment, but not ethanol or ethanol x treatment interaction (Table 3).
Figure 2: Choline chloride and daidzein+genistein (D+G) treatment effects on (A) GAPDH, (B) Tau, (C)
ChAT, and (D) AChE immunoreactivity in control and ethanol-exposed PNET2 CNS-derived neuronal cells
Immunoreactivity was measured by duplex ELISA (N=6 replicate cultures/group). Scatter plots within
each bar Reflect individual values. Inter-group comparisons were made by two-way ANOVA and repeated
measures Tukey tests (See Table 3). *P<0.05; **P<0.01.
Note: ( )-Ethanol and (
)-Ethanol and ( )-Control.
)-Control.
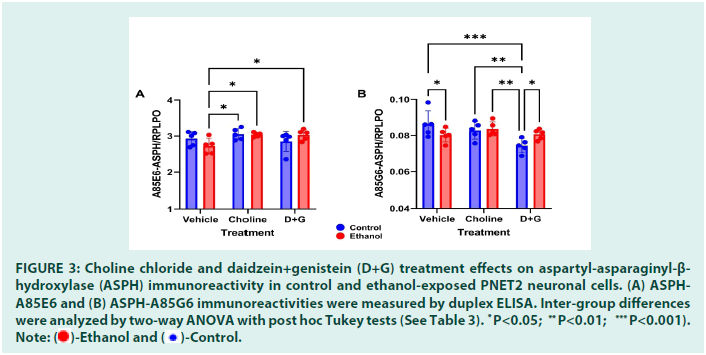
ASPH expression was measured with two different monoclonal antibodies: A85E6 and A85G6. A85E6 binds to a region within the N-terminus of the molecule, and detects fulllength ASPH. A85G6 binds to the C-terminus of ASPH which contains the catalytic domain needed for cell motility [36]. Ethanol caused modest reductions in A85E6-ASPH in vehicletreated cultures whereas Choline and D+G prevented the ethanol-associated decline, resulting in significantly higher levels of A85E6- ASPH immunoreactivity relative to Ethanolvehicle (Figure 3A). Two-way ANOVA detected a significant effect of treatment (Choline or D+G) and a statistical trend for ethanol × treatment interaction, but no significant effect of ethanol (Table 3).
Ethanol caused a significant reduction in the mean level of A85G6-ASPH in vehicle-treated cultures (Figure 3B). However, A85G6-ASPH was most prominently reduced in Control- D+G relative to Control-Vehicle, Controland Ethanol-Choline, and Ethanol-D+G. In contrast, Choline and D+G spared PNET2 cells from ethanol-mediated reductions in A85G6- ASPH expression. Two-way ANOVA detected significant effects of Choline/D+G treatment and ethanol × treatment interaction but not ethanol-only effects on ASPH-A85G6 (Table 3).
Figure 3:Choline chloride and daidzein+genistein (D+G) treatment effects on aspartyl-asparaginyl-β-hydroxylase (ASPH) immunoreactivity in control and ethanol-exposed PNET2 neuronal cells. (A) ASPH-A85E6 and (B) ASPH-A85G6 immunoreactivities were measured by duplex ELISA. Inter-group differences w * ere analyzed by two-way ANOVA with post hoc Tukey tests (See Table 3).
P<0.05; **P<0.01; ***P<0.001).
Note: ( )-Ethanol and (
)-Ethanol and ( )-Control.
)-Control.
Discussion
FASD comprises a group of developmental abnormalities linked to gestational alcohol misuse, and although it is preventable through abstinence, a host of social, cultural, and educational forces continue to challenge public health approaches. However, research in experimental models and humans has shown the value of choline supplementation for reducing FASD [73-77] and was built on ample evidence that choline is needed for neurodevelopment, energy metabolism, and brain functions, including the generation of acetylcholine [81,82], yet its deficiency is common [83]. Choline supplementation in pregnant women and infants at high risk for FASD has yielded promising results [76,77].
Previous studies focused on underlying mechanisms of alcohol-mediated neurodevelopmental defects identified inhibition of insulin signaling as a critical mediator of FASD-related pathologies including neuronal loss, impaired neuronal migration, increased oxidative stress, deficits in mitochondrial function and energy metabolism, and cognitive-motor dysfunctions [19,24- 31]. Preclinical studies showed that many adverse effects of prenatal alcohol exposure can be prevented or reduced by treatment with peroxisome Proliferator-Activated Receptor (PPAR) agonists which are small molecules with both insulin sensitizer and antioxidant actions [57,63-65]. Importantly, PPAR agonists that target both the delta and gamma receptors are highly protective for preventing or reducing permanent neurobehavioral and motor dysfunctions in FASD and other models with brain insulin resistance [57,64,65,84,85]. However, this treatment strategy is potentially problematic for humans due to unknown longterm effects on pregnant women and their offspring. Our alternative approach was to consider intervention with natural food, namely dietary soy, which like PPAR agonists, has known insulin-sensitizer and antioxidant actions [70,71,86].
Two recent preclinical studies support the use of dietary soy as a strategy for preventing longterm adverse effects of excessive alcohol exposure during development. In an adolescent model, dietary soy replacement of casein in the standard rodent diet prevented long-term neurocognitive and motor dysfunctions linked to chronic heavy ethanol exposure [15]. In a chronic gestational alcohol exposure model, dietary soy prevented FASD-associated impairments in placentation, and both craniofacial dysmorphic features and intrauterine growth restriction in the fetuses [72]. Mechanistically, dietary soy enhanced insulin and IGF-1 signaling through metabolic, growth, and antioxidant pathways required for placentation and fetal growth [15,72]. However, dietary soy contains abundant choline and conceivably, its therapeutic effects could be mediated by choline rather than soy. On the other hand, there is established evidence that soy isoflavones have positive effects on insulin-resistance diseases [67,68,70,71]. This study was designed to compare the supportive effects of choline and soy in a short-term in vitro human CNS neuronal model of early developmental alcohol exposure. However, to delineate the effects of soy, the study utilized purified soy isoflavones, namely daidzein and genistein. Daidzein and genistein (D+G) were administered together because exploratory studies showed greater efficacy with their combined versus individual use for supporting MTT activity in PNET2 neuronal cells.
Both choline and D+G enhanced MTT activity and MTT/H33342 relative to vehicle treatment, irrespective of ethanol exposure. The D+G effects were distinguished from those of choline based on the: 1) significantly greater increases in MTT/ H33342, a reflection of mitochondrial activity corrected for cell number; 2) significantly smaller increases in MTT and MTT/H33342 in choline- treated that D+G-treated ethanol-exposed cultures; 3) and the significantly lower mean cell densities (H33342) in D+G-treated versus choline- or vehicle-treated cultures. The lower cell densities in D+G-treated cultures, as well as in ethanol-exposed relative to corresponding choline- or D+G-treated cultures may reflect reduced cellular proliferation or increased cell loss and turnover due to enhanced metabolic activity (MTT/H33342). Together, the findings with respect to MTT and H33342 suggest that both choline and D+G support CNS immature neuronal function, but D+G is more effective and supportive of ethanol-exposed cells.
GAPDH is an insulin-responsive enzyme that has a key role in energy metabolism [87]. The inhibitory effects of ethanol on GAPDH expression observed in vehicle-treated cultures correspond with previous reports [29,44,88,89]. The major effects of choline and D+G were to normalize GAPDH expression in ethanol- exposed cultures. These findings reinforce the observations made with respect to the MTT/ H33342 responses and support the notion that both choline and D+G can enhance and normalize CNS neuronal metabolic function following ethanol exposure.
Tau is a major neuronal cytoskeletal protein that can accumulate with neurodegeneration or toxic responses leading to loss of axonal transport synaptic connections [90,91]. Intra- neuronal tau build-up can promote oxidative stress with attendant neuronal dysfunction [92]. Choline and D+G prevented intra-neuronal Tau build-up, and in that respect were similarly neuroprotective.
ChAT is enzymatically responsible for the generation of acetylcholine. Previous studies linked reductions in ChAT immunoreactivity to impairments in insulin signaling in the brain and cultured neuronal cells [25,39,57,93]. Corresponding with previous reports, ethanol significantly reduced ChAT expression in vehicle- treated cultures. Choline and D+G treatments prevented ethanol-mediated significant reductions in ChAT relative to all control groups suggesting that either treatment would support cognitive-motor functions in ethanol-exposed immature CNS neuronal cells.
AChE has an important role in modulating the degradation of acetylcholine. Previous studies showed reductions in temporal lobe AChE following chronic ethanol exposure [34,38,94]. However, following the short-term in vitro ethanol exposures, AChE immunoreactivity was not significantly altered relative to corresponding control cultures. The only significant effect detected was a modest reduction in AChE immunoreactivity in ethanol-exposed, D+G- treated relative to choline-treated cultures. Otherwise, the effects of ethanol, choline, and D+G exposures/treatments were nil.
ASPH immunoreactivity was assessed using two different monoclonal antibodies, A85G6-ASPH and A85E6-ASPH [36] that respectively bind to the C-terminal and N-terminal regions of the molecule. The rationale is that the C-terminus, which contains the Notch-activating catalytic domain, can be cleaved and function apart from the full-length protein [47,95]. Previous studies demonstrated ethanol inhibition of ASPH expression in FASD and other models of chronic ethanol exposure [36,96,97]. The ethanol-associated modest reductions in A85E6- ASPH and small but significant reductions in A85G6-ASPH, were not as robust as observed in previous reports [36], perhaps due to the short- term nature of the exposures utilized herein. Nonetheless, the studies showed that: 1) choline and D+G normalized A85E6-ASPH relative to the vehicle-treated control cells, and 2) the ethanol-vehicle suppression of A85G6-ASPH was abrogated by choline and D+G relative to their corresponding controls. In essence, ASPH’s functions in immature CNS neuronal cells would likely be supported by either choline or D+G treatment vis-à-vis ethanol exposure [98-100].
Conclusion
In a short-term in vitro ethanol exposure model utilizing CNS human immature neuronal cells (PNET2), both choline and D+G were effective in preventing several adverse effects of ethanol on metabolic function and neuronal protein expression. The use of D+G was designed to compare the therapeutic effects of soy isoflavones to those of choline which is abundantly present in dietary soy. The protective effects of choline and D+G were similar but not identical. In particular, for ethanol-exposed neuronal cells, the enhancements of MTT and MTT/H33342 were greater with D+G than choline, whereas GAPDH and ChAT expression were more prominently up-regulated by choline than D+G. Altogether, the results suggest that both approaches are neuroprotective vis-à-vis alcohol exposure, indicating that soy isoflavones in the absence of additional choline, can support neuronal functions. However, in vivo, dietary soy may be more effective given the combined administration of isoflavones (bioactive insulin sensitizers) with choline (a natural component of soy), and the added benefit of providing nutritional support beyond choline micronutrient supplementation, particularly in states of malnutrition.
Funding
This work was supported by grants from the National Institutes of Health, National Institutes on Alcohol Abuse and Alcoholism, AA-011431, AA-028408, AA-024092, the VA Biomedical Laboratory Research and Development (BLRD) Career Development Award-2 1IK2BX004961, and a Brown University Undergraduate Teaching and Research Award.
Conflicts of Interest
The authors have no conflicts of interest.
References
- Mukherjee RA, Hollins S, Turk J, et al. Fetal alcohol spectrum disorder: An overview. J R Soc Med. 99(6):298-302 (2006).
[Crossref] [Google Scholar] [Pubmed]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: An overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood). 230(6):357-365 (2005).
[Crossref] [Google Scholar] [Pubmed]
- Del Campo M, Jones KL. A review of the physical features of the fetal alcohol spectrum disorders. Eur J Med Genet. 60(1):55-64 (2017).
[Crossref] [Google Scholar] [Pubmed]
- Roozen S, Black D, Peters GY, et al. Fetal alcohol spectrum disorders (FASD): An approach to effective prevention. Curr Dev Disord Rep. 3:229-234 (2016).
[Crossref] [Google Scholar] [Pubmed]
- Marquardt K, Brigman JL. The impact of prenatal alcohol exposure on social, cognitive and affective behavioral domains: Insights from rodent models. Alcohol. 51:1-15 (2016).
[Crossref] [Google Scholar] [Pubmed]
- Murawski NJ, Moore EM, Thomas JD, et al. Advances in diagnosis and treatment of fetal alcohol spectrum disorders: from animal models to human studies. Alcohol Res. 37(1):97-108 (2015).
[Google Scholar] [Pubmed]
- Reid N, Dawe S, Shelton D, et al. Systematic review of fetal alcohol spectrum disorder interventions across the life span. Alcohol Clin Exp Res. 39(12):2283-2295 (2015).
[Crossref] [Google Scholar] [Pubmed]
- Roozen S, Peters GJ, Kok G, et al. Worldwide prevalence of fetal alcohol spectrum disorders: A systematic literature review including meta-analysis. Alcohol Clin Exp Res. 40(1):18-32 (2016).
[Crossref] [Google Scholar] [Pubmed]
- Lange S, Probst C, Gmel G, et al. Global prevalence of fetal alcohol spectrum disorder among children and youth: A systematic review and meta-analysis. JAMA pediatr. 171(10):948-956 (2017).
[Crossref] [Google Scholar] [Pubmed]
- de La Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathol. 127:71-90 (2014).
[Crossref] [Google Scholar] [Pubmed]
- Schmidt KS, Gallo JL, Ferri C, et al. The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dement Geriatr Cogn Disor. 20(5):286-291 (2005).
[Crossref] [Google Scholar] [Pubmed]
- Elofson J, Gongvatana W, Carey KB, et al. Alcohol use and cerebral white matter compromise in adolescence. Addict Behav. 38(7):2295-2305(2013).
[Crossref] [Google Scholar] [Pubmed]
- Jacobus J, Squeglia LM, Bava S, et al. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: A 3-year investigation. Psychiatry Res. 214(3):374-381(2013).
[Crossref] [Google Scholar] [Pubmed]
- Phillips SC, Harper CG, Kril J. A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain. 110(2):301-314 (1987).
[Crossref] [Google Scholar] [Pubmed]
- Tong M, Ziplow JL, Mark P, et al. Dietary Soy Prevents Alcohol-Mediated Neurocognitive Dysfunction and Associated Impairments in Brain Insulin Pathway Signaling in an Adolescent Rat Model. Biomolecules. 12(5):676 (2022).
[Crossref] [Google Scholar] [Pubmed]
- Zink M, Ferbert T, Frank ST, et al. Perinatal exposure to alcohol disturbs spatial learning and glutamate transmission-related gene expression in the adult hippocampus. Eur J Neurosci. 34(3):457-468 (2011).
[Crossref] [Google Scholar] [Pubmed]
- Goodlett CR, Thomas JD, West JR, et al. Long-term deficits in cerebellar growth and rotarod performance of rats following “binge-like” alcohol exposure during the neonatal brain growth spurt. Neurotoxicol Teratol. 13(1):69-74 (1991).
[Crossref] [Google Scholar] [Pubmed]
- Lupton C, Burd L, Harwood R et al. Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet. 127C(1):42-50 (2004).
[Crossref] [Google Scholar] [Pubmed]
- de la Monte SM, Wands JR. Role of central nervous system insulin resistance in fetal alcohol spectrum disorders. J Popul Ther Clin Pharmacol. 17(3):e390-404 (2010).
[Google Scholar] [Pubmed]
- Collins MA, Tajuddin N, Moon KH, et al. Alcohol, Phospholipase A 2-associated Neuroinflammation, and ω3 Docosahexaenoic Acid Protection. Mol Neurobiol. 50:239-45(2014).
[Crossref] [Google Scholar] [Pubmed]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 44(2):115-127 (2009).
[Crossref] [Google Scholar] [Pubmed]
- Eysseric H, Gonthier B, Soubeyran A, et al. Characterization of the production of acetaldehyde by astrocytes in culture after ethanol exposure. Alcohol Clin Exp Res. 21(6):1018-1023 (1997).
[Crossref] [Google Scholar] [Pubmed]
- Melgaard B. The neurotoxicity of ethanol. Acta Neurol Scand. 67(3):131-142 (1983).
[Crossref] [Google Scholar] [Pubmed]
- Carter JJ, Tong M, Silbermann E, et al. Ethanol impaired neuronal migration is associated with reduced aspartyl-asparaginyl-β-hydroxylase expression. Acta Neuropathol. 116(3):303-315 (2008).
[Crossref] [Google Scholar] [Pubmed]
- Cohen AC, Tong M, Wands JR, et al. Insulin and insulin-like growth factor resistance with neurodegeneration in an adult chronic ethanol exposure model. Alcohol Clin Exp Res. 31(9):1558-1573 (2007).
[Crossref] [Google Scholar] [Pubmed]
- De la Monte SM, Ganju N, Banerjee K, et al. Partial rescue of ethanol-induced neuronal apoptosis by growth factor activation of phosphoinositol-3-kinase. Alcohol Clin Exp Res. 24(5):716-726 (2000).
[Crossref] [Google Scholar] [Pubmed]
- De la Monte SM, Tong M, Bowling N, et al. si-RNA inhibition of brain insulin or insulin-like growth factor receptors causes developmental cerebellar abnormalities: Relevance to fetal alcohol spectrum disorder. Mol Brain. 4(1):13 (2011).
[Crossref] [Google Scholar] [Pubmed]
- De La Monte SM, Tong M, Cohen AC, et al. Insulin and insulin-like growth factor resistance in alcoholic neurodegeneration. Alcohol Clin Exp Res. 32(9):1630-1644 (2008).
[Crossref] [Google Scholar] [Pubmed]
- De la Monte SM, Wands JR. Chronic gestational exposure to ethanol impairs insulin-stimulated survival and mitochondrial function in cerebellar neurons. Cell Mol Life Sc. 59:882-893 (2002).
[Crossref] [Google Scholar] [Pubmed]
- Monte SD, Xu XJ, Wands JR, et al. Ethanol inhibits insulin expression and actions in the developing brain. Cell Mol Life Sci. 62:1131-1145 (2005).
[Crossref] [Google Scholar] [Pubmed]
- Ewenczyk A, Ziplow J, Tong M, et al. Sustained impairments in brain insulin/IGF signaling in adolescent rats subjected to binge alcohol exposures during development. J Clin Exp Pathol. 2(2)-106(2012).
[Crossref] [Google Scholar] [Pubmed]
- Nguyen VA, Le T, Tong M, et al. Impaired insulin/IGF signaling in experimental alcohol-related myopathy. Nutrients. 4(8):1058-1075 (2012).
[Crossref] [Google Scholar] [Pubmed]
- Resnicoff M, Rubini M, Baserga R, et al. Ethanol inhibits insulin-like growth factor-1-mediated signalling and proliferation of C6 rat glioblastoma cells. Lab Invest. 71(5):657-662 (1994).
[Google Scholar] [Pubmed]
- Tong M, Yu R, Deochand C, et al. Differential contributions of alcohol and the nicotine-derived nitrosamine ketone (NNK) to insulin and insulin-like growth factor resistance in the adolescent rat brain. Alcohol Alcoho. 50(6):670-679 (2015).
[Crossref] [Google Scholar] [Pubmed]
- Xu J, Yeon JE, Chang H, et al. Ethanol impairs insulin-stimulated neuronal survival in the developing brain: role of PTEN phosphatase. J Biol Chem. 278(29):26929-26937 (2003).
[Crossref] [Google Scholar] [Pubmed]
- De la Monte SM, Tong M, Carlson RI, et al. Ethanol inhibition of aspartyl-asparaginyl-β-hydroxylase in fetal alcohol spectrum disorder: Potential link to the impairments in central nervous system neuronal migration. Alcohol. 43(3):225-240 (2009).
[Crossref] [Google Scholar] [Pubmed]
- Luo J. Lithium-mediated protection against ethanol neurotoxicity. Front Neurosci. 4:41 (2010).
[Crossref] [Google Scholar] [Pubmed]
- Jamal M, Ameno K, Miki T, et al. Cholinergic alterations following alcohol exposure in the frontal cortex of Aldh2-deficient mice models. Brain Res. 1295:37-43 (2009).
[Crossref] [Google Scholar] [Pubmed]
- Soscia SJ, Tong M, Xu XJ, et al. Chronic gestational exposure to ethanol causes insulin and IGF resistance and impairs acetylcholine homeostasis in the brain. Cell Mol Life Sci. 63:2039-2056 (2006).
[Crossref] [Google Scholar] [Pubmed]
- Bordner KA, George ED, Carlyle BC, et al. Functional genomic and proteomic analysis reveals disruption of myelin-related genes and translation in a mouse model of early life neglect. Front Psychiatry. 2:18 (2011).
[Crossref] [Google Scholar] [Pubmed]
- Chiappelli F, Taylor AN, De Los Monteros AE, et al. Fetal alcohol delays the developmental expression of myelin basic protein and transferrin in rat primary oligodendrocyte cultures. Int J Dev Neurosci. 9(1):67-75 (1991).
[Crossref] [Google Scholar] [Pubmed]
- Lewohl JM, Wixey J, Harper CG, et al. Expression of MBP, PLP, MAG, CNP, and GFAP in the human alcoholic brain. Alcohol Clin Exp Res. 29(9):1698-1705 (2005).
[Crossref] [Google Scholar] [Pubmed]
- Tong M, Yu R, Silbermann E, et al. Differential contributions of alcohol and nicotine-derived nitrosamine ketone (NNK) to white matter pathology in the adolescent rat brain. Alcohol Alcohol. 50(6):680-689 (2015).
[Crossref] [Google Scholar] [Pubmed]
- De La Monte SM, Yeon JE, Tong M, et al. Insulin resistance in experimental alcohol-induced liver disease. J Gastroenterol Hepatol. 23(8pt2):e477-486 (2008).
[Crossref] [Google Scholar] [Pubmed]
- Gundogan F, Elwood G, Longato L, et al. Impaired placentation in fetal alcohol syndrome. Placenta. 29(2):148-157 (2008).
[Crossref] [Google Scholar] [Pubmed]
- Lavaissiere L, Jia S, Nishiyama M, et al. Overexpression of human aspartyl (asparaginyl) beta-hydroxylase in hepatocellular carcinoma and cholangiocarcinoma. J Clin Invest. 98(6):1313-1323 (1996).
[Crossref] [Google Scholar] [Pubmed]
- Jia S, VanDusen WJ, Diehl RE, et al. cDNA cloning and expression of bovine aspartyl (asparaginyl) beta-hydroxylase. J Biol Chem. 267(20):14322-14327 (1992).
[Crossref] [Google Scholar] [Pubmed]
- Wang QP, VanDusen WJ, Petroski CJ, et al. Bovine liver aspartyl beta-hydroxylase. Purification and characterization. J Biol Chem. 266(21):14004-14010 (1991).
[Crossref] [Google Scholar] [Pubmed]
- Lawton M, Tong M, Gundogan F, et al. Aspartyl-(asparaginyl) β-hydroxylase, hypoxia-inducible factor-1α and notch cross-talk in regulating neuronal motility. Oxid Med Cell Longev. 3(5):347-356 (2010).
[Crossref] [Google Scholar] [Pubmed]
- Sepe PS, Lahousse SA, Gemelli B, et al. Role of the aspartyl-asparaginyl-β-hydroxylase gene in neuroblastoma cell motility. Lab Invest. 82(7):881-891 (2002).
[Crossref] [Google Scholar] [Pubmed]
- Silbermann E, Moskal P, Bowling N, et al. Role of aspartyl-(asparaginyl)-β-hydroxylase mediated notch signaling in cerebellar development and function. Behav Brain Funct. 6(1):1-3 (2010).
[Crossref] [Google Scholar] [Pubmed]
- Borgas DL, Gao JS, Tong M, et al. Potential role of phosphorylation as a regulator of aspartyl-(asparaginyl)-β-hydroxylase: relevance to infiltrative spread of human hepatocellular carcinoma. Liver Cancer. 4(3):139-153 (2015).
[Crossref] [Google Scholar] [Pubmed]
- Tong M, Gao JS, Borgas D, et al. Phosphorylation Modulates Aspartyl-(Asparaginyl)-β Hydroxylase Protein Expression, Catalytic Activity and Migration in Human Immature Neuronal Cerebellar Cells. Cell Biol (Henderson, NV). 6(2): 133 (2013).
[Crossref] [Google Scholar] [Pubmed]
- Gilde AJ, Van Bilsen M. Peroxisome proliferator-activated receptors (PPARS): Regulators of gene expression in heart and skeletal muscle. Acta Physiol Scand. 178(4):425-434 (2003).
[Crossref] [Google Scholar] [Pubmed]
- Lee CH, Olson P, Evans RM , et al. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 144(6):2201-2207 (2003).
[Crossref] [Google Scholar] [Pubmed]
- Jiang G, Zhang BB. Modulation of insulin signalling by insulin sensitizers. Biochem Soc Trans. 33(2):358-361 (2005).
[Crossref] [Google Scholar] [Pubmed]
- Le T, Tong M, Nguyen V, et al. PPAR agonist rescue of ethanol-impaired brain insulin signaling: cerebellar slice culture model. J Drug Alcohol Res. 2(1):1-9 (2013).
- Pang M, de la Monte SM, Longato L, et al. PPARδ agonist attenuates alcohol-induced hepatic insulin resistance and improves liver injury and repair. J Hepatol. 50(6):1192-1201 (2009).
[Crossref] [Google Scholar] [Pubmed]
- de la Monte SM, Pang M, Chaudhry R, et al. Peroxisome proliferator-activated receptor agonist treatment of alcohol-induced hepatic insulin resistance. Hepatol Res. 41(4):386-98 (2011).
[Crossref] [Google Scholar] [Pubmed]
- Ramirez T, Tong M, Ayala CA, et al. Structural correlates of PPAR agonist rescue of experimental chronic alcohol-induced steatohepatitis. J Clinic Exp Pathol. 2(4) (2012).
[Crossref] [Google Scholar] [Pubmed]
- Enomoto N, Takei Y, Hirose M, et al. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-γ, pioglitazone. J Pharmacol Exp Ther. 306(3):846-854 (2003).
[Crossref] [Google Scholar] [Pubmed]
- Tomita K, Azuma T, Kitamura N, et al. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology. 126(3):873-885 (2004).
[Crossref] [Google Scholar] [Pubmed]
- Tong M, Dominguez C, Didsbury J, et al. Targeting Alzheimer’s disease neuro-metabolic dysfunction with a small molecule nuclear receptor agonist (T3D-959) reverses disease pathologies. J Alzheimers Dis Parkinsonism. 6(3):238 (2016).
[Crossref] [Google Scholar] [Pubmed]
- De la Monte SM, Tong M, Lester-Coll N, et al. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: Relevance to Alzheimer's disease. J Alzheimers Dis. 10(1):89-109 (2006).
[Crossref] [Google Scholar] [Pubmed]
- De la Monte SM, Tong M, Schiano I, et al. Improved brain insulin/IGF signaling and reduced neuroinflammation with T3D-959 in an experimental model of sporadic Alzheimer’s disease. J Alzheimers Dis. 55(2):849-864 (2017).
[Crossref] [Google Scholar] [Pubmed]
- Clark JL, Taylor CG, Zahradka P, et al. Rebelling against the (insulin) resistance: a review of the proposed insulin-sensitizing actions of soybeans, chickpeas, and their bioactive compounds. Nutrients. 10(4):434 (2018).
[Crossref] [Google Scholar] [Pubmed]
- Hassan, SM Soybean, Nutrition and Health. In Soybean - Bio-Active Compounds, El-Shemy, HA, Ed. IntechOpen: London, 2013.
- Heo G, Ko KS. Long-term feeding of soy protein attenuates choline deficient-induced adverse effects in wild type mice and prohibitin 1 deficient mice response more sensitively. Prev Nutr Food Sci. 24(1):32-40 (2019).
[Crossref] [Google Scholar] [Pubmed]
- Kim MH, Kang KS. Isoflavones as a smart curer for non-alcoholic fatty liver disease and pathological adiposity via ChREBP and Wnt signaling. Prev Med. 54:S57-63 (2012).
[Crossref] [Google Scholar] [Pubmed]
- Tovar AR, Torre-Villalvazo I, Ochoa M, et al. Soy protein reduces hepatic lipotoxicity in hyperinsulinemic obese Zucker fa/fa rats. J Lipid Res. 46(9):1823-1832 (2005).
[Crossref] [Google Scholar] [Pubmed]
- Wagner JD, Zhang L, Shadoan MK, et al. Effects of soy protein and isoflavones on insulin resistance and adiponectin in male monkeys. Metabolism. 57:S24-31 (2008).
[Crossref] [Google Scholar] [Pubmed]
- Qi W, Gundogan F, Gilligan J, et al. Dietary Soy Prevents Fetal Demise, Intrauterine Growth Restriction, Craniofacial Dysmorphic Features, and Impairments in Placentation Linked to Gestational Alcohol Exposure: Pivotal Role of Insulin and Insulin-Like Growth Factor Signaling Networks. Alcohol. S0741-8329(23): 00026-5 (2023).
[Crossref] [Google Scholar] [Pubmed]
- Ryan SH, Williams JK, Thomas JD, et al. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: Effects of varying the timing of choline administration. Brain Res. 1237:91-100 (2008).
[Crossref] [Google Scholar] [Pubmed]
- Thomas JD, Biane JS, O'Bryan KA, et al. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 121(1):120-130 (2007).
[Crossref] [Google Scholar] [Pubmed]
- Thomas JD, Idrus NM, Monk BR, et al. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol. 88(10):827-837 (2010).
[Crossref] [Google Scholar] [Pubmed]
- Wozniak JR, Fink BA, Fuglestad AJ, et al. Four-year follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder. J Neurodev Disord. 12(1):1-3 (2020).
[Crossref] [Google Scholar] [Pubmed]
- Wozniak JR, Fuglestad AJ, Eckerle JK, et al. Choline supplementation in children with fetal alcohol spectrum disorders has high feasibility and tolerability. Nutr Res. 33(11):897-904 (2013).
[Crossref] [Google Scholar] [Pubmed]
- De la Monte SM, Wands JR. Mitochondrial DNA damage and impaired mitochondrial function contribute to apoptosis of insulin-stimulated ethanol-exposed neuronal cells. Alcohol Clin Exp Res. 25(6):898-906 (2001).
[Crossref] [Google Scholar] [Pubmed]
- Andreani T, Tong M, Gundogan F, et al. Differential effects of 3rd trimester-equivalent binge ethanol and tobacco-specific nitrosamine ketone exposures on brain insulin signaling in adolescence. J Diabetes Relat Disord. 1(1):105-114 (2016).
[Google Scholar] [Pubmed]
- Deochand C, Tong M, Agarwal AR, et al. Tobacco smoke exposure impairs brain insulin/IGF signaling: Potential co-factor role in neurodegeneration. J Alzheimers Dis. 50(2):373-386 (2016).
[Crossref] [Google Scholar] [Pubmed]
- Zeisel SH. Choline: An important nutrient in brain development, liver function and carcinogenesis. J Am Coll Nutr. 11(5):473-481 (1992).
[Crossref] [Google Scholar] [Pubmed]
- Phillis JW. Acetylcholine release from the central nervous system: A 50-year retrospective. Crit Rev Neurobiol. 17(3-4): 161-217 (2005).
[Crossref] [Google Scholar] [Pubmed]
- Secades JJ, Frontera G. CDP-choline: Pharmacological and clinical review. Crit Rev Neurobiol. 17:1-54 (1995).
[Crossref] [Google Scholar] [Pubmed]
- Reich D, Gallucci G, Tong M, et al. Therapeutic Advantages of Dual Targeting of PPAR-δ and PPAR-γ in an Experimental Model of Sporadic Alzheimer’s Disease. J Parkinsons Dis Alzheimers Dis. 5(1) (2018).
[Crossref] [Google Scholar] [Pubmed]
- Tong M, Deochand C, Didsbury J, et al. T3D-959: A multi-faceted disease remedial drug candidate for the treatment of Alzheimer’s disease. J Alzheimers Dis. 51(1):123-138 (2016).
[Crossref] [Google Scholar] [Pubmed]
- Chiang YF, Shaw HM, Yang MF, et al. Dietary oxidised frying oil causes oxidative damage of pancreatic islets and impairment of insulin secretion, effects associated with vitamin E deficiency. Br J Nutr. 105(9):1311-1319 (2011).
[Crossref] [Google Scholar] [Pubmed]
- Alexander-Bridges M, Dugast I, Ercolani L, et al. Multiple insulin-responsive elements regulate transcription of the GAPDH gene. Adv Enzyme Regul. 32:149-159 (1992).
[Crossref] [Google Scholar] [Pubmed]
- Banerje K, Mohry L, Wands JR, et al. Ethanol inhibition of insulin signaling in hepatocellular carcinoma cells. Alcohol Clinic Exp Res. 22(9):2093-2101(1998).
[Crossref] [Google Scholar] [Pubmed]
- DeNucci SM, Tong M, Longato L, et al. Rat strain differences in susceptibility to alcohol-induced chronic liver injury and hepatic insulin resistance. Gastroenterol Res Pract. 2010:312790 (2010).
[Crossref] [Google Scholar] [Pubmed]
- Mandelkow EM, Stamer K, Vogel R, et al. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 24(8):1079-1085 (2003).
[Crossref] [Google Scholar] [Pubmed]
- Moroz N, Tong M, Longato L, et al. Limited Alzheimer-type neurodegeneration in experimental obesity and type 2 diabetes mellitus. J Alzheimers Dis. 15(1):29-44 (2008).
[Crossref] [Google Scholar] [Pubmed]
- Haque MM, Murale DP, Kim YK, et al. Crosstalk between oxidative stress and tauopathy. Int J Mol Sci. 20(8):1959 (2019).
[Crossref] [Google Scholar] [Pubmed]
- Rivera EJ, Goldin A, Fulmer N, et al. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 8(3):247-268 (2005).
[Crossref] [Google Scholar] [Pubmed]
- Pires RG, Pereira SR, Oliveira-Silva IF, et al. Cholinergic parameters and the retrieval of learned and re-learned spatial information: a study using a model of Wernicke–Korsakoff Syndrome. Behav Brain Res. 162(1):11-21 (2005).
[Crossref] [Google Scholar] [Pubmed]
- Ince N, de la Monte SM, Wands JR, et al. Overexpression of human aspartyl (asparaginyl) β-hydroxylase is associated with malignant transformation. Cancer Res. 60(5):1261-1266(2000).
[Google Scholar] [Pubmed]
- Gundogan F, Elwood G, Greco D, et al. Role of aspartyl-(asparaginyl) β-hydroxylase in placental implantation: relevance to early pregnancy loss. Hum Pathol. 38(1):50-59 (2007).
[Crossref] [Google Scholar] [Pubmed]
- Tong M, Gonzalez-Navarrete H, Kirchberg T, et al. Ethanol-Induced White Matter Atrophy Is Associated with Impaired Expression of Aspartyl-Asparaginyl-β-Hydroxylase (ASPH) and Notch Signaling in an Experimental Rat Model. J Drug Alcohol Res. 6: 236033 (2017).
[Crossref] [Google Scholar] [Pubmed]
- DaDalt AA, Bonham CA, Lotze GP, et al. Src-mediated phosphorylation of the ribosome biogenesis factor hYVH1 affects its localization, promoting partitioning to the 60S ribosomal subunit. J Biol Chem. 298(12): 102679 (2022).
[Crossref] [Google Scholar] [Pubmed]
- Liu HT, Zou YX, Zhu WJ, et al. lncRNA THAP7-AS1, transcriptionally activated by SP1 and post-transcriptionally stabilized by METTL3-mediated m6A modification, exerts oncogenic properties by improving CUL4B entry into the nucleus. Cell Death Differ. 29(3):627-641 (2022).
[Crossref] [Google Scholar] [Pubmed]
- Xie JJ, Jiang YY, Jiang Y, et al. Super-enhancer-driven long non-coding RNA LINC01503, regulated by TP63, is over-expressed and oncogenic in squamous cell carcinoma. Gastroenterology. 154(8):2137-2151 (2018).
[Crossref] [Google Scholar] [Pubmed]